Robotics in uro-oncologic surgery
Elisa De Lorenzis, Carlotta Palumbo, Gabriele Cozzi, Michele Talso, Marco Rosso, Beatrice Costa, Franco Gadda and Bernardo Rocco
Department of Specialist Surgical Sciences, University of Milan, Urology Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Correspondence to: Bernardo Rocco. Email: bernardo.rocco@gmail.com
Abstract
In urology, the main use for the robotic technique has been in radical prostatectomy for prostate cancer. Robotic surgery for other organs, such as the kidneys and bladder, has been less explored. However, partial nephrectomy or radical nephroureterectomy can be difficult for inexperienced laparoscopic surgeons. The advent of the da Vinci robot, with multijointed endowristed instruments and stereoscopic vision, decreases the technical difficulty of intracorporeal suturing and improves the reconstructive steps.
The objective of this article is to offer an overview of all robotic procedures recently developed in the field of urology. We evaluate the feasibility of these procedures and their potential advantages and disadvantages. We also describe perioperative, postoperative, and oncologic outcomes of robot-assisted surgery as well as perform a comparison with open and laparoscopic techniques.
Comparative data and an adequate follow-up are needed to demonstrate equivalent oncologic outcomes in comparison with traditional open or laparoscopic procedures.
Keywords: robotics, minimally invasive surgery, cystectomy, nephroureterectomy, nephrectomy, partial nephrectomy
Introduction
Laparoscopic surgery represented a major breakthrough in the urologic field, due to the decreased intraoperative estimated blood loss (EBL), shorter hospital stay, and quicker return of the patient to function [1]. The main obstacle that prevented the widespread use of the laparoscopic approach is the steep learning curve required for a surgeon to achieve proficiency [2].
The advent of robotic-assisted laparoscopic surgery represented a great advantage for experienced laparoscopic surgeons and for laparoscopically naïve ones.
Urology is the surgical specialty that really introduced the possibilities of robot-assisted surgery and ensured its very rapid development with its application to the radical prostatectomy procedure.
Urologists experienced in laparoscopy found in the robotic-assisted approach a better quality of vision, with 3D resolution, precise movements, and no limitations on movements. On the other hand, open surgeons were provided with a minimally invasive technique with a simpler and faster learning curve [3].
In the field of oncologic urologic surgery, the robotic approach has been applied to radical prostatectomy, radical cystoprostatectomy, partial and radical nephrectomy, and nephroureterectomy [2].
Robot-assisted radical cystoprostatectomy
Radical cystectomy is the gold standard of treatment for patients with muscle-invasive bladder cancer (BC) and for those with high-risk recurrent noninvasive disease, in terms of local control and long-term disease-free survival [4].
In recent years, a case series has been reported in the literature of radical cystectomy performed with minimally invasive approaches such as laparoscopic and robotic-assisted techniques [5]. These reports have demonstrated the feasibility of robot-assisted radical cystectomy (RARC); the potential benefits of this procedure are lower surgical blood loss, more rapid return of bowel function, and a shorter hospitalisation [6, 7].
However, due to the lack of sufficient comparative data and long-term follow-up, there is diffidence about the real advantages of the laparoscopic and robotic approaches in comparison with traditional open radical cystectomy (ORC).
Currently, high levels of clinical evidence of the benefits of RARC are lacking in peer-reviewed literature, because the majority of the reports are single-institution case series and because randomised clinical trials are lacking or with a limited number of patients enrolled [8].
For these reasons, both RARC and laparoscopic radical cystectomy (LRC) are still considered experimental [9].
Indications for RARC
Indications for RARC are the same as those for ORC contemplated in the European Association of Urology Guidelines: ‘localized BC (cT2N0M0) or locally advanced BC (cT3a-T4a, N0-NX, M0). Radical cystectomy may be an appropriate initial treatment for some patients with first occurrence of high-risk non muscle invasive BC or recurrent non muscle invasive BC. Patients with high-grade, non muscle invasive disease (Ta-T1) or carcinoma in situ that recurs following treatment with intravesical bacillus Calmette-Guérin or patients with intermediate-risk papillary disease that cannot be controlled with transurethral resection and intravesical therapy can be candidated to radical cystectomy’ [10].
Often patients undergoing RARC represented a highly selected population as compared with concurrent patients undergoing an open approach.
The presence of bulky lymphadenopathy, locally advanced disease (T4), uncorrected coagulopathy, and morbid obesity [body mass index (BMI) > 35] is a strong contraindication to minimally invasive radical cystectomy. In addition, patients with significant prior abdominal surgery and/or prior radiation therapy are often avoided because of concerns about prolonged laparoscopic adhesiolysis and the consequent risks of bowel injury and poor anastomotic healing [11, 12].
During RARC, ileal conduit or orthotopic bladder substitution can be performed with intracorporeal or extracorporeal techniques. Laparoscopic intracorporeal construction of urinary diversion with or without robotic assistance has been tested in small series only. It is a challenging and lengthy procedure with the current technical equipment available. This technique is considered an experimental technique, in the Guidelines of the European Association of Urology, while laparoscopic cystectomy and pelvic lymphadenectomy (with or without robotic assistance) with extracorporeal construction of urinary diversion are considered an option for surgical treatment [level of evidence (LE): 3] [10].
Robotic-assisted lymph node dissection during RARC
During radical cystectomy, along with the bladder and the surrounding perivesical tissues and pelvic lymph nodes, the adjoining organs are also removed. In men, the prostate and the seminal vesicles are removed, while in women, the ovaries, fallopian tubes, and uterus may be removed. Thus, RARC must be able to replicate the lymphadenectomy performed with open surgery to be considered as an alternative technique [13].
The feasibility of pelvic lymph node dissection during RARC as well as the adequacy of nodal yield has been a matter of concern. However, the improved range of movement of the da Vinci S and Si systems has placated many of these preoccupations. Acceptable lymph node yields have been reported in RARC series, with mean lymph node counts up to 18 (range: 6–43) [14].
A non-randomised comparative study has reported equivalence between ORC and RARC lymph node yields (15 versus 16) [15]. Another randomised, prospective trial showed equivalence in node counts with a mean of 18 nodes harvested in the open group and19 nodes in the robotic group [8].
A prospectively maintained but retrospectively analysed study based on the International Robotic Cystectomy Consortium database concluded that the rates of lymphadenectomy at RARC for advanced BC are similar to those of ORC series [16 ]. The learning curve could certainly play a role in the number of nodes removed during pelvic lymph node dissection, meaning that robotic surgeons remove more nodes as their case series increase.
Perioperative and postoperative outcomes of RARC
There is a lack of consensus in the way of reporting complications in urological surgery. For this reason, comparisons of surgical results between different techniques can be difficult [17]. With the introduction and the spread of the Clavien classification, the system of complications reporting is now defined and standardised [18].
The perioperative outcomes of RARC seemed to be comparable with ORC series [19]. A study comparing postoperative complications in 104 ORCs versus 83 RARCs has suggested a decrease in the rate of high-grade complications compared with ORC, with a 17% major complication rate in patients undergoing RARC compared with a 31% high-grade complication rate observed in ORC patients. The authors concluded that RARC is an independent predictor of fewer overall and major complications [20].
Smith et al [21] reviewed RARC’s technique and associated preoperative and oncological outcomes. They concluded that, although long-term data are not yet available, RARC is effective in decreasing hospital stay, analgesic requirement, time to return of bowel function, and improving health-related quality of life.
A recent prospective cohort comparison of 158 patients undergoing ORC (n = 52), LRC (n = 58), or RARC (n = 48) showed that RARC had the lowest transfusion and complication rates and the shortest length of stay. However, operating time was shorter in ORC and LRC compared with RARC [22].
The only prospective randomised controlled noninferiority study [8] that compared ORC versus RARC procedures demonstrated lower mean EBL (575 versus 258 mL), lower analgesic requirements (147.4 versus 89.0 mg), and earlier return to bowel function (4.3 versus 3.2 days) in favour of robotic assistance. The authors observed similar complication rates between the two procedural groups and significantly longer operating room time in the robotic group (4.20 hours) versus the open cohort (3.51 hours).
Oncologic outcomes after RARC
Long-term oncologic outcomes to define the place of the robot-assisted approach for BC are awaited; there are concerns over the ability of minimally invasive radical cystectomy to treat bulky and locally advanced disease, making careful patient selection vital. More definitive long-term data are still needed, and ultimately head-to-head randomised comparisons will provide the most useful data [23].
In accordance with Dasgupta et al [24], intermediate oncological outcomes are encouraging and comparable with the contemporary ORC series. Indeed, the authors reported 100% overall and recurrence-free survival at 2 years, while at a maximum follow-up of 3.5 years, the overall and recurrence-free survival were 95% and 90%, respectively.
A recent collaborative review [23] seems to indicate that in patients with BC ≤ pT2, LRC and RARC achieve comparable, acceptable positive surgical margins (PSMs) to ORC. However, in those with higher-stage disease (pT4) and perhaps in those with pT3 disease, PSM rates are higher, and the loss of tactile feedback or inexperience with the technique may contribute to this rise.
Robotic-assisted radical nephrectomy
Open radical nephrectomy (ORN) is the gold standard of treatment for large renal tumours and has demonstrated good long-term oncologic outcomes. Since the first laparoscopic nephrectomy in 1991 [25], the use of a minimally invasive approach has become the preferred treatment modality for most nephrectomy cases.
The potential benefits of the da Vinci surgery can be more evident during reconstructive rather than during ablative procedures. For this reason, there is less appeal to use this technology for the ablative surgery of the upper urinary tract (UUT) [26–28].
Potential advantages of robotic assistance for performing a radical nephrectomy are a magnified 3D high-definition vision and the movements of the EndoWrist instruments that can facilitate a careful dissection and ligation of the renal hilum. Nevertheless, robotic-assisted radical nephrectomy (RARN) can be used as training during the learning curve for acquiring the ability needed for more complex types of kidney surgery, such as a partial nephrectomy [29]. However, RARN has certain definite disadvantages in the form of high cost, the set-up time, need for training in its use, lack of tactile feedback, and the need for an experienced laparoscopic assistant [30].
Indications for RARN
The principles for RARN are primarily the same as those for laparoscopic radical nephrectomy (LRN). In the Guidelines of the European Association of Urology, LRN is considered the standard of care for patients with T2 tumours and smaller renal masses not treatable by nephron-sparing surgery (NSS) [31].
Few reports discuss robotic nephrectomy, and those that do are based on small patient cohorts [32].
Perioperative, postoperative, and oncological outcomes after RARN
The surgical approach for robotic nephrectomy included both transperitoneal and retroperitoneal approaches.
RARN for renal cancer has been reported in the literature in very few published series that demonstrated the feasibility and viability of RARN, as an alternative for performing radical nephrectomy [27, 32].
Nazemi et al [30] compared radical nephrectomy performed with open, laparoscopic, hand-assisted laparoscopic, or robotic-assisted approach. No differences in terms of perioperative complications were detected between the methods. While EBL, postoperative narcotic doses, and hospitalisation were significantly higher in the open group, the median operative time was significantly longer for RARN.
Only one small, non-randomised prospective cohort study [33] compared RARN with LRN. The groups were similar at baseline in terms of tumour size, stage, and grade, histological cell type, and age, but the study provided no baseline data on necrosis, ethnicity, performance status, and co-morbidity and was therefore assessed at high risk of confounding from these factors. Follow-up was short. There was no clear difference between the groups in terms of EBL, need for blood transfusion, analgesic requirements, hospital stay, and convalescence time. The mean operating time was significantly longer with the use of the robot. Both RARN and LLR cases had no recurrence or metastasis. No survival analysis was reported. The authors concluded that in their comparative study, there were no extraordinary benefits of RARN observed over LRN for localised renal tumour.
Murphy and Dasgupta [26] concluded that in their article about robotic approaches to renal cancer the small number of reported cases managed with RARN suggests that this procedure is unlikely to enter standard practice.
Robotic-assisted partial nephrectomy
Surgical therapy is the only curative therapeutic approach for the treatment of renal cell cancer. For T1 tumours, radical nephrectomy is no longer the gold standard treatment [31].
Robot-assisted partial nephrectomy (RAPN) allows magnified stereoscopic visualisation and the use of articulated robotic instruments under precise control [34]. The advent of robot assistance has facilitated the technical performance of partial nephrectomies by simplifying and speeding up two important parts of the procedure: dissection of the renal parenchyma and suturing after tumour resection. The use of a robot facilitates the dissection and mobilisation of the kidney, especially for the approach to the upper pole, which is sometimes difficult in standard laparoscopy.
Since its introduction by Gettman et al in 2004 [35], there has been a steady increase in RAPN, with more than 300 cases reported between 2009 and 2010 [36–39]. It is becoming the technique of choice for most stage T1a tumours, where the technology and expertise are available.
Indications for RARN
As indicated in the European Association of Urology Guidelines, for elective indications, NSS (partial tumour resection) for tumours limited in diameter (T1a) provides recurrence-free and long-term survival rates similar to those observed after radical surgery (LE: 2b). For larger tumours (T1b), partial nephrectomy has demonstrated feasibility and oncological safety in carefully selected patients [31].
NSS is recommended when a small renal mass (defined as a ‘contrast-enhancing mass within the kidney with the largest dimension ≤4 cm’ [40]) was detected in anatomically or functionally solitary kidneys (absolute indications), in patients with a contralateral functioning kidney with a condition that might impair renal function in the future, or in the presence of multiple bilateral tumours or hereditary forms of renal cell cancer that represent a high risk of developing a tumour in the contralateral kidney (relative indications) [41]. In the last few years, the evidence derived from high-quality clinical research and randomised trials has increased the use of partial nephrectomy in the presence of localised unilateral renal cell cancer with a healthy contralateral kidney (elective indications) [34].
However, in the European Association of Urology Guidelines on Renal Cell Carcinoma, RAPN is considered a novel technique that is still undergoing evaluation [31].
Perioperative and postoperative outcomes of RAPN
Overall, RALPN seemed to afford significant improvements in operative time, warm ischaemia time (WIT), and EBL. These improvements have been demonstrated by several authors [39, 42–46].
One small, non-randomised study [47] conducted a matched-pair study on the basis of age, gender, BMI, ASA score, tumour size, location, and specific technique used (early versus conventional unclamping) in 12 patients who underwent robotic and in 12 who had standard LPN. Perioperative outcomes were comparable for operative blood loss, operation time, WIT, and length of hospital stay. Two patients in the RAPN group were converted to standard LPN, one for a positive focal margin on frozen-section analysis, and the other for a robotic camera malfunction. The renal functional outcomes as measured by 3-month serum creatinine and estimated GFR were comparable between the matched groups.
The first large, comparative, multi-institutional study was reported by Benway et al in 2009 [42]. They evaluated the outcomes of three experienced minimally invasive surgeons by analysing 118 consecutive LPNs and 129 consecutive RAPNs. No significant differences were detected in overall operative time or PSM rate. They also found that increased tumour complexity resulted in increased LPN operative time. This was not the case for RAPN, perhaps indicating that the ease of tumour dissection and repair allows for a more uniform experience with RAPN than for LPN. These findings support the idea that the learning curve can be shortened using robotics.
A very recent comparative analysis of a large series of laparoscopic and robotic partial nephrectomies performed by a high volume single surgeon at a tertiary care institution [48] showed that the RAPN group had lower operative time, WIT, rates of intraoperative complications, and conversion. The RAPN group also showed a lower rate of postoperative complications with a lower rate of high grade (Clavien III–V) complications.
Pierorazio et al [43] in their comparison of outcomes and evaluation of learning curve of 48 RAPNs and 102 LPNs concluded that progress in perioperative outcomes in RAPN is due to the improved visualisation and excision of tumour and to the following reconstruction facilitated by the endowristed robotic instruments.
Actually, WIT is a matter of discussion in the literature because the reduction of the ischaemia time can preserve the global renal function and subsequently avoid renal injury, chronic renal disease, and the related morbidity [49, 50]. Thus, RALPN may be a means to reduce WIT further and protect long-term renal function in patients in whom NSS can be safely performed.
Oncologic outcomes of RAPN
It has been demonstrated that LPN can guarantee equivalent intermediate and long-term oncologic outcomes (in terms of surgical margin status and local recurrence) in comparison with OPN [51]. In large single-institution series [42], PSM rate was 3.9%, which is consistent with historical rates for OPN (1.3%) and LPN (2.9%) in experienced hands [52].
In an analysis of 40 RAPN and 62 LPN cases, no significant difference was found in the rate of focal positive margins between the two modalities [39].
A recent retrospective analysis of 500 consecutive LPNs and RAPNs performed by a single surgeon showed that LPN cases had significantly more positive margins than RAPN cases overall (5.6% versus 2.9%, p < 0.001) [48].
The longest duration of median follow-up in the reported RAPN literature is less than 24 months [53], which is inadequate to state that oncologic efficacy. However, in this study, none of the patients had evidence of local recurrence or metastatic disease at median follow-up of 22 months.
Although the first experiences of RAPN are encouraging, oncologic outcomes are still immature, and a larger series with longer follow-up are awaited to confirm the preliminary results [34].
Robotic-assisted nephroureterectomy
Open radical nephroureterectomy (ORNU) with excision of the ipsilateral bladder cuff has long been considered the standard management for upper urinary tract urothelial cell carcinomas (UUT-UCCs) [54].
Multiple minimally invasive techniques have been introduced with the goal of replicating these open procedures.
Robot-assisted laparoscopic nephroureterectomy (RALNU) is a relatively new procedure with promising early results. Being essentially a laparoscopic procedure, it is a minimally invasive surgery that may minimise surgical morbidity for the patient. In addition, the da Vinci surgical robot provides some additional advantage over a standard laparoscopic nephroureterectomy, namely increased degrees of freedom, 3D vision, movement scaling, and tremor filtration, and as such, it has the potential for a more widespread adoption by urologists.
Indications for RALNU
Indications for RALNU are the same as those for ORNU: ORNU with excision of the bladder cuff is the gold standard treatment for UUT-UCCs, regardless of the location of the tumour in the UUT (LE: 3), especially for muscle-invasive and/or high-grade disease. Resection of the distal ureter and its orifice is performed because it is a part of the urinary tract with considerable risk of recurrence [54].
In the European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinomas, however, the use of robotic approach for nephroureterectomy is not mentioned [54].
Perioperative, postoperative, and oncological outcomes after RALNU
The feasibility of RALNU by the retroperitoneal route was first described by Rose et al in 2006 [55].
Nanigian et al [56] described their experience in a series of ten patients undergoing RALNU for UUT-UCCs. A transperitoneal approach was used. No surgical complications were reported. They showed promising short-term oncologic outcomes.
Eandi et al [57] reported their initial experience with 11 patients who underwent to complete robotic-assisted nephroureterectomy and showed promising short intraoperative and postoperative outcomes with regard to oncological outcomes, hospital stay, and operative time. In these cases, the robotic approach required undocking and redocking of the da Vinci system during the procedure in order to reach the bladder for the excision of the bladder cuff.
Later, other authors described several robotic approaches in which the repositioning of the patient from flank to supine and movement of the patient cart are unnecessary: keeping the patient in the flank position not only shortens the operative duration but also improves exposure of the distal ureter and closure of the bladder cuff [56, 59].
More recently, Hemal et al [59] described their new technique in which ports were strategically placed to allow access to the kidney, ureter, and bladder: as described the excision of the bladder cuff does not require undocking the robot without the need to reposition the patient.
Despite the documented benefits of minimally invasive RALNU in perioperative morbidity, the oncological equivalence of this procedure to ORNU remains to be completely established even if overall, in the last decade, numerous retrospective studies have demonstrated oncological equivalence for laparoscopic and open nephroureterectomies [60, 61].
Short-term oncologic outcomes of RALNU have been encouraging, and it seems reasonable to expect long-term outcomes as good as those seen with traditional laparoscopy, which in turn has been comparable with the outcomes of open surgery.
Murphy et al [26] concluded in his article about robotic approaches to renal cancer that RALNU is technically feasible, but there are no prospective comparisons with the conventional laparoscopic approach. Randomised trials with long-term follow-up comparing the outcomes of open and robotic nephroureterectomies are needed.
Conclusion
Advances in minimally invasive surgery have allowed urologists to use robotic assistance in developing new techniques in extirpative and reconstructive surgery.
The relatively short learning curve of robotic surgery could represent a potential advantage over laparoscopic techniques and could make robotics the minimally invasive modality of choice.
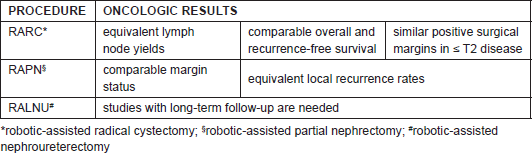
Long-term follow-up of procedures such as RARC, RAPN, and RALNU are obviously needed, but the short and intermediate parameters of efficacy of these procedures are encouraging and can predict the increased dissemination and utilisation of robotics in uro-oncologic surgery (Table 1).
TABLE 1. Oncologic results in comparison with the open/laparoscopic procedure.
References
1. Hemal AK and Menon M (2004) Robotics in urology Curr Opin Urol 14(2) 89–93 DOI: 10.1097/00042307-200403000-00007 PMID: 15075836
2. Babbar P and Hemal AK (2011) Robot-assisted urologic surgery in 2010 – advancements and future outlook Urol Ann 3(1) 1–7 DOI: 10.4103/0974-7796.75853 PMID: 21346825 PMCID: 3036993
3. Piechaud P (2011) State of the art: urologic surgery J Visc Surg 148(Suppl 5) e27–9 DOI: 10.1016/j.jviscsurg.2011.08.004 PMID: 22056796
4. Huang GJ and Stein JP (2007) Open radical cystectomy with lymphadenectomy remains the treatment of choice for invasive bladder cancer Curr Opin Urol 17 369–75 DOI: 10.1097/MOU.0b013e3282dc95b5 PMID: 17762633
5. Haber GP, Crouzet S and Gill IS (2008) Laparoscopic and robotic assisted radical cystectomy for bladder cancer: a critical analysis Eur Urol 54 54–64 DOI: 10.1016/j.eururo.2008.03.076 PMID: 18403100
6. Guru KA, Kim HL, Piacente PM, et al (2007) Robot-assisted radical cystectomy and pelvic lymph node dissection: initial experience at Roswell Park Cancer Institute Urology 69 469–74 DOI: 10.1016/j.urology.2006.10.037 PMID: 17382147
7. Hemal AK (2009) Robotic and laparoscopic radical cystectomy in the management of bladder cancer Curr Urol Rep 10 45–54 DOI: 10.1007/s11934-009-0009-8 PMID: 19116095
8. Nix J, Smith A, Kurpad R, et al (2010) Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results Eur Urol 57 196–201 DOI: 10.1016/j.eururo.2009.10.024
9. Khan MS, Elhage O, Challacombe, et al (2011) Analysis of early complications of robotic-assisted radical cystectomy using a standardized reporting system Urology 77 357–62 DOI: 10.1016/j.urology.2010.04.063
10. Stenzl A, Witjes JA, Compérat E, et al (2012) Guidelines on bladder cancer muscle-invasive and metastatic. European Association of Urology Web site. http://www.uroweb.org/gls/pdf/07_Bladder%20Cancer_LR%20II.pdf
11. Haber GP and Gill IS (2007) Laparoscopic radical cystectomy for cancer: oncological outcomes at up to 5 years BJU Int 100 137–42 DOI: 10.1111/j.1464-410X.2007.06865.x PMID: 17552961
12. Yuh BE, Ciccone J, Chandrasekhar R, et al (2009) Impact of previous abdominal surgery on robot-assisted radical cystectomy JSLS 13 398–405 PMID: 19793483 PMCID: 3015968
13. Herr H, Lee C, Chang S, et al (2004) Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer. A collaborative group report J Urol 171 1823–8 DOI: 10.1097/01.ju.0000120289.78049.0e
14. Guru KA, Sternberg K, Wilding GE, et al. (2008) The lymph node yield during robot-assisted radical cystectomy BJU Int 102 231–4 DOI: 10.1111/j.1464-410X.2008.07589.x PMID: 18325058
15. Richards KA, Hemal AK, Kader AK, et al (2010) Robot assisted laparoscopic pelvic lymphadenectomy at the time of radical cystec-tomy rivals that of open surgery: single institution report Urology 76 1400–4 DOI: 10.1016/j.urology.2010.01.019 PMID: 20350755
16. Hellenthal NJ, Hussain A, Andrews PE, et al (2011) Lymphadenectomy at the time of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium BJU Int 107 642–6 DOI: 10.1111/j.1464-410X.2010.09473.x
17. Donat SM (2007) Standards for surgical complication reporting in urologic oncology: time for a change Urology 69 221–5 DOI: 10.1016/j.urology.2006.09.056 PMID: 17320654
18. Dindo D, Demartines N and Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey Ann Surg 240 205–13 DOI: 10.1097/01.sla.0000133083.54934.ae PMID: 15273542 PMCID: 1360123
19. Orvieto MA, DeCastro GJ, Trinh QD, et al (2011) Oncological and functional outcomes afterrobot-assisted radical cystectomy: critical review of current status Urology 78 977–84 DOI: 10.1016/j.urology.2011.04.073 PMID: 21890182
20. Ng CK, Kauffman EC, Lee MM, et al (2010) A comparison of postoperative complications in open versus robotic cystectomy Eur Urol 57 274–82 DOI: 10.1016/j.eururo.2009.06.001
21. SmithAB, Raynor MC and Pruthi RS (2011) Peri- and postoperative outcomes of robot-assisted radical cystectomy (RARC) BJU Int 108 969–75 DOI: 10.1111/j.1464-410X.2011.10456.x PMID: 21917099
22. Khan MS, Challacombe B, Elhage O, et al (2012) A dual-centre, cohort comparison of open, laparoscopic and robotic-assisted radical cystectomy. Int J Clin Pract 66(7) 656–62 DOI: 10.1111/j.1742-1241.2011.02888.x PMID: 22507234
23. Challacombe BJ, Bochner BH, Dasgupta P, et al (2011) The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications. Eur Urol 60 767–75 DOI: 10.1016/j.eururo.2011.05.012 PMID: 21620562
24. Dasgupta P, Rimington P, Murphy D, et al (2008) Robotically assisted radical cystectomy BJU Int 101 1489–92 DOI: 10.1111/j.1464410X.2008.07564.x PMID: 18384638
25. Clayman RV, Kavoussi LR and Soper NJ (1991) Laparoscopic nephrectomy: initial case report J Urol 146(2) 278–82 PMID: 1830346
26. Murphy D and Dasgupta P(2007) Robotic approaches to renal cancer. Curr Opin Urol 17327–30 DOI: 10.1097/MOU.0b013e328277f1b3 PMID: 17762625
27. Hoznek A, Hubert J, Antiphon P, et al (2004) Robotic renal surgery Urol Clin North Am 31 731–6 DOI: 10.1016/j.ucl.2004.06.015 PMID: 15474599
28. Kumar R, Hemal AK and Menon M (2005) Robotic renal and adrenal surgery: present and future BJU Int 96 244–9 DOI: 10.1111/ j.1464-410X.2005.05611.x PMID: 16042710
29. Rogers C, Laungani R and Krane LS (2008) Robotic nephrectomy for the treatment of benign and malignant disease BJU Int 102(11) 1660–5 DOI: 10.1111/j.1464-410X.2008.07895.x PMID: 18671787
30. Nazemi T, Galich A, Sterrett S, et al (2006) Radical nephrectomy performed by open, laparoscopy with or without hand-assistance or robotic methods by the same surgeon produces comparable perioperative results Int Braz J Urol 32 15–22 DOI: 10.1590/S1677-55382006000100003 PMID: 16519823
31. Ljungberg B, Cowan N and Hanbury DC (2012). Guidelines on renal cell carcinoma European Association of Urology Web site: http://www.uroweb.org/gls/pdf/NEW09_Renal_Cell_Carcinoma_LR.pdf
32. Klingler DW, Hemstreet GP and Balaji KC. (2005) Feasibility of robotic radical nephrectomy – initial results of single-institution pilot study Urology 65(6) 1086–9 DOI: 10.1016/j.urology.2004.12.020 PMID: 15913733
33. Hemal AK and Kumar A. (2009) A prospective comparison of laparoscopic and robotic radical nephrectomy for T1-2N0M0 renal cell carcinoma World J Urol 27 89–94 DOI: 10.1007/s00345-008-0321-9
34. Volpe A, Cadeddu JA, Cestari A, et al (2011) Contemporary management of small renal masses Eur Urol 60 501–15 DOI: 10.1016/j. eururo.2011.05.044 PMID: 21664040
35. Gettman MT, Blute ML, Chow GK, et al (2004) Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system Urology 64 914–8 DOI: 10.1016/j.urology.2004.06.049 PMID: 15533477
36. Benway BM, Wang AJ, Cabello JM, et al (2009) Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes Eur Urol 55 592–9 DOI: 10.1016/j.eururo.2008.12.028 PMID: 19144457
37. Patel MN, Krane LS, Bhandari A, et al (2010) Robotic partial nephrectomy for renal tumors larger than 4 cm Eur Urol 57 310–6 DOI: 10.1016/j.eururo.2009.11.024
38. Michli EE and Parra RO (2009) Robotic-assisted laparoscopic partial nephrectomy: initial clinical experience Urology 73 302–5 DOI: 10.1016/j.urology.2008.09.056
39. Wang AJ and Bhayani SB (2009) Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures Urology 73 306–10 DOI: 10.1016/j.urology.2008.09.049
40. Gill IS, Aron M, Gervais DA, et al (2010) Clinical practice. Small renal mass N Engl J Med 362 624–34 DOI: 10.1056/NEJMcp0910041 PMID: 20164486
41. Uzzo RG and Novick AC (2001) Nephron sparing surgery for renal tumors: indications, techniques and outcomes J Urol 166 6–18 DOI: 10.1016/S0022-5347(05)66066-1 PMID: 11435813
42. Benway BM, Bhayani SB, Rogers CG, et al (2009) Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes J Urol 182 866–72 DOI: 10.1016/j.juro.2009.05.037 PMID: 19616229
43. Pierorazio PM, Patel HD and Feng T (2011) Robotic-assisted vs. traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve Urology 78(4) 813–819 DOI: 10.1016/j.urology.2011.04.065 PMID: 21802120 PMCID: 3190025
44. Deane LA, Lee HJ, Box GN, et al (2008) Robotic versus standard laparoscopic partial/wedgenephrectomy: a comparison of intraoperative and perioperative results from a single institution J Endourol 22 947–52 DOI: 10.1089/end.2007.0376 PMID: 18397157
45. DeLong JM, Shapiro O and Moinzadeh A (2010) Comparison of laparoscopic versus robotic assisted partial nephrectomy: one surgeon’s initial experience Can J Urol 17 5207–12 PMID: 20566016
46. Kural AR, Atug F, Tufek I, et al (2009) Robot-assisted partial nephrectomy versus laparoscopic partial nephrectomy: comparison of outcomes J Endourol 23 1491–7 DOI: 10.1089/end.2009.0377 PMID: 19694519
47. Aron M, Koenig P, Kaouk JH, et al (2008) Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre BJU Int 102 86–92 DOI: 10.1111/j.1464-410X.2008.07580.x PMID: 18336600
48. Khalifeh A, Autorino R, Hillyer SP, et al (2013) Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomies: a single surgeon experience J Urol 189 1236–42 DOI: 10.1016/j.juro.2012.10.021
49. Thompson RH, Lane BR, Lohse CM, et al (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy Eur Urol 58 340–5 DOI: 10.1016/j.eururo.2010.05.047 PMID: 20825756
50. Go AS, Chertow GM, Fan D, et al (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization N Engl J Med 351 1296–305 DOI: 10.1056/NEJMoa041031 PMID: 15385656
51. Lane BR and Gill IS (2010) 7-year oncological outcomes after laparoscopic and open partial nephrectomy J Urol 183 473–9 DOI: 10.1016/j.juro.2009.10.023
52. Gill IS, Kavoussi LR, Lane BR, et al (2007) Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors J Urol 178 41–6 DOI: 10.1016/j.juro.2007.03.038 PMID: 17574056
53. Gupta GN, Boris R, Chung P, et al (2013) Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up Urol Oncol 31 51–6 DOI: 10.1016/j.urolonc.2010.10.008
54. Rouprêt M, Zigeuner R, Palou J, et al (2012) Guidelines on upper urinary tract urothelial cell carcinomas European Association of Urology Web site: http://www.uroweb.org/gls/pdf/06_UUTUCC.pdf
55. Rose K, Khan S, Godbole H, et al (2006) Robotic assisted retroperitoneoscopicnephroureterectomy – first experience and the hybrid port technique Int J Clin Pract 60(1) 12–4 DOI: 10.1111/j.1368-5031.2006.00703.x PMID: 16409422
56. Nanigian DK, Smith W and Ellison LM (2006) Robot-assisted laparoscopic nephroureterectomy J Endourol 20 463–5 DOI: 10.1089/ end.2006.20.463 PMID: 16859455
57. Eandi JA, Nelson RA, Wilson TG, et al (2010) Oncologic outcomes for complete robot-assisted laparoscopic management of upper-tract transitional cell carcinoma J Endourol 24 969–75 DOI: 10.1089/end.2009.0340 PMID: 20210537
58. Park SY, Jeong W, Ham WS, et al (2009) Initial experience of robotic nephroureterectomy: a hybrid-port technique BJU Int 104 1718–21 DOI: 10.1111/j.1464-410X.2009.08671.x PMID: 19519762
59. Hemal AK, Stansel I, Babbar P, et al (2011) Robotic-assisted nephroureterectomy and bladder cuff excision without intraoperative repositioning Urology 78(2) 357–64 DOI: 10.1016/j.urology.2010.12.075 PMID: 21680014
60. Capitanio U, Shariat SF, Isbarn H, et al (2009) Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases Eur Urol 256(1) 1–9 DOI: 10.1016/j.eururo.2009.03.072
61. Favaretto RL, Shariat SF, Chade DC, et al (2010) Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique Eur Urol 58(5) 645–51 DOI: 10.1016/j.eururo.2010.08.005 PMID: 20724065






