Vanishing clear cell carcinoma of the kidney presenting with skin metastases – a case report
Sidhart Misra1, Zainab Yusufali Motiwala2, Ayyaz Mulla3, Jagatheswaran Chinnathambi4 and Danny Darlington Carbin5
1Armed Forces Medical College, Pune 411040, India
2Medical Intern, JNMCH, Aligarh Muslim University, Aligarh 202001, India
3United Hospital, Jayanagar, Bengaluru 560011, India
4Assistant Professor of Urology, Manakkula Vinayagar Medical College, Pondicherry 605107, India
5Locum Consultant Urologist, Ashford and St Peters NHS Foundation Trust, Chertsey, UK
Abstract
Renal cell carcinoma (RCC) is one of the common genitourinary malignancies that has an increasing incidence. RCC presents a diagnostic challenge due to its wide range of clinical manifestations, often leading to delays in diagnosis and complicating management strategies. These tumours have clear cells in 70% of cases and have a high preponderance of haematogenous metastases to distant organs such as lungs, bones and liver. Skin metastases of RCC in the absence of an obvious renal tumour are rare. We report a young woman with clear cell renal carcinoma with skin metastasis who presented with left loin pain and acute kidney injury, prompting a series of comprehensive investigations, including imaging studies and laboratory tests. Despite these efforts, a primary tumour remained elusive. However, a breakthrough occurred when histopathological examination of a skin nodule biopsy, alongside cytological analysis of nephrostomy fluid, ultimately identified the underlying cause as malignant RCC. Despite commencing palliative Sunitinib therapy based on intermediate risk criteria, the patient died from lung metastases after 6 months of systemic medication. Here is a more succinct version. This case report emphasises the need to investigate renal primaries in unknown-origin metastases and the importance of a thorough diagnostic approach for RCC.
Keywords: renal cell carcinoma, metastasis, unknown primary neoplasms, lung, liver, skin
Correspondence to: Sidharth Misra
Email: misra.sidharth.afmc@gmail.com
Published: 07/08/2025
Received: 08/12/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Renal cell carcinoma (RCC) predominates as the primary type of urogenital cancer, showing a greater prevalence in men than in women, and it carries a mortality rate ranging from 30% to 40% [1]. For RCC, metastasis rates were noted as 3.6% for tumours measuring ≤4 cm, 13.1% for tumours ranging from 4 to 7 cm, 30.3% for those between 7 and 10 cm and 45.1% for tumours larger than 10 cm [2]. Bone metastasis is common, followed by brain, pancreas, adrenal gland, gallbladder, liver and lymph nodes [3, 4]. RCC presenting with skin metastases is uncommon but not a rare occurrence. The incidence of skin metastases in patients with RCC ranges from 3.3% to 6.8% [5, 6]. The timing of metastases could be before, after or at the same time as the diagnosis of the primary renal cell carcinoma [5, 7]. It has been observed that the overall prognosis of patients with RCC and skin metastases is generally poor with survival ranging from 7 to 36 months after detection [5–7].
Vanishing tumours are lesions that disappear or reduce in size spontaneously without treatment. Some of the common locations include the brain and kidneys. In the brain, two common diagnoses for vanishing tumours are malignant tumours or multiple sclerosis [8]. In the kidney, the main associations with renal cell carcinoma are malignant peripheral nerve sheath tumours and xanthogranulomatous pyelonephritis [9, 10]. There have been reports of vanishing tumours in cases of cerebral metastases of unknown primary [11].
This case study presents a unique case of cutaneous metastases in a patient with vanishing RCC. The interesting rare aspect of this case was that the renal tumour underwent complete regression due to spontaneous necrosis and disappeared. As a consequence, the tumour was not detected on imaging. However, the biopsy of the skin lesion revealed clear cell RCC, which is rather unusual. The regression of the renal mass combined with the confirmation of RCC on skin biopsy makes this case a rare occurrence.
Case presentation
This is a case of a 45-year-old woman who presented to our emergency department with left loin pain and acute kidney injury (AKI). She had a past medical history of diabetes but otherwise, she was generally fit. On examination, she was febrile with stable vital signs and her performance status was 0. Physical examination was positive for tenderness in the left loin region and a soft abdomen on palpation. Her hematological investigations revealed low hemoglobin, elevated white cell count and thrombocytopenia. Other relevant findings include hypoalbuminemia and hypocalcemia. Collectively, the patient was diagnosed with stage 3 AKI.
An urgent non-contrast CT scan showed a left hydronephrotic kidney, with no renal parenchyma. With the patient being in sepsis, a prompt left-sided nephrostomy was done which drained brown fluid (Figure 1). The cytological analysis of the fluid revealed pleomorphic malignant clear cells with abundant nucleoli and nuclear atypia. Following the resolution of the acute kidney injury, a contrast-enhanced CT scan was done which showed multiple liver and lung metastasis (Figures 2 and 3), as well as para-aortic lymphadenopathy (Figure 4). In addition, several skin nodules were noted. On examination, one of the nodules was located in the right hypochondrium which was mobile, hard and measured 3 × 4 cm. Subsequently, a biopsy was taken under local anesthesia and the histology confirmed clear cell RCC. Considering the lesion to be intermediate risk based on the parameters given by the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) such as platelet count, albumin, calcium, prior nephrectomy, haemoglobin and metastatic presentation, the patient was deemed unsuitable for nephrectomy [12, 13]. The multidisciplinary oncology team decided to initiate palliative Sunitinib therapy [14–16]. Despite receiving palliative treatment for 6 months, the patient succumbed to complications arising from lung metastasis and passed away due to acute massive hemoptysis.
Discussion
RCC is a heterogeneous group of cancers arising from renal tubular epithelial cells, with clear cell renal cell carcinoma (ccRCC) originating from proximal tubules with a histological presentation of thin-walled cells filled with lipids and glycogen [17, 18]. It is a common urinary tract malignancy that has witnessed a rise in incidence worldwide [19]. At a global level, the incidence and mortality have been reported to be 434,840 and 155,953, respectively, according to WHO [20]. Higher rates have been noticed in developed countries [21]. 15% of clear cell carcinomas have the potential to metastasise [22, 23]. The majority of ccRCC cases originate from mutations in the Von Hippel-Lindau gene, which is located on the short arm of chromosome 3 [24].
Carcinoma of unknown primary accounts for about 15% of all solid tumours, and it poses a diagnostic challenge [25]. To determine its primary site, immunohistochemistry (IHC) staining patterns, particularly cytokeratin 7 and 20 play a crucial role [26]. They use specific antibody panels to identify the various skin cancers such as melanoma, lymphoma and sarcomas. In addition, they aim to determine the primary sites of the tumour (breast, prostate and thyroid). CK7+/CK20+ marker is commonly observed in urothelial cancers [25]. In instances where the primary origin remains unknown even after IHC analysis, additional diagnostic procedures such as cytopathological specimens and gene expression studies can be carried out [26, 27].

Figure 1. Left-sided nephrostomy drained brown hemo-serous fluid.
Lung involvement of metastatic ccRCC accounts for approximately 20%–30% of metastatic occurrences with a projected 5-year survival rate ranging from 21% to 60% in pulmonary metastasectomy cases, in contrast to 11% for non-operated patients [28]. Lung metastasis has also been reported in a patient 16 years post radical nephrectomy [29]. This emphasis is on the high post nephrectomy recurrence rate within the lungs which are the most common site for distant metastasis (50%–60%).
This case report describes a very rare instance of metastatic RCC (mRCC) presenting without an obvious primary renal mass/tumour and the histology of the skin lesions confirmed the origin of the tumour. This histological diagnosis is related to an important phenomenon called Azzopardi effect which was first described in 1959 [30]. It is defined as the deposition of basophilic nuclear material in the blood vessel wall which shows a positive feulgen reaction suggestive of DNA material. It has been described in various types of cancer such as Burkitt lymphoma, small cell carcinoma, Merkel cell carcinoma and medulloblastoma [31]. The underlying mechanism behind this phenomenon is the release of nucleic acid from degenerating cancer cells. It has been noticed more commonly with testicular; cancer however, our case depicted the Azzopardi effect with mRCC which is a rare occurrence. Additionally, spontaneous primary tumour regression is also a rare phenomenon observed in this case that can be attributed to the different mechanisms like tumour necrosis, angiogenesis inhibition and apoptosis [32].
The given case demonstrated metastasis to the skin, liver and, lung with no primary tumour identified. A similar case was reported by Razi et al [33] demonstrating the presence of mRCC in para-aortic lymph node where the origin of the tumour could not be determined. The IHC of the node was positive for CK20, CD10, AMACR, PAX8, CAIX and CK8/18 which indicated renal origin. Another case report demonstrated biopsy proven RCC in the bones like scapula, ribs and pelvis with no evidence of the primary tumour [34]. Furthermore, a study presented 2 cases of mRCC to the pubo-iliac bone and knee, with no parenchymal solid renal lesion observed on CT imaging [35]. The diagnostic challenges presented by mRCC of unknown primary origin and the role of IHC in diagnosis were highlighted in the cases.
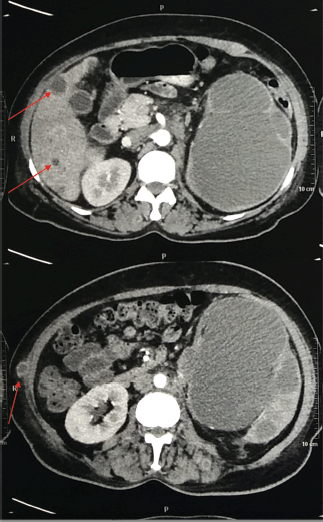
Figure 2. Contrast-enhanced CT scan of the abdomen and pelvis (Transverse section) representing liver and skin metastasis (red arrow).
Skin metastases originating from RCC manifested as nodular growths that proliferate swiftly, exhibiting round or oval shapes with varying colours from skin tone to hues of red-purple [36]. The IMDC criteria are considered the standard for stratifying risk in mRCC patients [37]. For individuals classified as intermediate- or poor-risk, the preferred first-line therapies include ipilimumab + nivolumab or pembrolizumab + axitinib, while alternative options such as avelumab/axitinib and targeted therapy (sunitinib or pazopanib) are available, particularly for patients unsuitable for or intolerant to combination therapy or immunotherapy [12]. Interleukin-2 or interferon alfa are commonly employed as primary therapies for mRCC due to its high resistance to chemotherapy [38]. Sunitinib, a tyrosine kinase inhibitor with multi-targeted activity against VEGFR, PDGFR, c-Kit, RET and Flt3, demonstrates notable efficacy as a first-line treatment option for patients diagnosed with mRCC as evidenced by higher response rates, prolonged progression-free survival and improved overall survival [39, 40]. Other treatment options for mRCC include surgical debulking and radiotherapy [41].
The prognosis depends on the extent of metastatic spread and it is associated with poor outcomes in cases of spread to the lung, liver and skin as seen in the given case. Risk stratification with IMDC criteria and using IHC for histological confirmation are essential for initiation of appropriate therapy [42]. Therefore, this case highlights the need for further research on this rare phenomenon and the need for standardisation of management strategies. Additionally, in the era of immunotherapy, it is ideal to get a biopsy with immunohistochemistry to identify the protein expression as well as molecular and cytogenetics studies that can guide future personalised treatments [20].
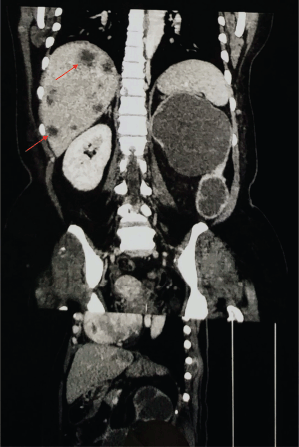
Figure 3. Contrast-enhanced CT scan of the abdomen and pelvis (Coronal section) representing metastasis (red arrows).
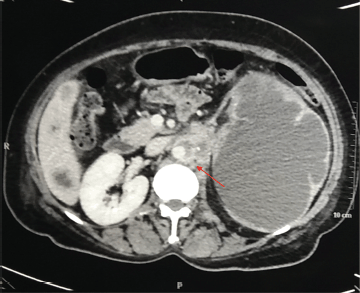
Figure 4. CT scan of the abdomen and pelvis (Transverse section) representing para aortic lymphadenopathy (red arrow).
Conclusion
Clinicians should have a high degree of suspicion for RCC, especially in patients with carcinoma of unknown origin. Skin metastases should be considered as a potential manifestation of RCC even in the absence of an obvious renal mass. When considering RCC in the context of unexplained metastatic disease, cytological analysis of fluids such as nephrostomy fluid can also provide important insights.
Acknowledgment
None.
Conflicts of interest
None.
Funding
None.
Informed consent
Informed consent was obtained for anonymous publication of the images, surgical videos, details of the clinical presentation, surgery and follow-up.
Author contributions
SM- manuscript writing, data collection, literature search, ZYM- manuscript writing, data collection, literature search, AM- manuscript writing, data collection, literature search, images, primary surgical team, JC- manuscript review, literature search, primary surgical team, follow up, images, DDC- manuscript review, data collection, literature search, primary surgeon.
References
1. Bahadoram S, Davoodi M, and Hassanzadeh S, et al (2022) Giornale Italiano di Nefrologia in depth review renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment G Ital Nefrol 39(3) 2022 PMID: 35819037
2. Monda SM, Lui HT, and Pratsinis MA, et al (2023) The metastatic risk of renal cell carcinoma by primary tumor size and subtype Eur Urol Open Sci 52 137–144 https://doi.org/10.1016/j.euros.2023.04.015 PMID: 37284045 PMCID: 10240521
3. Mikami S, Oya M, and Mizuno R, et al (2014) Invasion and metastasis of renal cell carcinoma Med Mol Morphol 47 63–67 https://doi.org/10.1007/s00795-013-0064-6
4. Kanwal R (2023) Metastasis in renal cell carcinoma: biology and treatment Adv Cancer Biol Metastasis 7 100094 https://doi.org/10.1016/j.adcanc.2023.100094
5. Dorairajan LN, Hemal AK, and Aron M, et al (1999) Cutaneous metastases in renal cell carcinoma Urol Int 63 164–167 https://doi.org/10.1159/000030440
6. Kouroupakis D, Patsea E, and Sofras F, et al (1995) Renal cell carcinoma metastases to the skin: a not so rare case? Br J Urol 75 583–585 https://doi.org/10.1111/j.1464-410X.1995.tb07411.x PMID: 7613792
7. Koga S, Tsuda S, and Nishikido M, et al (2000) Renal cell carcinoma metastatic to the skin Anticancer Res 20 1939–1940 PMID: 10928130
8. Okita Y, Narita Y, and Miyakita Y, et al (2012) Long-term follow-up of vanishing tumors in the brain: how should a lesion mimicking primary CNS lymphoma be managed? Clin Neurol Neurosurg 114 1217–1221 https://doi.org/10.1016/j.clineuro.2012.02.053 PMID: 22445618
9. Rai Bansal A, Singh Griwan M, and Rajan Karthikeyan Y, et al (2013) Vanished kidney by pheripheral nerve seath tumor: a rare case report Nephrourol Mon 5 843–846 https://doi.org/10.5812/numonthly.8029 PMID: 24282798 PMCID: 3830914
10. Amini F, Onur MR, and Kosemehmetoglu K (2024) Vanishing kidney: on the far end of the spectrum of xanthogranulomatous pyelonephritis Int J Surg Pathol 32 359–361 https://doi.org/10.1177/10668969231171938
11. Wijesundara D and Senanayake B (2021) Vanishing cerebral ring enhancing lesions; a three year mystery J Neurol Sci 429 118926 https://doi.org/10.1016/j.jns.2021.118926
12. Ko JJ, Xie W, and Kroeger N, et al (2015) The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study Lancet Oncol 16 293–300 https://doi.org/10.1016/S1470-2045(14)71222-7 PMID: 25681967
13. Motzer RJ, Mazumdar M, and Bacik J, et al (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma J Clin Oncol 17 2530–2530 https://doi.org/10.1200/JCO.1999.17.8.2530 PMID: 10561319
14. Choueiri TK, Tomczak P, and Park SH, et al (2021) Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma N Engl J Med 385 683–694 https://doi.org/10.1056/NEJMoa2106391 PMID: 34407342
15. Bex A, Mulders P, and Jewett M, et al (2019) Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib JAMA Oncol 5 164 https://doi.org/10.1001/jamaoncol.2018.5543
16. Méjean A, Ravaud A, and Thezenas S, et al (2018) Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma N Engl J Med 379 417–427 https://doi.org/10.1056/NEJMoa1803675 PMID: 29860937
17. di Meo NA, Lasorsa F, and Rutigliano M, et al (2022) Renal cell carcinoma as a metabolic disease: an update on main pathways, potential biomarkers, and therapeutic targets Int J Mol Sci 23 14360 https://doi.org/10.3390/ijms232214360 PMID: 36430837 PMCID: 9698586
18. Nabi S, Kessler ER, and Bernard B, et al (2018) Renal cell carcinoma: a review of biology and pathophysiology F1000Res 7 307 https://doi.org/10.12688/f1000research.13179.1 PMID: 29568504 PMCID: 5850086
19. Kim JH and Hwang JW (2024) Global renal cell carcinoma research trends over 30 years: a PRISMA-compliant bibliometric analysis J Urol Oncol 22 42–51 https://doi.org/10.22465/juo.244600120006
20. EAU Guidelines on RCC - EPIDEMIOLOGY AETIOLOGY AND PATHOLOGY - Uroweb [Internet] Uroweb - European Association of Urology [https://uroweb.org/guidelines/renal-cell-carcinoma/chapter/epidemiology-aetiology-and-pathology]
21. Adibi M, Karam JA, and Wood CG (2015) Reporting geographic and temporal trends in renal cell carcinoma: why is this important? Eur Urol 67 531–532 https://doi.org/10.1016/j.eururo.2014.10.030
22. Low G, Huang G, and Fu W, et al (2016) Review of renal cell carcinoma and its common subtypes in radiology World J Radiol 8 484 https://doi.org/10.4329/wjr.v8.i5.484 PMID: 27247714 PMCID: 4882405
23. Palumbo C, Pecoraro A, and Rosiello G, et al (2020) Renal cell carcinoma incidence rates and trends in young adults aged 20–39 years Cancer Epidemiol 67 101762 https://doi.org/10.1016/j.canep.2020.101762
24. Linehan WM, Schmidt LS, and Crooks DR, et al (2019) The metabolic basis of kidney cancer Cancer Discov 9 1006–1021 https://doi.org/10.1158/2159-8290.CD-18-1354 PMID: 31088840
25. Rubin BP, Skarin AT, and Pisick E, et al (2001) Use of cytokeratins 7 and 20 in determining the origin of metastatic carcinoma of unknown primary, with special emphasis on lung cancer Eur J Cancer Prev 10 77–82 https://doi.org/10.1097/00008469-200102000-00009 PMID: 11263595
26. Mokhtari M, Safavi D, and Soleimani N, et al (2022) Carcinoma of unknown primary origin: application of immunohistochemistry with emphasis to different cytokeratin 7 and 20 staining patterns Appl Immunohistochem Mol Morphol 30 623–634 https://doi.org/10.1097/PAI.0000000000001054 PMID: 36036642
27. Doxtader EE and Chute DJ (2018) Evaluation of carcinoma of unknown primary on cytologic specimens Surg Pathol Clin 11 545–562 https://doi.org/10.1016/j.path.2018.04.006 PMID: 30190140
28. Shields LBE and Rezazadeh Kalebasty A (2020) Spontaneous regression of delayed pulmonary and mediastinal metastases from clear cell renal cell carcinoma Case Rep Oncol 13 1285–1294 https://doi.org/10.1159/000509509 PMID: 33250744 PMCID: 7670320
29. Singh M, Aryal V, and Dangol AMS, et al (2021) Lung metastasis from renal cell carcinoma 16 years after nephrectomy: a case report and review of the literature Clin Case Rep 9(11) e05033 https://doi.org/10.1002/ccr3.5033
30. Azzopardi JG (1959) Oat‐cell carcinoma of the bronchus J Pathol Bacteriol 78 513–519 https://doi.org/10.1002/path.1700780218 PMID: 13795444
31. Zustin J, Skinner JA, and Hart AJ (2016) Azzopardi phenomenon in cystic pseudotumours associated with retrieved metal-on-metal arthroplasty Hum Pathol 51 134–137 https://doi.org/10.1016/j.humpath.2016.01.009 PMID: 27067791
32. Papac RJ (1998) Spontaneous regression of cancer: possible mechanisms In Vivo 12 571–578
33. Razi B, Cole-Clark D, and Self D, et al (2024) Renal cell carcinoma metastasis without a primary: a case report Urol Case Rep 53 102658 PMID: 38348274 PMCID: 10859299
34. Hlaing SS, Desai D, and Goyal A, et al (2022) Wandering cancer cells: metastatic renal cell carcinoma without evidence of a primary tumor Cureus 14(6) e26305 https://doi.org/10.7759/cureus.26305 PMID: 35898368 PMCID: 9309091
35. Kumar RM, Aziz T, and Jamshaid H, et al (2014) Metastatic renal cell carcinoma without evidence of a primary renal tumour Curr Oncol 21 e521–e524 https://doi.org/10.3747/co.21.1914 PMID: 24940113 PMCID: 4059817
36. de Paula TA, da Silva PSL, and Berriel LGS (2010) Renal cell carcinoma with cutaneous metastasis: case report J Bras Nefrol 32 213–215 PMID: 21103681
37. Martini DJ, Liu Y, and Shabto JM, et al (2020) Novel risk scoring system for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors Oncologist 25 e484–e491 https://doi.org/10.1634/theoncologist.2019-0578 PMID: 32162798 PMCID: 7066702
38. McDermott DF, Regan MM, and Clark JI, et al (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and Interferon in patients with metastatic renal cell carcinoma J Clin Oncol 23 133–141 https://doi.org/10.1200/JCO.2005.03.206
39. Motzer RJ, Hutson TE, and Tomczak P, et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma N Engl J Med 356 115–124 https://doi.org/10.1056/NEJMoa065044 PMID: 17215529
40. Moran M, Nickens D, and Adcock K, et al (2019) Sunitinib for metastatic renal cell carcinoma: a systematic review and meta-analysis of real-world and clinical trials data Target Oncol 14 405–416 https://doi.org/10.1007/s11523-019-00653-5 PMID: 31301015 PMCID: 6684538
41. Bimbatti D, Cavasin N, and Galuppini F, et al (2023) Liver metastases of unknown primary renal cell carcinoma treated with immune checkpoint inhibitors plus tyrosine kinase inhibitors: a case report and literature review Anticancer Res 43 2359–2365 https://doi.org/10.21873/anticanres.16401 PMID: 37097698
42. Massari F, Di Nunno V, and Guida A, et al (2021) Addition of primary metastatic site on bone, brain, and liver to IMDC criteria in patients with metastatic renal cell carcinoma: a validation study Clin Genitourin Cancer 19 32–40 https://doi.org/10.1016/j.clgc.2020.06.003






