Understanding the causes of high mortality among adult acute leukaemia patients in Armenia
Astghik Voskanyan1,2, Lusine Harutyunyan1,2, Arusyak Ivanyan2, Alisa Movsisyan2, Nerses Ghahramanyan2, Lusine Sahakyan2, Shushan Hovsepyan1,2,3,4, Samvel Danielyan1, Hayk Grigoryan2 and Gevorg Tamamyan1,4
1Immune Oncology Research Institute, Yerevan 0014, Armenia
2Yeolyan Hematology and Oncology Center, Yerevan 0014, Armenia
3Pediatric Cancer and Blood Disorders Center of Armenia, Yeolyan Hematology and Oncology Center, Yerevan 0014, Armenia
4Yerevan State Medical University, Yerevan 0025, Armenia
Abstract
Background: Acute leukaemias (AL) are a group of heterogeneous malignancies characterised by clonal hematopoietic progenitor cell proliferation. Despite advances in the therapy of acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML) in developed countries, outcomes in developing countries like Armenia are thought to be substantially worse.
Aims: The aim of this article is to understand how the limitations in diagnostic and treatment modalities in Armenia impact the clinical outcomes of AL patients and by presenting it to health authorities contribute to the development of evidence-based policies and regulatory improvements to enhance patient care.
Methods: We interrogated data from 431 adults representing all cases of adult AL in Armenia at the Hematology Centre of Armenia from 1 January 2016, to 31 December 2020. Death data were obtained from the United Information System of Electronic Healthcare in the Republic of Armenia. There were some limitations with data collection due to the absence of a unified electronic database and detailed paper records.
Results: Over the 5 years a total of 431 patients were diagnosed with AL at the Hematology Centre of Armenia of which 310 (72%) died. Male patients’ number was 131 (54%). Median age was 59 years (Interquartile Range = 50, 18–85 years). A morphological complete remission before death was reached in 82 (34%) patients, including 24 dying without relapse. Additionally, 50 subjects (20%) died during induction chemotherapy including 9 with ALL, 37 with AML and 1 with mixed-lineage leukaemia. Causes of death included no response to treatment (N = 29) or therapy-related complications including septic shock (N = 5), acute heart failure (N = 5), brain hemorrhage (N = 2) and acute respiratory failure (N = 1). Causes of death were unclear in eight patients. Thirty subjects failed induction therapy and declined further treatment before starting the induction. In 24 subjects’ remission, state and death causes were unclear. Before starting chemotherapy 58 subjects died, 26 of whom refused therapy and 24 had leukaemia progression. A 5-year survival was 22% including 26% for ALL and 21% for AML.
Conclusion: The results of AL therapy in Armenia are worse than those reported in developed countries, where overall survival is about 60%. The major reasons are leukaemia progression and treatment-related complications.
Keywords: leukaemia, hematology, mortality, low-middle income country, resource-limited setting
Correspondence to: Lusine Harutyunyan
Email: lucy.harutyunyan.md@gmail.com
Published: 23/07/2025
Received: 19/11/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Acute leukaemias (AL) are a group of heterogeneous malignancies characterised by clonal hematopoietic progenitor cell proliferation. The incidence rates of AL vary depending on various factors, including leukaemia subtype, gender, age and geographic location. There are two major categories, acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL), each representing distinct epidemiological patterns. AML typically affects individuals with a median age of 66, with an incidence of 1.78 per 100,000 in those under 65. This rate escalates to 16.8 per 100,000 in those aged 65 and older, as reported in a 4-year dataset from the USA [1]. Conversely, ALL has a higher incidence in children, characterised by an age-adjusted rate of 1.5 per 100,000 in the USA [1].
Historically, in the absence of chemotherapy, individuals diagnosed with AL faced a dismal prognosis, with life expectancy limited to 6 weeks [2, 3]. However, the landscape of AL has undergone a remarkable transformation in recent years, thanks to the advent of chemotherapy and other therapeutic approaches, including allogeneic stem cell transplantation (allo-SCT). As a result, a significant proportion of patients now achieve complete remission (CR) and experience long-term survival, akin to achieving a potential cure [1, 2, 4]. Remarkably, the 5-year overall survival (OS) rate for paediatric ALL patients has reached an impressive 90%, while among adults, it ranges from 47% to 51% [4, 5]. For AML patients below the age of 45, the 5-year OS rate stands at 50%, but it diminishes significantly to a mere 2% for patients aged 75 and above [2]. These advancements underscore the evolving landscape of AL management.
Over the past few decades, the landscape of AL management has been transformed with the introduction of several Food and Drug Administration (FDA)-approved targeted therapies. These advancements have significantly improved the prognosis for patients with AL, particularly in cases of AML. Notable targeted therapies include FLT3 inhibitors (such as midostaurin, gilteritinib and quizartinib), isocitrate dehydrogenase (IDH) inhibitors (ivosidenib and enasidenib) and the use of the CD33-targeted monoclonal antibody gemtuzumab ozogamicin (GO) in specific cases [6]. In the context of ALL, several new therapies, including blinatumomab, inotuzumab ozogomycin and CAR-T therapy, have been introduced mainly as bridge therapies to allo-SCT [7–10]. However, it is important to recognise that AML and ALL continue to represent significant public health challenges globally [1], with more favourable outcomes typically observed in developed countries. Alarmingly, approximately 70% of cancer-related deaths occur in low- and middle-income countries [11], often due to limited access to modern treatment modalities. Developed regions allocate 5 to 10 times the per capita resources for cancer control compared to their counterparts in low- and middle-income countries. Importantly, the financial gap between developed and developing nations in this context is widening [11]. The primary objective of our study is to present a comprehensive assessment of the leukaemia landscape in Armenia while highlighting the substantial disparities that profoundly affect treatment outcomes. Through an analysis of the challenges faced in managing AL patients in Armenia, we aim to contribute to the development of holistic strategies aimed at ensuring equitable access to appropriate care for individuals, irrespective of their socioeconomic status.
Materials and methods
This retrospective cohort study encompasses a cohort of 431 AL patients diagnosed at the Hematology Centre between 2016 and 2020, which draws on data from diverse sources, including outpatient and dispensary records, hospitalisation logs and clinical data obtained from the Registry of Blood Diseases of the Centre. The Hematology Centre stands as the only institution in Armenia dedicated to the treatment of leukaemia, thereby our dataset represents the entire nation. Death dates were taken from the United Information System of Electronic Healthcare in the Republic of Armenia. Survival analysis was performed through the Kaplan–Meier method.
Results
Over the 5 years between 1 January 2016, and 31 December 2020, a total of 431 patients were diagnosed with AL at the Hematology Centre of Armenia. Among this cohort, a substantial proportion, comprising 310 (71.9%) patients died. Our comprehensive analysis focused on a subset of 244 available patients with a median age of 59 years (age range 18–85 years) at the time of diagnosis and 112 (46%) of the patients were female. Among the diagnosed cases, 158 (65%) had AML, 56 (23%) ALL and 2 (1%) mixed phenotypes, while in 28 (11%) cases, the AL type remained unspecified (Table 1). A notable proportion accounting for 24% (58), passed away before frontline chemotherapy. Among these patients, 45% (26) refused treatment, while others experienced various complications due to disease progression. Among the patients who received chemotherapy, 34% (82) achieved CR. However, 29% (24) of these patients died during the first remission. The causes of death in these cases varied (acute heart failure, COVID-19, septic shock, pulmonary aspergillosis and acute respiratory failure). Notably, 20% (50) died during induction therapy. Of these cases, 29 (58%) were attributed to chemotherapy. The remaining patients passed away due to various reasons, including septic shock, acute heart failure, brain hemorrhage, acute respiratory failure, mesenteric venous thrombosis and multiple organ failure. Furthermore, 30 patients demonstrated resistance to the first-line therapy chose to discontinue further treatment and refused second-line therapy. In 10% (24), it was not possible to determine whether these patients achieved remission and what was the exact cause of death, underscoring the need for comprehensive documentation and standardised protocols for data collection (Figure 1). Among the patients who experienced relapse, only 7 (12%) patients reached second remission, indicating the challenges faced in managing recurrent disease. The 5-year OS was evaluated at 22% (Figure 2).
Table 1. Demographic and diagnostic profile of patients with AL.
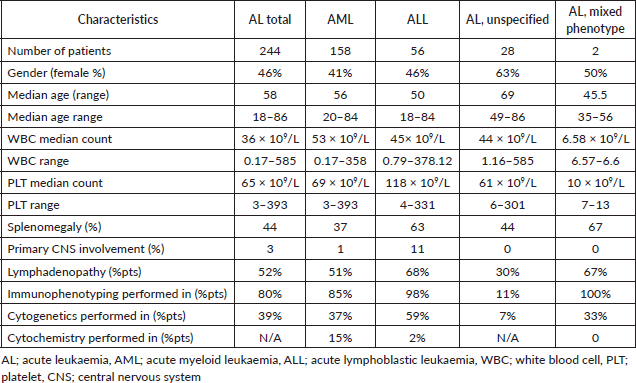
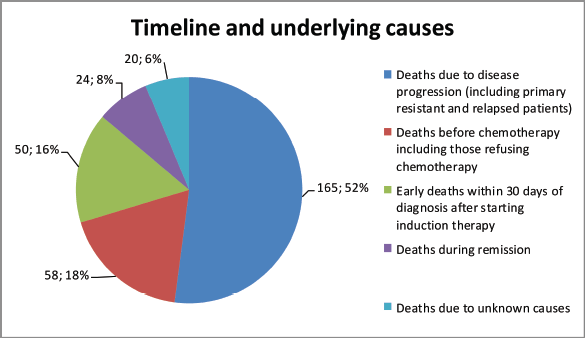
Figure 1. Mortality patterns and causes in AL patients.
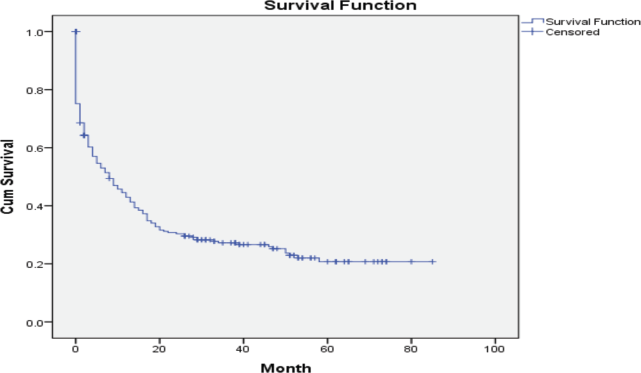
Figure 2. Five-year OS of patients with AL in Armenia.
Discussion
Challenges of management of AML in Armenia
The incidence of AML in Armenia was evaluated at 1.12 per 100,000 which is notably lower compared with European developed countries and the United States (3–20 per 100,000) [11–14]. Among developing countries, Serbia has a higher incidence compared with Armenia (2.73 versus 1.12 per 100,000), and another developing country, Croatia, has a similar incidence with Armenia [14]. In our cohort, the incidence was 1.9 per 100,000. The prevalence of AML in Armenia was estimated at 7.4 per 100,000 populations as of January 1st, 2021, which reflects a relatively low but stable disease burden. This rate is lower than those reported in many European and North American populations, where prevalence often ranges from 12 to 20 per 100,000 [1, 14]. The observed difference may be attributed to factors such as younger population structure, underdiagnoses in older adults or variations in registry coverage and reporting practices.
Traditionally, the diagnosis of AML in Armenia relied on bone marrow cytochemistry. However, since 2010, the introduction of flow cytometry and the gradual adoption of immunophenotyping have made the latter the primary diagnostic method for AML, despite not being covered by state funding. In our cohort, out of 158 AML cases, 135 were diagnosed using immunophenotyping. Presently, cytogenetics and molecular genetics play pivotal roles in AML diagnostics, classification, risk stratification, prognosis and treatment protocol selection. While cytogenetic methods are available in Armenia, in our cohort they have been conducted in only 37% of patients due to high costs. Unfortunately, the availability of molecular genetic methods in Armenia is limited, making it impossible to detect gene mutations such as FLT3 and IDH, which play an important role in determining treatment strategies. FLT3 and IDH mutations occur in approximately 30% and 10%–15% of AML cases respectively [15, 16].
Several studies have demonstrated that FLT3- and IDH-targeted treatment modalities such as midostaurin, gilteritinib, quizartinib, enasidenib and ivosidenib prolong median OS [17–20]. Another essential option for AML management is core binding factor (CBF)-targeted therapy with GO. This targeted therapy has shown the potential to significantly increase long-term survival rates from less than 50% to 75% or more, making it a compelling option for all CBF AML patients [3]. The risk of CBF AML relapse can be reduced with additional allo-SCT, which is also beneficial for high-risk AML patients, those with relapsed/refractory disease and younger patients with intermediate/poor risk profiles [6].
In Armenia, the standard induction therapy for AML continues to be the time-tested ‘7 + 3’ regimen, consisting of 7 days of cytarabine and 3 days of anthracycline. This regimen, which has been a mainstay since the 1970s, is still widely used in many countries, including Armenia. Consolidation therapy primarily involves either 3–4 courses of high-dose cytarabine (HiDAC) or ‘7 + 3’ followed by maintenance therapy with the ‘5 + 5’ regimen (5 days of cytarabine + 6-mercaptopurine) every 6 weeks for 2 years.
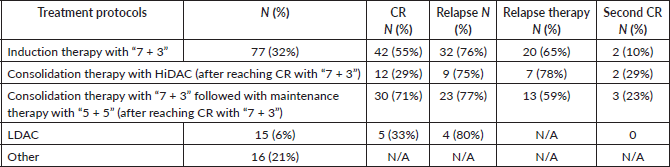
The choice of treatment strategy often depends on the patient's socio-economic status, given that chemotherapy is only partially covered by the state. In our cohort, 32% received induction therapy with ‘7 + 3,’ and of those who achieved CR, 29% underwent consolidation therapy with HiDAC, while 71% followed the ‘7 + 3’ regimen, coupled with maintenance therapy. The relapse rates were 75% and 77%, respectively. A small fraction, 6% of patients, received treatment with low-dose cytarabine (LDAC) and 21% underwent other treatment regimens (Table 2). Unfortunately, the abovementioned targeted treatment modalities are not currently available in Armenia, and none of the patients in our cohort received targeted therapy.
Table 2. Management and outcomes of AML.
In summary, the integration of targeted therapies with induction/consolidation chemotherapy holds promise for improving outcomes in AML patients, particularly those with specific genetic mutations and the absence of these diagnostic and treatment modalities in Armenia has led to a decrease in OS compared to the developed world, with a 5-year OS rate of 21% (Figure 3).
Challenges of management of ALL in Armenia
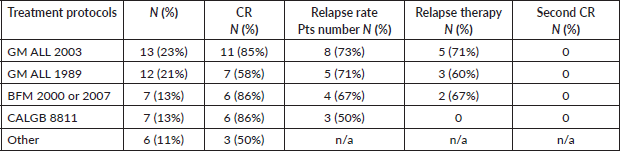
The incidence of ALL in Armenia was evaluated at 0.6 per 100,000, which is notably lower than in other countries, where it ranges from 2 to 3.5 per 100,000, including Australia, China, Costa Rica, Germany, Italy, Japan and the US [13, 21]. In our cohort, the incidence was 0.68 per 100,000, and the prevalence reached 10.8 per 100,000 as of 1st January 2021. These findings highlight a relatively low rate of newly diagnosed cases, yet a higher cumulative burden possibly reflecting improved survival and treatment outcomes over time. Similar to AML patients, those with ALL face challenges in diagnostics and risk stratification due to limitations in cytogenetic and molecular genetic methods. In our cohort of 56 ALL patients, 98% received their diagnosis through immunophenotyping. The evaluation of Philadelphia chromosome positivity using the FISH method was conducted in 59% of patients. The mainstay treatment protocols during this period were GM ALL-2003 and GM ALL-1989. In our cohort, 23% of patients received GM ALL-2003, with 85% achieving CR. Additionally, 21% were treated with GM ALL-1989 13% with BFM-2000 or BFM-2007, 13% with CALGB 8811 and 11% with other regimens, with CR rates of 85%, 58%, 86%, 86% and 50%, respectively. Unfortunately, 59% of patients experienced relapse and only 50% of them received relapse therapy, with none achieving a second remission (Table 3).
The primary challenges in ALL treatment protocols lie in the high cost of drugs that are not covered by the state. Specifically, essential components of ALL treatment protocols, such as peg-asparaginase, rituximab and nelarabine are unaffordable for many patients, leading to some receiving treatment without these critical components. In the case of Ph-positive ALL patients, tyrosine kinase inhibitors are provided through the Max Foundation.
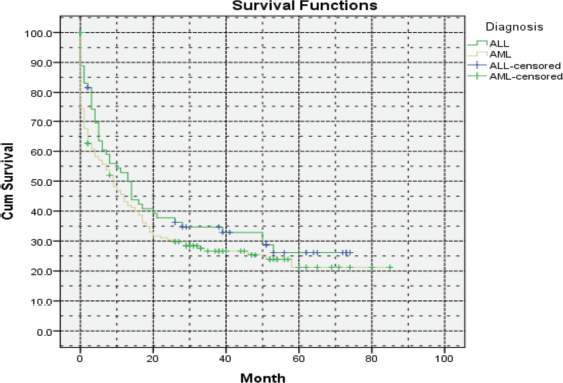
Figure 3. Five-year OS of ALL and AML patients in Armenia.
Table 3. Management and outcomes of ALL.
In the context of ALL, several new therapies, including bispecific T-cell engager blinatumomab, anti-CD22 antibody inotuzumab ozogomycin and CAR-therapy Tisagenlecleucel have been introduced and got FDA approvement. These treatment modalities have shown significant improvement in median OS and high remission rate [8–11, 22, 23].
Unfortunately, the unavailability of these crucial medications presents extreme challenges in the management of ALL, particularly in cases of early relapses and refractory disease. The 5-year OS in Armenia was evaluated at 26%, which is lower compared to the developed world (Figure 3).
Based on our analysis of the cohort, the challenges in AL management in Armenia can be categorised into two primary aspects:
Equipment: The limited availability of cytogenetic and molecular genetic methods poses significant challenges in achieving an accurate diagnosis, risk stratification and the utilisation of FDA-approved targeted therapies for ALs.
Treatment access: The absence of critical treatment options, including allo-SCT, GO, CPX-351, midostaurin, gilteritinib, quizartinib, enasidenib, ivosidenib, blinatumomab, inotuzumab ozogomycin, nelarabine and CAR-T therapy, as well as limited availability of essential chemotherapy drugs, deviates from standard treatment regimens, ultimately impacting the outcomes of AL. Here, it should be mentioned that within our cohort, 24% (58) of patients died of the disease before starting chemotherapy. Among them, 45% (26) declined treatment, primarily due to financial constraints. In some cases, patients were unaware of their diagnosis, and decisions were made by their relatives. Others faced various complications resulting from disease progression. Among the 82 patients who achieved CR, 29% (24) died during the first remission due to various treatment-related complications. Limited access to appropriate s care also played a role in these challenges. Access to supportive care also plays an important role in outcomes, such as the use of granulocyte colony-stimulating factors has shown to be highly effective in shortening the duration of chemotherapy-induced neutropenia, which is associated with a 20%–25% reduction in the incidence of febrile neutropenia. This reduction in febrile episodes translates to fewer hospitalisations and a decrease in the need for broad-spectrum antibiotics, further improving patient outcomes [24]. Antibiotics and antifungal treatments, such as posaconazole, are not consistently accessible or cost-effective for the country.
In this study, comprehensive data were collected from all cases of AL diagnosed at our centre, the only diagnostic institution for hematologic malignancies in Armenia. However, we should consider the potential for selection bias: the dataset does not account for possible cases of AL that remain undiagnosed, particularly in less accessible regions of the country. The absence of these cases from our analysis represents a notable limitation, as barriers like socio-economic constraints and limited disease awareness could influence the diagnosis and subsequent inclusion in our research. Consequently, while the study describes the situation for a part of the population, it simultaneously shows a selection bias. This underscores a need for expanded healthcare outreach and improved awareness of the disease throughout Armenia. Our findings, therefore, provide an essential, albeit incomplete, perspective on the mortality of AL in Armenia.
In conclusion, the limited availability of modern diagnostic and treatment modalities in Armenia has resulted in inferior outcomes of AL with a lower median OS compared with the developed world. Addressing these challenges requires efforts to enhance diagnostic and treatment accessibility giving all patients an equal chance of overcoming this malignant disease regardless of their socio-economic background, and trying to achieve outcomes comparable to those in the developed world.
List of abbreviations
AL, acute leukaemia; ALL, acute lymphoblastic leukaemia; allo-SCT, allogeneic stem cell transplantation; AML, acute myeloid leukaemia; CNS, central nervous system; OS, overall survival; PLT, platelets; WBC, white blood cells.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest
The authors have stated that they have no conflicts of interest.
References
1. Du M, Chen W, and Liu K, et al (2022) The global burden of leukaemias and its attributable factors in 204 countries and territories: findings from the Global Burden of Disease 2019 Study and Projections to 2030 J Oncol 2022 1612702 https://doi.org/10.1155/2022/1612702 PMID: 35509847 PMCID: 9061017
2. Rose-Inman H and Kuehl D (2017) Acute leukaemias Hematol Oncol Clin North Am 31(6) 1011–1028 https://doi.org/10.1016/j.hoc.2017.08.006 PMID: 29078921
3. Kantarjian H, Kadia T, and DiNardo C, et al (2021) Acute myeloid leukaemias: current progress and future directions Blood Cancer J 11(2) 41 https://doi.org/10.1038/s41408-021-00425-3 PMID: 33619261 PMCID: 7900255
4. Terwilliger T and Abdul-Hay MJ (2017) Acute lymphoblastic leukaemias: a comprehensive review and 2017 update Blood Cancer J 7(6) e577 https://doi.org/10.1038/bcj.2017.53 PMID: 28665419 PMCID: 5520400
5. Canadian Cancer Statistics Advisory Committee (2019) Canadian Cancer Statistics 2019 (Toronto: Canadian Cancer Society) [https://cancer.ca/en/research/cancer-statistics/past-editions]
6. Leotta S, Condorelli A, and Sciortino R, et al (2022) Prevention and treatment of acute myeloid leukaemias relapse after hematopoietic stem cell transplantation: the state of the art and future perspectives J Clin Med 11(1) 253 https://doi.org/10.3390/jcm11010253 PMID: 35011994 PMCID: 8745746
7. Pawinska-Wasikowska K, Wieczorek A, and Balwierz W, et al (2022) Blinatumomab as a bridge therapy for hematopoietic stem cell transplantation in pediatric refractory/relapsed acute lymphoblastic leukaemias Cancers 14(2) 458 https://doi.org/10.3390/cancers14020458 PMID: 35053619 PMCID: 8773605
8. Franquiz MJ and Short NJ (2020) Blinatumomab for the treatment of adult B-cell acute lymphoblastic leukaemias: toward a new era of targeted immunotherapy Biologics 14 23–34 PMID: 32103893 PMCID: 7027838
9. Kantarjian HM, Stock W, and Cassaday RD, et al (2021) Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukaemias in the INO-VATE trial: CD22 pharmacodynamics, efficacy, and safety by baseline CD22 Clin Cancer Res 27(10) 2742–2754 https://doi.org/10.1158/1078-0432.CCR-20-2399 PMID: 33602684
10. Sheykhhasan M, Manoochehri H, and Dama P (2022) Use of CAR T-cell for acute lymphoblastic leukaemias (ALL) treatment: a review study Cancer Gene Ther 29(8-9) 1080–1096 [doi: 10.1038/s41417-021-00418-1] https://doi.org/10.1038/s41417-021-00418-1 PMID: 34987176 PMCID: 9395272
11. Tebbi CK (2021) Etiology of acute leukaemias: a review Cancers (Basel) 13(9) 2256 [doi: 10.3390/cancers13092256] https://doi.org/10.3390/cancers13092256 PMID: 34066700 PMCID: 8125807
12. Ming Y, Anping L, and Linghui Z, et al (2020) The global burden and attributable risk factor analysis of acute myeloid leukaemias in 195 countries and territories from 1990 to 2017 J Hematol Oncol 13(1) 72 https://doi.org/10.1186/s13045-020-00908-z
13. Harutyunyan L, Sahakyan L, and Hakobyan Y (2022) PB1761: acute leukaemias statistics in Armenia during 2010-2020 HemaSphere 6(Suppl) 1642–1643 https://doi.org/10.1097/01.HS9.0000849900.03921.06 PMCID: 9429519
14. Maksimovic N, Zaric M, and Gazibara T, et al (2018) Incidence and mortality patterns of acute myeloid leukaemias in Belgrade, Serbia (1999–2013) Medicina 54(1) 5 https://doi.org/10.3390/medicina54010005
15. Kiyoi H, Kawashima N, and Ishikawa Y (2020) FLT3 mutations in acute myeloid leukaemias: therapeutic paradigm beyond inhibitor development Cancer Sci 111(2) 312–322 https://doi.org/10.1111/cas.14274 PMCID: 7004512
16. Patel KP, Ravandi F, and Ma D, et al (2011) Acute myeloid leukaemias with IDH1 or IDH2 mutation: frequency and clinicopathologic features Am J Clin Pathol 135(1) 35–45 https://doi.org/10.1309/AJCPD7NR2RMNQDVF
17. Molica M, Perrone S, and Rossi M (2023) Gilteritinib: the story of a proceeding success into hard-to-treat FLT3-mutated AML patients J Clin Med 12(11) 3647 https://doi.org/10.3390/jcm12113647 PMID: 37297842 PMCID: 10253262
18. Zhao JC, Agarwal S, and Ahmad H, et al (2022) A review of FLT3 inhibitors in acute myeloid leukaemias Blood Rev 52 100905 https://doi.org/10.1016/j.blre.2021.100905
19. Cortes JE, Khaled S, and Martinelli G, et al (2019) Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial Lancet Oncol 20(7) 984–997 https://doi.org/10.1016/S1470-2045(19)30150-0 PMID: 31175001
20. Larson RA, Mandrekar SJ, and Sanford BL, et al (2017) An analysis of maintenance therapy and post-midostaurin outcomes in the international prospective randomized, placebo-controlled, double-blind trial (CALGB 10603/RATIFY [Alliance]) for newly diagnosed acute myeloid leukaemias (AML) patients with FLT3 mutations Blood 130 145
21. Katz AJ, Chia VM, and Schoonen WM, et al (2015) Acute lymphoblastic leukaemias: an assessment of international incidence, survival, and disease burden Cancer Causes Control 26 1627–1642 https://doi.org/10.1007/s10552-015-0657-6 PMID: 26376890
22. Zheng PP, Kros JM, and Li J (2018) Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts Drug Discov Today 23(6) 1175–1182 https://doi.org/10.1016/j.drudis.2018.02.012 PMID: 29501911
23. Whittington MD, McQueen RB, and Ollendorf DA, et al (2018) Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukaemias JAMA Pediatr 172(12) 1161–1168 https://doi.org/10.1001/jamapediatrics.2018.2530 PMID: 30304407 PMCID: 6583018
24. Crawford J, Caserta C, and Roila F (2010) Hematopoietic growth factors: ESMO clinical practice guidelines for the applications Ann Oncol 21(Suppl 5) v248–v251 https://doi.org/10.1093/annonc/mdq195 PMID: 20555091






