Filgrastim XM02 (Tevagrastim®) after autologous stem cell transplantation compared to lenograstim: favourable cost-efficacy analysis
A Gardellini1, F Gigli1, A Babic3, G Andreola1, D Radice2, S Sammassimo1, G Martinelli1 and D Laszlo3
1 Division of Haematoncology, European Institute of Oncology, Milan, Italy
2 Division of Statistics, European Institute of Oncology, Milan, Italy
3 Stem Cell Collection Unit, European Institute of Oncology, Milan, Italy
Correspondence to: Gardellini Angelo. Email: angelo.gardellini@ieo.it
Abstract
Purpose: Granulocyte colony-stimulating factors (G-CSFs), filgrastim and lenograstim, are recognised to be useful in accelerating engraftment after autologous stem cell transplantation. Several forms of biosimilar non-glycosylated G-CSF have been approved by the European Medicines Agency, with limited published data supporting the clinical equivalence in peripheral blood stem cell mobilisation and recovery after autologous stem cell transplantation.
Method: With the aim of comparing cost-effective strategies in the use of G-CSF after autologous stem cell transplantation, we retrospectively evaluated 32 patients consecutively treated with biosimilar filgrastim XM02 (Tevagrastim) and 26 with lenograstim. All patients received G-CSF (biosimilar or lenograstim) at a dosage of 5 mcg/kg/day subcutaneously from day 5 to absolute neutrophil count of 1500/mmc for three days.
Results: The median time to absolute neutrophil count engraftment was 11 days for the filgrastim XM02 group and 12 days for the lenograstim group. As for platelets recovery, the median time was 12 days in both groups. The median number of G-CSF vials used for patients was 9.5 for Tevagrastim and 10.5 for lenograstim, reflecting a mean estimated cost of about 556.1 euros for Tevagrastim versus 932.2 euros for lenograstim (p < 0.001). The median days of febrile neutropenia were 1.5 and 1 for filgrastim XM02 and lenograstim, respectively. No adverse event related to the use of XM02 filgrastim was recorded.
Conclusion: In our experience, filgrastim XM02 and lenograstim showed comparable efficacy in shortening the period of neutropenia after cytoreduction and autologous stem cell transplantation, with a favourable cost effect for filgrastim XM02.
Keywords: biosimilar G-CSF, autologous bone marrow transplantation, engraftment, filgrastim, lenograstim
Introduction
Granulocyte colony-stimulating factors (G-CSFs), lenograstim and filgrastim, are biological growth factors that promote the proliferation, differentiation, and activation of neutrophils in the bone marrow. Lenograstim is derived from Chinese hamster ovary cells consisting of 174 amino acids with 4% carbohydrate, indistinguishable from native G-CSF, whereas filgrastim is produced in Escherichia coli differing from lenograstim in being non-glycosylated and in having an extra methionine group at the N-terminal end of the peptide chain [1–3].
Both are recognised to be useful in the treatment in chemotherapy-induced febrile neutropenia, in peripheral blood stem cells (PBSCs) mobilisation and in promoting recovery after autologous stem cell transplantation [1, 2, 4–8].
The American Society of Clinical Oncology’s 2006 update on the use of white blood cell growth factors provides recommendations for primary and secondary G-CSF prophylaxis [10–12]; they confirm the positive impact of G-CSF in mobilising PBSCs and in accelerating recovery after cytoreduction chemotherapy treatment and PBSC transplantation.
The clinical efficacy of lenograstim and filgrastim has been compared in a systematic review of 16 studies, showing no differences between the two growth factors in any of the approved indications [13].
Biosimilars are non-identical versions of originator biopharmaceuticals. They differ from originator drugs in the size of the active substance, complexity, and nature of the manufacturing process. Several biosimilar G-CSFs are approved in Europe: Biograstim®/Filgrastim, ratiopharm/Ratiograstim®/Tevagrastim® (XM02), Zarzio® and Nivestim®. Recently, biosimilars have been routinely introduced in clinical practice, in particular in the treatment of cancer neutropenia [14]. On the basis of these results, the European Medical Association (EMA) extrapolated the therapeutic equivalence of the biosimilars for PBSC mobilisation and recovery after autologous stem cell transplantation despite the fact that there is little data supporting clinical equivalence. The relevance of biosimilars is mainly related to cost-efficient analysis despite the limited experience at the time of approval of these products in terms of efficacy, safety, and immunogenicity.
Here, we report our experience of the use of G-CSF biosimilar Tevagrastim (filgrastim XM02), compared with branded lenograstim (Myelostim) in the recovery after PBSC transplantation, taking into account their cost-efficacy profile.
Patients and methods
Patient characteristics
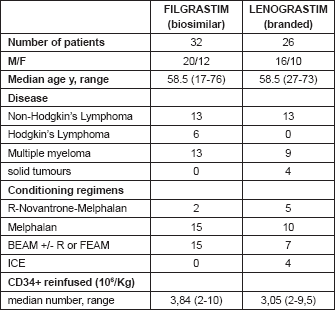
From November 2010 to December 2011, 26 consecutive patients with haematological disease (13 with non-Hodgkin’s lymphoma, nine with multiple myeloma) and four patients with solid tumours (testicular seminoma) underwent PBSC transplantation in our haematoncology division and received lenograstim after PBSC reinfusion to accelerate engraftment. The median age was 58 (range 18–73). Thirty-two patients with haematological disease (six with Hodgkin’s lymphoma, 13 with non-Hodgkin’s lymphoma, 13 with multiple myeloma), with similar median age (58 years, range 17–76) received G-CSF biosimilar (filgrastim, Tevagrastim) at the same dosage. The conditioning regimen was ICE (ifosfamide, citarabine, etoposide) for testicular seminoma, R-FEAM or R-BEAM (Rituximab, fotemustine or carmustine, etoposide, citarabine, melphalan) for high-grade B cell lymphomas or for Hodgkin lymphoma, Melaphalan for multiple myeloma, and Rituximab–Novantrone–melphalan for follicular lymphomas. Clinical characteristics are reported in Table 1.
All patients received Tevagrastim or lenograstim after PBSC reinfusion at the dosage of 5 mcg/kg/day subcutaneously from day 5 until absolute neutrophil count (ANC) of 1500/mm3 for three days.
Engraftment was defined as ANC > 500/mmc and platelet count > 20,000/mmc.
Febrile neutropenia was defined as grade 4 neutropenia (ANC<500/mm3) with an axillary temperature ≥ 38.5 °C or two or more febrile episodes at > 38 °C within a 12-h period.
Table 1: Patients’ characteristics.
The primary data from clinical records were stored in the divisional database containing detailed information of patients hospitalised at the European Institute of Oncology for autologous transplantation and including demographic and diagnostic variables as well as conditioning regimen and data regarding post-transplant complications and haematological recovery.
A cost-effective analysis considering engraftment, incidence of febrile neutropenia, number of days of hospitalisation, and number of vials of G-CSF administered was performed.
Statistical analysis
The patients’ characteristics have been summarised and tabulated using either counts and percentages for categorical data or count, mean, median standard error, min and max for continuous variables. The median days to reach the ANC cut-off values of 0.5 x 109/L by treatment have been estimated by the Kaplan–Meier method (log-rank test for treatments comparison) and tabulated with 95% confidence intervals. The hazard ratios have been calculated taking lenograstim as reference. All other treatment comparisons were done using the two-sample Wilcoxon test or the unpaired t-test as appropriate. All tests were considered statistically significant at the 5% level and two tailed. Statistical analyses were performed using the SAS 9.2 (Cary, North Carolina, United States).
Results
Engraftment
The median number of CD34 cells reinfused was 3.05 x 106/kg (2.0–9.5) and 3.84 x 106/kg (2.0–10.0) in the lenograstim group and filgrastim group, respectively (p = 0.437).
The median number of days to ANC recovery was 12 days (10–12) in the lenograstim group and 11 (10–11) in the tevagrastim group without statistically significant difference (p = 0.055) (Table 2). Regarding platelets recovery, median days were 12 in both group with a range of 7–22 days for the tevagrastim group and 9–30 days for the lenograstim group.
Table 2: Median days to neutrophiles count cut-off values by treatment.

Safety
The median number of days of febrile neutropenia was 1 (range 0–10) for the lenograstim group and 1.5 (range 0–5) for the tevagrastim group without statistically significant difference; the median days of antibiotic therapy was 3.5 (range 0–12 ) for the filgrastim group and 4 (range 0–22) for the tevagrastim group (p ns). With a median follow-up of 14 months (range 3–23), neither systemic or local side effects nor immunogenicity related to the filgrastim was observed.
Cost analysis evaluation
The median number of G-CSF vials administered of lenograstim and tevagrastim was, respectively, 10.5 (11.7 ± 0.7) and 9.5 (9.6 ±0.3), with a statistically significant difference (p 0.027). Performing a cost analysis evaluation, we found a mean estimated cost about of 932.2 ± 54.4 euros for filgrastim and 556.1 ± 20.0 euros for tevagrastim (p < 0.001) (Table 3).
Discussion
Different studies have evaluated the impact of lenograstim in the treatment of chemotherapy-induced febrile neutropenia [15], in peripheral stem cells mobilisation and engraftment [16–18]. In addition, in 2011, Sourgens et al [13] performed a systematic review of 16 studies comparing filgrastim with lenograstim. No clinically remarkable differences between filgrastim and lenograstim in chemotherapy-induced neutropenia and mobilisation of peripheral blood progenitor cells in patients and healthy donors were observed. In conclusion, there is no reason to prefer lenograstim over filgrastim in any of the approved indications for both.
Biosimilars are non-identical versions of originator biopharmaceuticals. From their introduction to clinical practice, a cost-efficient impact has been evaluated in different onco-haematological fields.
The first application of biosimilars was the chemotherapy-induced febrile neutropenia. Salesi et al [19] reported that the use of biosimilar G-CSF is safe and effective in reducing neutropenic complications in patients with solid tumours and may be associated with cost saving. Also, Aapro et al [20] in a comparative cost-efficiency analysis across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim and peg-filgrastim reported that Zarzio® is the most cost-efficient approach in reducing the incidence of febrile neutropenia in chemotherapy-treated patients. Kotwica et al [21] in their experience of 23 consecutive patients concluded that biosimilar G-CSF appeared to be effective in reducing the duration of neutropenia in patients undergoing myeloablative therapy followed by autologous Stem cell Transplantation with cost savings in cancer supportive care budgets.
Table 3: Multivariate analysis.

Regarding the application of biosimilars in peripheral stem cells mobilisation, our group reported their experience on the use of Plerixafor in 28 patients treated with the G-CSF originator or with G-CSF biosimilar (Tevagrastim) in patients with lymphoma or myeloma candidate to autologous stem cell transplantation. In this report, no difference was observed with the use of G-CSF or biosimilar Tevagrastim, and no major side effect was reported either with G-CSF or biosimilar Tevagrastim [22].
Finally, the impact of biosimilars was evaluated in the engraftment after PBSC reinfusion. Publicover [23] demonstrated no differences between the originator versus G-CSF biosimilar (ratiopharm) not only in CD34 mobilisation and harvest but also in median days to obtain neutrophil engraftment (13 ± 2.4 vs 13 ± 2.9 , p 0.26) and platelet engraftment (12 ± 3.9 vs 12 ± 4.1, p 0.33), without any increased toxicity with the biosimilar ratiopharm.
Lefrère et al [24] enrolled 40 patients affected by haematological malignancy to receive biosimilar G-CSF (Zarzio) after the first cycle of chemotherapy. These patients were compared with a historical control group treated with G-CSF (Neupogen) in the same centre and clinical protocol. No significant differences were observed between groups in median CD34 cells mobilised and harvested or the number of G-CSF injections to obtain at least 3 x 106 CD34 cells/kg (minimal CD34 cells count considered successful). The benefit of biosimilar G-CSF in PBSC mobilisation with regard to safety and cost saving was unquestionable due to the identical median consumption of G-CSF doses and leukapheresis procedures noted to reach the target of a minimal count.
Finally, Czerw et al [25] showed the results of 35 consecutive patients with multiple myeloma treated with Neupogen and 55 with Zarzio: in this analysis, GCSF biosimilar provides equivalent efficacy in accelerating neutrophil recovery after ASCT compared with the original formulation.
In our analysis, patients with haematological and non-haematological disease have been included. Two different agents have been compared in the post-autologous transplantation: the originator lenograstim, (myelostim) and biosimilar filgrastim (Tevagrastim) used in our institution.
In our experience, tevagrastim and myelostim were both used at 5 mcg/kg/die. In particular, we did not find a significant statistical difference in terms of ANC recovery between filgrastim biosimilar and lenograstim originator. Nevertheless, this result has been obtained with a lower median number of G-CSF biosimilar vials (9.5) than G-CSF originator (10.5) with a statistically significant difference.
Both treatments were safe. With a median follow-up of 14 months we did not observe any deaths related to the autologous procedure.
According to multivariate analysis, no statistically significant differences were observed in the two groups in terms of number of febrile neutropenia; engraftment and days of hospitalisation were similar. Nevertheless, the cost between biosimilar G-CSF and originator is very different, biosimilars being cheaper than G-CSF originators. This could allow a significant cost reduction at our centre.
To the best of our knowledge, this is the first experience comparing lenograstim versus biosimilar filgrastim, showing no differences in activity and toxicity, with a 27% cost savings in favour of biosimilar.
Disclosures
The authors have declared no conflicts of interest.
Acknowledgment
The cost of publication was kindly donated by Gruppo Italiano Infermieristico Mobilizzazione e Aferesi (GIIMA).
References
1. Mostyn G and Burges AW (1988) Hematopoietic growth factors: a review Cancer Res 48 5624–37
2. Lieschke GJ and Burgess AW (1992) Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor N Engl J Med 327 28–35, 99–106 DOI: 10.1056/NEJM199207093270207 PMID: 1376442
3. Kubota N, Orita T and Hattori K (1990) Structural characterization of natural and recombinant human granulocyte colony-stimulating factor J Biochem 107 486–92 PMID: 1692828
4. Huttmann A, Schirsafi K, Seeber S and Bojto P (2005) Comparison of lenograstim and filgrastim: effects on blood cell recovery after high-dose chemotherapy and autologous peripheral stem cell transplantation J Cancer Res Clin Oncol 131 152–6 DOI: 10.1007/s00432-004-0636-x
5. Kim IH, Park SK, Suh OK and Oh JM (2003) Comparison of lenograstim and filgrastim on haematological effects after autologous peripheral blood stem cell transplantation with high-dose chemotherapy Curr Med Res Opin 19 753–759 DOI: 10.1185/030079903125002531 PMID: 14687447
6. Lefrere F, Bernard M, Audat F, Cavazzana-Calvo M, et al (1999) Comparison of lenograstim vs filgrastim administration following chemotherapy for peripheral blood stem cell (PBSC) collection: a retrospective study of 126 patients Leuk Lymphoma 35 501–5 DOI: 10.1080/10428199909169614 PMID: 10609787
7. Teschendorf C, Karaivanova D, Koldehoff M and Schmiegel W (2010) Lenograstim and filgrastim are equally effective for the hematopoietic recovery after high-dose chemotherapy and autologous peripheral blood stem cell transplantation Onkologie 33(suppl 2) 140
8. Ria R, Gasparre T, Mangialardi G, Bruno A, et al (2010) Comparison between filgrastim and lenograstim plus therapy for mobilization of PBPCs Bone Marrow Tranplant 45 277–81 DOI: 10.1038/bmt.2009.150
9. Saccardi R, Avanzi G, Bezzini R, Bosi A, et al (1997) Mobilization of PBPC for hematological rescue: comparison between glycosylated and non-glycosylated G-CSF Bone Marrow Transplant 19(suppl 1; Abstr 43) S11
10. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, et al (2006) Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline J Clin Oncol 24 3187–205 DOI: 10.1200/JCO.2006.06.4451 PMID: 16682719
11. Aapro MS, Cameron DA, Pettengell R, Bohlius J, et al (2006) EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumors Eur J Cancer 42 2433–53 DOI: 10.1016/j.ejca.2006.05.002 PMID: 16750358
12. Aapro MS, Bohlius J, Cameron DA, et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours Eur J Cancer DOI: 10.1016/j.ejca.2010.10.013
13. Sourgens H and Lefrère F (2011) A systematic review of available clinical evidence – filgrastim compared with lenograstim Int J Clin Pharmacol Ther 49 510–8 PMID: 21781651
14. Gascon P (2012) Presently available biosimilars in hematology-oncology: G-CSF (2012) Target Oncol (suppl 1) S29–34 DOI: 10.1007/s11523-011-0190-9
15. Schmitz N, Ljungman P, Cordonnier C, Kempf C, et al (2004) Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial Bone Marrow Transplant 34 955–62 DOI: 10.1038/sj.bmt.1704724 PMID: 15489865
16. Gisselbrecht C, Prentice HG, Bacigalupo A, Biron P, et al (1994) Placebo-controlled phase III trial of lenograstim in bone-marrow transplantation J Clin Oncol 12 1931–8
17. Stahel RA, Jost LM, Cerny T, Pichert G, et al (1995) Randomized study of recombinant human granulocyte colony-stimulating factor after high-dose chemotherapy and autologous bone marrow transplantation for high-risk lymphoid malignancies J Clin Oncol 13 1323–7 PMID: 7521907
18. Klumpp TR, Mangan KF, Goldberg SL and Pearlman ES (1995) Granulocyte colony-stimulating factor accelerates neutrophil engraftment following peripheral-blood stem-cell transplantation: a prospective, randomized trial J Clin Oncol 13 1323–7 PMID: 7538555
19. Salesi N, Di Cocco B, Colonna M and Veltri E (2012) Biosimilar medicines in oncology: single-center experience with biosimilar G-CSF Future Oncol 8 625–50 DOI: 10.2217/fon.12.32 PMID: 22401144
20. Aapro M, Cornes P and Abraham I (2012) Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia J Oncol Pharm Pract 18 171–9 DOI: 10.1177/1078155211407367
21. Kotwica K, Cioch M, Wach M, Manko J, et al (2012) Biosimilar G-CSF is effective in reducing the duration of neutropenia ater autologous bone marrow transplantation Bone Marrow Transplantation P873 47(suppl 1)
22. Andreola G, Babic A, Rabascio C, Negri M, et al (2012) Plerixafor and Filgrastim XM02 (Tevagastrim) as a first line peripheral blood stem cell mobilisation strategy in patients with multiple myeloma and lymphoma candidated to autologous bone marrow transplantation Eur J Haematol 88 154–8 DOI: 10.1111/j.1600-0609.2011.01719.x. Epub 2011 Nov 17
23. Publicover F, Richardson D, Hill K, Jenner M, et al (2012) Use of a biosimilar granulocyte colony stimulating factor (G-CSF) for peripheral blood stem cell mobilisation prior to autologous stem cell transplantation: an analysis of mobilisation and engraftment 17th of the European Hematology Association: Poster 0452 PMCID: 3492921
24. Lefrère F, Brignier AC, Elie C, Ribeil JA, et al (2011) First experience of autologous peripheral blood stem cell mobilization with biosimilar granulocyte colony-stimulating factor M. Adv Ther 28 304–10 DOI: 10.1007/s12325-011-0009-1
25. Czerw T, Kruzel T, Sadus-Wojcciechowska M, Naida J, et al (2012) Comparison of two formulations of filgrastim, Neupogen (Amgen) and Zarzio (Sandoz), used to accelerate neutrophil recovery after autologous blood stem cell transplantation Bone Marrow Transplantation P872 47(suppl 1)






