Sociodemographic factors and treatment effects on quality of life in locally advanced breast cancer: a cross-sectional study
Sasongko Hadi Priyono1,a, Winardi Budiwinata1,b, Budianto Tedjowitono1,c, Effendy2,d and Muhamad Daffa Ibnurasy Pratama3,e
1Department of Surgical Oncology, Ulin General Hospital, Faculty of Medicine, Universitas Lambung Mangkurat, Banjarmasin 70123, Indonesia
2Department of Surgery, Ulin General Hospital, Faculty of Medicine, Univeritas Lambung Mangkurat, Banjarmasin 70123, Indonesia
3Faculty of Medicine, Universitas Lambung Mangkurat, Banjarmasin 70123, Indonesia
ahttps://orcid.org/0009-0006-0212-2513
bhttps://orcid.org/0009-0005-8754-0302
chttps://orcid.org/0009-0002-9763-8862
dhttps://orcid.org/0000-0003-3094-4646
ehttps://orcid.org/0009-0001-5277-3369
Abstract
Purpose: This study aimed to identify key aspects of health-related quality of life in women with locally advanced breast cancer (LABC) and analyse their links to factors and treatment modalities.
Method: A cross-sectional study was conducted from August to October 2023 in Ulin Regional Public Hospital, Banjarmasin, Indonesia, involving LABC women whose quality of life (QoL) was assessed using Quality-of-Life Questionnaire Breast Cancer 23. Data were analysed using ANOVA, independent t-tests for parametric data, Kruskal-Wallis and Mann-Whitney tests for non-parametric data and significant variables (p < 0.05) included in a final regression model for identifying predictors.
Results: Of 100 participants (mean age 50 years), most had low education levels (41%), were unemployed (74%) and had stage IIIB cancer. Body image score was the highest, while systemic therapy side effect was the lowest. Better sexual enjoyment was reported in post-menopausal women (p = 0.043), those with higher education (p = 0.036) and married individuals (p = 0.021). Higher economic status was associated with better sexual enjoyment (p = 0.008) and fewer breast symptoms (p = 0.011); however, economic status was negatively associated with employment status (p = 0.043). Worsening arm symptoms were associated with prolonged illness (p = 0.022). Surgical intervention was associated with higher body image (p = 0.010) and lower systemic side effects (p = 0.023). Traditional medicine was associated with lower arm symptoms (p = 0.026). Economic/occupational status explained 10.5% of sexual functioning scores.
Conclusion: Poor QoL in LABC patients overall was associated with low sociodemographic conditions, late presentation and chemotherapy-related side effects.
Keywords: breast neoplasmsm, quality of life, neoplasm
Correspondence to: Muhamad Daffa Ibnurasy Pratama
Email: daffaibnurasy@gmail.com
Published: 15/08/2025
Received: 27/09/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer is the most common cancer in women globally, with 2.3 million cases diagnosed in 2022, causing 665,000 deaths globally, as reported by the WHO. Up to 2022, half of the breast cancer cases were diagnosed in Asia, where it ranked second after lung cancer but was the most common cancer among women [1, 2]. In Indonesia, breast cancer accounted for 30.8% of all female cancer cases and 20.4% of cancer-related deaths in 2020, with projected increases in incidence and mortality rates regarding late diagnosis and limited access to treatment [3].
In low- and middle-income countries (LMICs), breast cancer often presents at a locally advanced stage (LABC) and research on quality of life in women with LABC in LMICs, particularly in Indonesia, where breast cancer is frequently diagnosed at later stages with poorer prognosis-remains limited [4–7]. LABC, classified into stages IIIA, IIIB and IIIC according to the TNM system (T = tumour, N = regional lymph nodes spread and M = metastasis), is characterised by tumours larger than 5 cm, skin or pectoral muscle involvement, lymph node involvement or inflammatory breast cancer, without distant metastases [8].
Despite being classified as ‘locally advanced,’ LABC often signifies early rapid metastasis. Breast cancer development is multifactorial, significantly impacting patient quality of life (QoL). [9] Health-related quality of life (HR-QoL) assessments are crucial for evaluating disease severity, progression and treatment impact. QoL may be influenced by clinical manifestations and psychological effects, including depression [10]. Advanced malignancies often correlate with impaired functioning and cancer-related life disruption, requiring rehabilitation, yet in developing nations like Indonesia, these services are often inaccessible or insufficient due to provider awareness [11, 12].
At the regional level, Asian patients have a lower HR-QoL than their Western counterparts and Asian patients tend to avoid private topics and dislike discussing them in public, which presents a challenge for healthcare professionals [13]. Present studies regarding QoL on breast cancer in Indonesia are mostly done in the central administrative area of Java, which culminated in a lack of data from patients from outside of Java and are mostly done on unspecified breast cancer presentations [14]. Social structure, culture and beliefs amongst the populace differed across Asian countries, notably Indonesia, with its diverse population, which often contrasts with the more sophisticated Java, and to add, scarce data obtained from Kalimantan may provide further studies regarding cancer in Indonesia [15].
This study aims to evaluate how different factors, including sociodemographic characteristics and disease progression, are associated with changes in the QoL of women with LABC and analyse the association between QoL and these parameters.
Materials and Methods
Study design and population characteristics
This is an analytical cross-sectional study involving women aged 30 years and older admitted and diagnosed with LABC across all stages (IIIA, IIIB and IIIC). The study was performed at the Regional Public Hospital Ulin in Banjarmasin, Indonesia, a tertiary hospital that provided services for two provinces at once. Despite this, there is still no multidisciplinary team approach from the beginning of patient care towards cancer case management; cases are often handled only by a single department. Our center also has both rehabilitation and psychological services available; however, it should be noted that these services are not integrated regularly into patient management. As of our research, no specific palliative care unit was available exclusively for both early and end-stage breast cancer patients.
The calculated sample size for this study was 90, obtained from the Lemeshow formula. A post-hoc power analysis was conducted to statistically verify the use of a sample size of 100 participants. This result was validated under the conditions of having an alpha level of 0.05, assuming adherence to statistically significant thresholds of 0.80 and identification of a medium-sized effect (f = 0.25). This analysis confirmed that the achieved power was adequate to identify large, medium-sized effects in group comparisons of quality-of-life parameters using the Quality-of-Life Questionnaire Breast Cancer 23 (QLQ-BR23) instrument.
By examining the sample population in the regional health survey data, it was found that Southeast Asia experiences significant late-stage presentation burdens, as evidenced in the 2020 study conducted by the ASEAN Cost in Oncology project, which found that socioeconomic disparities, low screening uptake and late health-seeking behaviours were among the most significant contributors to the advanced stage presentation in Southeast Asia [16].
Our findings provide evidence that our sample population is a true representation of the wider regional population, both in age and socioeconomic status and educational disparities.
The study population consisted of women with breast cancer admitted to the surgical oncology service between August and October 2023, totaling 100 patients. It was suggested that younger women report significantly different perspectives on body image and distinct psychological conditions; hence, to avoid bias, we excluded women younger than 30 years old [17]. The inclusion criteria, therefore, were women aged 30 years or older, diagnosed with locally advanced breast cancer, i.e., in clinical stages III (A, B and C)–while patients with distant metastases were excluded. Our study population primarily consisted of patients from low-income households, both urban and rural, as our center serves as the only end-referral hospital in the region.
Anamnesis, physical examination, imaging studies and histopathological examinations were done to diagnose them as eligible for the study.
Patients were recruited during their first appointment at the surgical oncology service. Those who agreed to participate in the study signed an informed consent form and were interviewed using the QLQ-BR23 questionnaire by designated researchers. The QLQ-C30 was excluded due to the high number of English terms in the CLC-Q30, which were difficult for the Indonesian population, particularly those with limited education, to understand. These terms often did not align with the Bahasa Indonesia translation or local language equivalents. As a result, fatigue and global health status were not assessed in this study. We achieved an 80% response rate, with refusals primarily due to participants’ difficulty understanding the questions or lack of time for the interview.
The administered QLQ-BR23, used as a QoL response parameter to the disease, is a supplementary questionnaire for breast cancer patients using the Bahasa Indonesia version. This questionnaire has 23 questions, divided into two dimensions: a functional scale (body image, sexual functioning, sexual enjoyment and future perspective) and a symptom scale (systemic therapy side effects, breast symptoms, arm symptoms and upset by hair loss). The Bahasa Indonesia version of the questionnaire has been proven to be valid and reliable for usage [18].
Clinical data, including time from initial clinical presentation until the last period of treatment, is also collected along with the history of medication that the patient has taken: hormonal therapy, chemotherapy, surgical procedures and other unconventional medicines not prescribed by the clinician, limited to the usage of traditional and herbal medicines. Our data regarding these were collected during our primary verbal interview and several data were confirmed with medical records. Sociodemographic data was also collected during the primary verbal interview obtained from patients and their families to provide clarity on several key items such as monthly incomes, decline in savings and livelihood difficulties. For this, we provided separate specified questionnaires in addition to the BR-23 questionnaire, with question items such as: (1) What is your average family monthly income?
(2) Does breast cancer impact your savings?
(3) Has your capability to purchase daily goods been impaired since contracting cancer?
Data analysis
The data were coded and analysed with the Statistical Package for the Social Sciences (SPSS) using IBM SPSS Version 26. EORTC BR-23 was scored according to the rules established in the EORTC manual.
The analysis of the results targeted points by which QoL is/are affected. This evaluation is then compared between three stages of LABC (IIIA, IIIB and IIIC); hypothesis tests were proposed for more than two populations, in which values of p < 0.05 (established significance level) were considered as significant results.
The Kolmogorov-Smirnov test was performed to verify the assumption of data normality, in addition to graphical analysis using histograms to analyse questionnaire responses.
The scores obtained from both functional scale and symptom scale parameters were the dependent variables in the study, while the basic cancer staging and several specified parameter groups, ranging from age, menopausal status, marital status, therapeutic surgery and sociodemographic status, were the independent variables. The ANOVA and independent t-test parametric tests were used for the categorical variables and the Kruskal-Wallis and Mann-Whitney tests were used for non-parametric tests.
Additional analytical testing was done to measure each predictor parameter's significant correlation and to calculate the coefficient of determination using a linear regression test. The dependent variables were the symptom scale and functional scale. While cancer staging, age, menopausal status, marital status, educational status, occupational status, economic status and in clinical parameter group, were chosen as independent variables and were labeled into each of their own group variables and considered as the models’ predictors. The value of R squared was calculated, and p ≤ 0.05 was taken as significant where the comparison was conducted.
Ethical considerations
An exploratory interview was done, including but not limited to informed consents, which included that the patient’s data from the interview may be utilised for research purposes. The data obtained from interviews using the QLQ-BR23 questionnaire has been fully anonymised for each patient. Patients were recruited at their first appointment at the Oncology Surgery service. Those who agreed to participate in the study were asked to sign the informed consent form and were interviewed by appointed researchers.
The joint ethics committee from the Faculty of Medicine of Universitas Lambung Mangkurat/Ulin Regional Public Hospital has acknowledged and agreed on this use of primary data.
Results
Sociodemographic and epidemiological characteristics
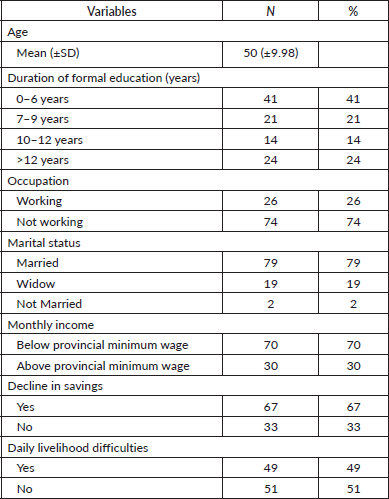
In total, 100 women were interviewed for their responses. The mean age of participants was 50 years (SD ± 9.98 years).
The participants exhibited diverse educational backgrounds, with categorisation aligned with the Indonesian formal education system. A significant proportion of the female participants (41%) attained the lowest strata of education, completing 0–6 years of schooling (Table 1).
Analysis of sociodemographic elements, including occupational status, revealed a high unemployment rate (74%). The financial element, using 3 parameters, showed that 70% reported income below minimum wage, two-thirds (67% versus 33%) with reduced savings and near-equal reports on daily livelihood difficulties (49% versus 51%). These indicate that most participants experience financial hardship (Table 1).
Table 1. Sociodemographic characteristics of LABC patients (N = 100).
Clinical data
Clinical data revealed that nearly all the patients had stage IIIB disease (89%) and approximately half were diagnosed between 1 and 5 years. Regarding treatment modalities, chemotherapy was administered for most subjects. Modified-radical mastectomy was performed on 44% of the patients. Notably, 21% of the patients had resorted to non-clinician-recommended treatments during their disease course (Table 2).
Assessment of quality of life using QLQ-BR-23 questionnaires
Overall, QLQ-BR-23 variables are depicted in Table 3. Most participants consistently reported higher scores for body image, as evidenced by a high mean score and a low standard deviation. A high standard deviation suggests that both sexual enjoyment and functioning afflicting these women differ greatly from one another. Future perspective declined in the future perspective group with advancing clinical stage (Figure 1).
We also classified our patients into two groups: Scores of ≤ 33 in functional scales indicated problematic function, while scores ≥ 66 suggested better QoL; this scoring was reversed in symptom scale assessment based on grouping method done by Imran et al [19] on their earlier study on breast cancer patient population as to better identify patients with more QoL issues with score-based approach. Based on that, patients exhibited better QoL during the treatment. [18] However, sexual-related QoL was lower, with a high standard deviation indicating significant inter-patient variability.
Comparative analysis of QLQ-BR-23 quality of life scores between parameters
QLQ-BR-23 QoL scores were compared across demographic and clinical groups. Functional scale depicted similar trends, showing higher body image and future perspective in stage IIIA compared to other stages, though declines with advancing stages were not statistically significant. Likewise, arm and breast symptoms increased with advancing clinical stage, though not significantly.
Table 2. Clinical characteristics of study participants (N =100).
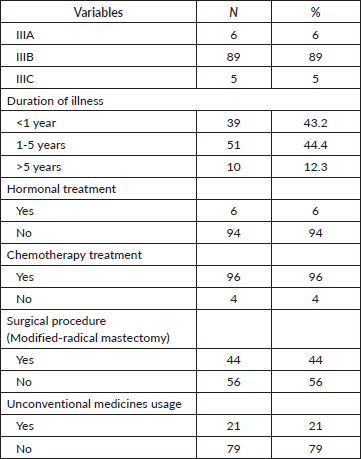
Table 3. Assessment of quality of life in locally advanced breast cancer patient by using QLQ-BR-23 questionnaire (N = 100).
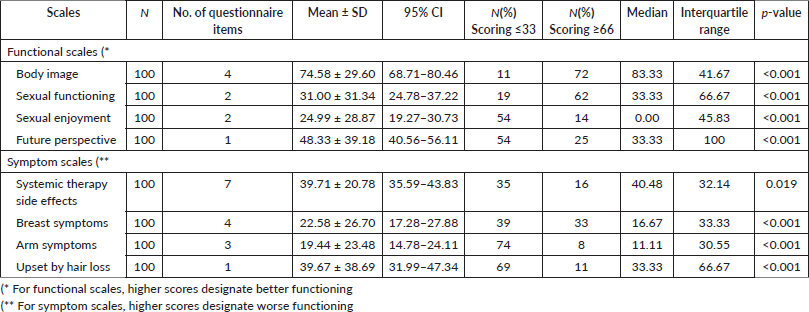
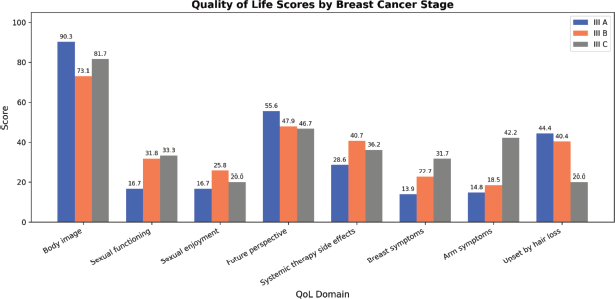
Figure 1. Results of both functional scales and symptom scales between clinical stages of locally advanced breast cancer.
Age did not considerably affect QoL scoring; however, the ≥50 years subgroup experienced lower functional scores and higher symptom scores. Illness duration correlated with decreased symptom severity, relative to those without symptoms, over time, except for arm symptoms, which worsened significantly after 5 years (p = 0.026). Patients treated with surgical interventions demonstrated better QoL: higher functional scores, particularly for body image (p = 0.010) and reduced incidence of systemic therapy side effects (p = 0.023) and breast symptoms (p = 0.035).
Demographic, socioeconomic and net health status factors significantly influenced sexual enjoyment. Relationship status has a significant effect, with married participants reporting higher sexual satisfaction than unmarried ones (p = 0.000). Menopausal status correlated with increased sexual enjoyment (p = 0.043), suggesting hormonal or life stage influences. Sexual enjoyment increased with education duration (highest scores = highest education; p = 0.036) [27] with a slight decline at the highest education level. Higher income (above minimum wage) was associated with greater sexual enjoyment (p = 0.008). Conversely, employment status negatively impacted sexual enjoyment (p = 0.043) (Table 4).
Table 4. Comparison of quality-of-life scores by using QLQ-BR-23 questionnaire between variable groups (N = 100).
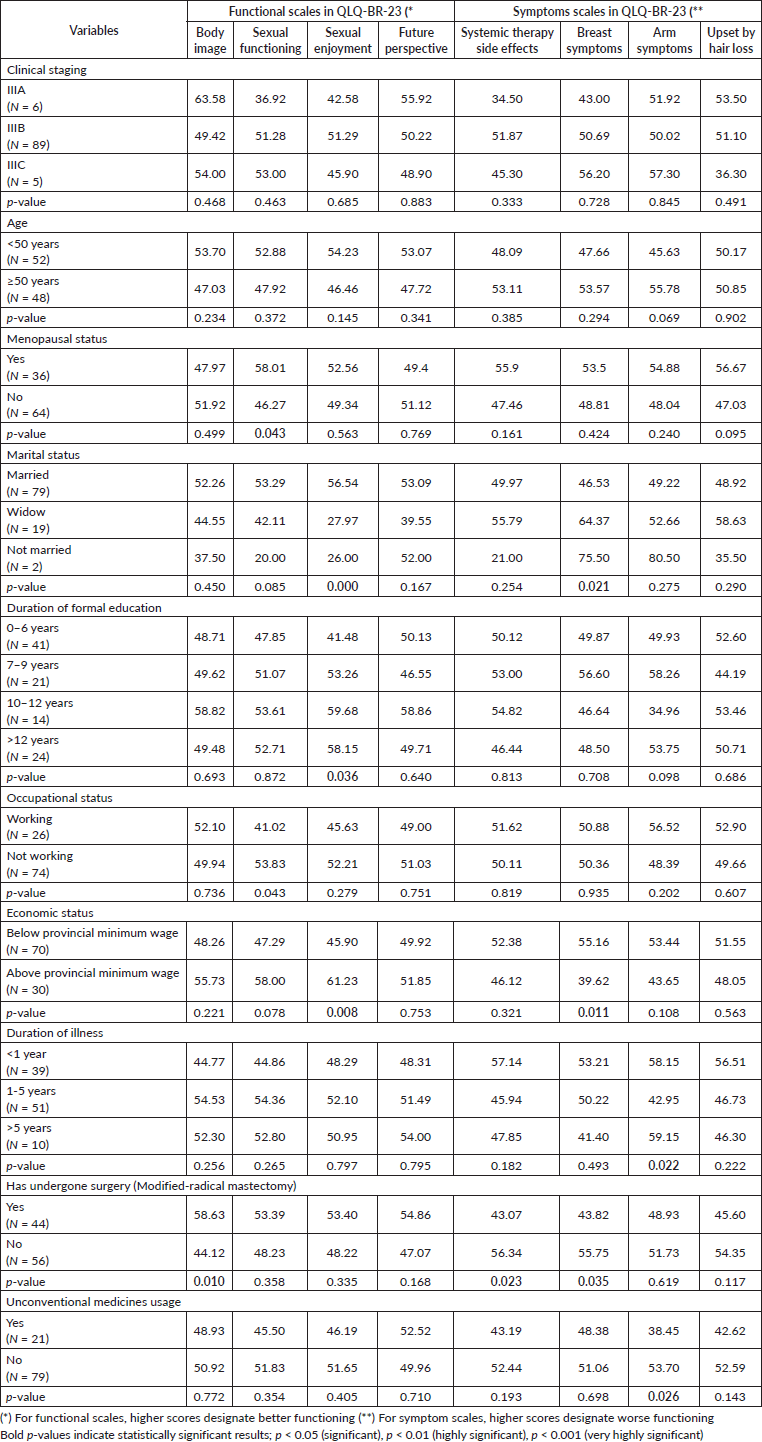
Table 5. Effect sizes analysis of key statistical findings of table 6 depicting linear regression model with parameter for QLQ-BR-23 score schemes between variable groups (N = 100).
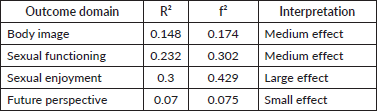
Multivariate analysis showed that occupational and economic status explained 10.5% of the variance in sexual functioning (adjusted R-squared = 0.105). Marital status was the most significant indicator of sexual enjoyment, explaining 18.5% of the variation (p = 0.004) (Tables 5 and 6).
Use of alternative therapies (traditional or herbal medicines) showed no correlation with functional scale scores but was associated with lower arm symptoms (p = 0.026) and a better future perspective.
Quality of life is determined by a wide variety of factors, such as surgical intervention and sociodemographic factors. These findings will aid in the development of targeted interventions aimed at enhancing individual quality of life, sexual well-being and symptom management.
Table 6. Linear regression model with parameter for QLQ-BR-23 score schemes between variable groups (N = 100).
Discussion
Our study found that body image was the least disturbing on the functional scale, while sexual functioning and enjoyment had the lowest scores and highest variability. We identified several HR-QoL aspects that were associated with sexual aspects of QoL in our patients. Early menopause and being not married were linked to both reduced sexual functioning and enjoyment, in addition to worse breast symptoms, respectively. Higher education level and economic status were associated with better sexual enjoyment and breast symptoms, while higher symptoms were associated with unmarried patients. Modified radical mastectomy (MRM) patients were also associated with higher body image, in addition to lower breast symptoms and chemotherapy side effect score. Higher arm symptoms were associated with longer illness duration, and unconventional medicine usage was associated with lower arm symptom scores.
Our findings on improved body image and varied sexual aspect scores align with a Saudi Arabian study that also found higher scores in body image [19]. Similarly, a Chinese study showed high sexual functioning and enjoyment scores with a high standard of deviation, suggesting hesitation in discussing sexual topics [20]. Bobrie et al [21] emphasised that sexual issues are often overlooked in breast cancer care, exacerbated by inadequate communications. In Indonesia, hesitance by professionals was also found in addressing sexual issues [22]. Inadequate professional awareness can exacerbate proof sexual QoL, potentially leading to a cluster of psychological issue, including sexual dysfunction and depression [23]. Other studies also found that psychological issues persist in long-term breast cancer survivors, even with good QoL, underscoring the importance of addressing emotional needs in palliative care settings. Thus, interventions aiming to address psychological issues and sexual problems that are focused on the active participation and counselling by psychologist, sexual health counsellors and psychiatrists, may be beneficial towards improving QoL in breast cancer patients [24, 25]. In this aspect, there was still no integrated multidisciplinary approach towards psychological supports in Indonesia as of during the time of our study.
Several HR-QoL aspects correlated with sexual aspects of QoL in our patients. Our findings on menopausal status align with Park and Yoon [26], who found that early menopause triggered by acute ovarian failure due to chemotherapy adversely affected sexual function and increased depression symptoms. Marschner et al [27] also reported that premenopausal women undergoing chemotherapy experienced more severe symptoms, including body image and emotional and social functioning. This again underscores the importance of physician and psychologist follow-up on post-chemotherapy sexual functioning in tackling other QoL-related problems. An Indonesian study found no significant association between menopausal status and sexual parameters but concurred that sexual issues remain inadequately addressed in Indonesia; however, the nature of cross-sectional model of our study should also be considered as to interpret this result despite various parameters associated [28]. A Malaysian study also suggested that many challenges to sexual wellbeing remained as an issue for cancer survivors living in settings with limited supportive care, citing that sexual issues are often overlooked in the planning of supportive care in LMIC settings [29]. These findings highlighted the need for professional counseling and the adoption of couple-focused sex therapy interventions, as they have been shown to be beneficial in fostering communication between couples and improving sexual relationships in both cancer patients and their partners [30, 31]. A couple-based approach is important in LMIC countries, mainly Asian countries, as marital relationships are often considered the primary framework for sexual activity and emotional intimacy in traditional societies. Chang et al [32] also suggested that emotional support from spouses enhanced QoL for breast cancer patients in China. However, other studies also indicated the need for more research to explore socio-economic issues like spousal dynamics, family economy and overall well-being related to a couple's stability [9, 33].
Our patients with higher education were associated with better sexual QoL, consistent with Telli [34], who associated this with better perception and an open perspective toward sexual practice. Some Indonesian studies done in central administration provinces did not find a significant impact of education on sexual enjoyment, possibly due to differences in patients’ demographics and regional educational distribution. These findings highlighted that breast cancer patients’ characteristics can also have further variations based on demographic status in each of a country’s regions, especially in LMICs, where both economic and social disparities are commonly found in contrast between central and peripheral regions, requiring a tailored approach to addressing multi-factorial QoL problems in local breast cancer patients [30, 35].
Our findings regarding chemotherapy aligned with studies done by Nguyen et al [36] and Cardoso et al [37], which noted that chemotherapy patients reported significant side effects, often worsened with disease progression. A Nigerian study linked worsening systemic therapy side effects to non-compliance with chemotherapy, often due to chemotherapy-induced anemia, neutropenia, lymphopenia and thrombocytopenia. [38] Prophylactic measures thus may be recommended to be integrated, but issues such as administration practices and high costs further complicate these treatment options in low-to-middle-income settings.
A review of studies involving breast cancer patients in low-to-middle-income Asian settings found that lower income significantly correlated with poorer overall QoL, consistent with our findings [39]. This contradicts Yusoff et al [40], who reported higher discomfort in higher socioeconomic groups. However, their study lacked specification of patient clinical stages, which may affect symptom presentation. A longitudinal study further addressed that low socioeconomic status significantly impaired follow-up care, HR-QoL and increased psychological distress in Southeast Asia populations [16]. Additionally, findings by Clegg-Lamptey et al [41] and Bichoo et al [42], further emphasised that fungating breast cancer, which often arises in developing countries, requires more complex treatment, raising costs and highlighted the need of coordinated multidisciplinary effort in addition to government initiative to alleviate economic burden and integration of social and occupational support groups tailored to LMICs breast cancer care.
MRM was associated with improved QoL in our study, potentially due to lower patient expectations in advanced disease stages and inherent expectations regarding MRM results compared to other types of surgeries [43, 44]. While MRM with neoadjuvant chemotherapy (NAC) improved certain QoL aspects, NAC alone was associated with diminished QoL due to increased toxicity, as observed in our patients [45, 46]. Thus, regular QoL assessments during chemotherapy are essential for monitoring and managing side effects, but it should also be noted that being a cross-sectional study, we cannot ascertain on the certainty that better QoL is always associated with combined therapy.
Arm symptoms showed mixed results: they decreased after 5 years of illness, contrasting with Norman et al [47], who reported worsening symptoms with prolonged duration. This discrepancy may stem from our patients’ arm symptoms being linked to reduced movement and subsequent inflexibility, which tends to improve with time [48]. Thus, additional tools may enhance arm symptom assessment in breast cancer studies. Traditional medicine use correlated with reduced arm symptoms in our study. Some studies suggested that this could be a perceived effect rather than an actual therapeutic benefit, acting as a coping mechanism. Others emphasized potential therapeutic effects, but further research is needed to validate the effectiveness of traditional medicines for LABC patients [49, 50]. Our patients utilised honey and Phaleria macrocarpa mixtures with several other unidentified substances for wound dressing for breast and flaky skin on their breast and arm. A randomised study done by Lund-Nielsen et al [51, 52] found honey-coated bandages effective for wound control; however, while these dressings reduced wound size, antimicrobial properties were not directly linked to improvement. The usage of P. macrocarpa was not directed by our physician; an in-silico study by Christina et al [53] suggested that bioactive compounds from P. macrocarpa leaf extract might potentially modulate signaling pathways related to cancer apoptosis and cell growth; however, they also noted that further research still should be performed to validate its efficacy for target cancer development. These findings implied that while usage of traditional medicines is often discouraged, mixed results implied that further research is needed to offer clarity, since evidence remains limited and caution is advised pending further research on its usage, since we cannot ascertain the causality of this association in our cross-sectional study.
Linear regression showed that marital status, occupation and economic status significantly predicted sexual functioning, with marital status being a strong predictor of sexual enjoyment. Our regression model findings align with Getu et al [54], where active marriage status predicted better sexual experience. Additionally, predictors for sexual functioning, such as menopausal status and sociodemographic factors, align with Smedsland et al [55] and another study from Saudi Arabia, where lower QoL was linked to worse sociodemographic conditions, where economic factors and occupational status also impacted sexual functioning. Patients with higher incomes and job stability experienced better sexual QoL [56]. These findings highlight the importance of socioeconomic stability in coping with breast cancer and sexual management should not be overlooked in breast cancer patients’ management and must be integrated to better improve patients’ overall well-being.
Strength and Limitation
This is the first study in Indonesia to analyse multiple sociodemographic and disease parameters in relation to QoL, focusing on LABC in an LMIC context. However, limitations include the cross-sectional design and lack of a control group, which may affect the analysis. Furthermore, the usage of multiple comparisons is still a concern for further analysis adjustments. The exclusion of additional questionnaire items in our study is due to English terms that do not conform to terms contained in the Bahasa Indonesia further translation to local languages; thus, additional authorised questionnaire remained as a challenge, and thus, a questionnaire tailored to local languages can be considered in future research. Additionally, Indonesia's diverse sociodemographic makeup means these results may not be fully generalisable to the entire country.
Conclusion
Our study found that our patients reported high body image scores, but diminished sexual functioning and enjoyment, per QLQ-BR-23. Early menopause and single status were associated with lower sexual QoL, while higher education and economic status improved sexual outcomes. Chemotherapy side effects were the most debilitating. MRM surgery was associated with higher body image, lower systemic therapy side effects and breast symptoms. Longer illness duration worsened arm symptoms, though unconventional treatments correlated with lower arm symptom scores.
However, the nature of the cross-sectional design of our study must always be considered when interpreting the results since causality cannot be established. These issues warranted the need for holistic, multidisciplinary care aimed at both medical and psychological support, especially on sexual issues for breast cancer patients, emphasising the need of a multidisciplinary approach that integrated oncological service, psychological services and supportive cares in addition to integrated economic support from both government and occupation-related social groups towards improving breast cancer patients’ QoL. Further studies can be performed to evaluate specified LABC care and usage of unconventional treatments in the future.
List of abbreviations
HR-QoL, Health-related quality of life; LABC, locally advanced breast cancer; QLQ-BR23, The Quality-of-Life Questionnaire Breast Cancer 23; WHO, World Health Organisation; LMIC, Low-and middle-income countries; SPSS, Statistical Package for the Social Sciences; EORTC, European Organisation for Research and Treatment of Cancer; ANOVA, Analysis of Variance; MRM, Modified radical mastectomy; US, United States; NAC, Neoadjuvant chemotherapy.
Acknowledgment and Funding
This research received neither specific grant nor funding from interest groups, public, commercial or profit agencies. The author would like to thank all participants who participated in this study.
Ethics approval and consent to participate
The study obtained approval from the institutional ethics of the joint ethics committee from the Faculty of Medicine of Universitas Lambung Mangkurat/Ulin Regional Public Hospital with registry number 048/KEPK-FKIK ULM/EC/IV/2024. Written consent with the addition of a written signature from each of the participants had been taken prior to the interviews.
All methods were carried out in accordance with relevant guidelines and regulations. Both digital and written documents were collected and stored by the corresponding author and not shared to any third party outside our study group.
Conflict of interest
The authors declare no competing interests.
Author contributions
SHP: conceptualisation, methodology, critical reviewing, overall supervision; WB: methodology, critical reviewing, overall supervision, literature review, technical guidance; BT: methodology, critical reviewing, overall supervision; E: editing, data management, quality control of counselling, data quality assurance; MDIP: writing, data collection, data management.
References
1. World Health Organization (2023) Breast Cancer: Scope of the Problem Geneva: WHO [https://www.who.int/news-room/fact-sheets/detail/breast-cancer] Date accessed: 30/03/24
2. Bray F, Laversanne M, and Sung H, et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 74(3) 229–263 https://doi.org/10.3322/caac.21834 Date accessed: 30/03/24 PMID: 38572751
3. Ferlay J, Laversanne M, and Ervik M, et al (2020) Global Cancer Observatory: Cancer Tomorrow Lyon, France: International Agency for Research on Cancer [https://gco.iarc.fr/tomorrow]
4. Anwar SL, Raharjo CA, and Herviastuti R, et al (2019) Pathological profiles and clinical management challenges of breast cancer emerging in young women in Indonesia: a hospital-based study BMC Womens Health 19(1) 28 https://doi.org/10.1186/s12905-019-0724-3 PMID: 30728000 PMCID: 6364389
5. Devi BC, Tang TS, and Corbex M (2007) Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia Ann Oncol 18(7) 1172–1176 https://doi.org/10.1093/annonc/mdm105 PMID: 17434897
6. Ogundiran TO, Ayandipo OO, and Ademola AF, et al (2013) Mastectomy for management of breast cancer in Ibadan, Nigeria BMC Surg 13 59 https://doi.org/10.1186/1471-2482-13-59 PMID: 24354443 PMCID: 3878251
7. Ermiah E, Abdalla F, and Buhmeida A, et al (2012) Diagnosis delay in Libyan female breast cancer BMC Res Notes 5 452 https://doi.org/10.1186/1756-0500-5-452 PMID: 22909280 PMCID: 3542159
8. Jagsi R, King TA, and Burstein HJ (2015) Malignant tumors of the breast Cancer: Principles and Practice of Oncology 12th edn, eds VT Devita, S Hellman, and SA Rosenberg (Philadelphia: Wolters Kluwer Health)
9. De Aguiar SS, Bergmann A, and Mattos IE (2014) Quality of life as a predictor of overall survival after breast cancer treatment Qual Life Res 23 627–637 https://doi.org/10.1007/s11136-013-0476-8
10. Çömez A and Karayurt Ö (2016) We as spouses have experienced a real disaster! a qualitative study of women with breast cancer and their spouses Cancer Nurs 39(5) 19–28 https://doi.org/10.1097/NCC.0000000000000306
11. Anwar SL, Adistyawan G, and Wulaningsih W, et al (2018) Rehabilitation for cancer survivors: how we can reduce the healthcare service inequality in low- and middle-income countries Am J Phys Med Rehabil 97(10) 764–771 https://doi.org/10.1097/PHM.0000000000000982 PMID: 29905600
12. Deliana M, Suza DE, and Tarigan R (2019) Advanced stage cancer patients experience in seeking treatment in Medan, Indonesia Open Access Maced J Med Sci 7(13) 2194–2203 https://doi.org/10.3889/oamjms.2019.590 PMID: 31456851 PMCID: 6698112
13. Chen X, Wu C, and Bai D, et al (2022) Health-related quality of life in breast cancer patients in Asia: a meta-analysis and systematic review Front Oncol 12 1–15
14. Prabandari YS, Hartanti W, and Syafriani, et al (2022) “Alas … my sickness becomes my family’s burden”: a nested qualitative study on the experience of advanced breast cancer patients across the disease trajectory in Indonesia Breast 63 168–176 https://doi.org/10.1016/j.breast.2022.04.001 PMID: 35413611 PMCID: 9010781
15. Puspitaningtyas H, Espressivo A, and Hutajulu SH, et al (2021) Mapping and visualization of cancer research in Indonesia: a scientometric analysis Cancer Control 28 1–13 https://doi.org/10.1177/10732748211053464
16. The Action Study Group (2017) Health-related quality of life and psychological distress among cancer survivors in Southeast Asia: results from a longitudinal study in eight low- and middle-income countries BMC Med 15(1) 1–10
17. Maly RC, Liu Y, and Liang LJ, et al. (2015) Quality of life over 5 years after a breast cancer diagnosis among low-income women: effects of race/ethnicity and patient-physician communication Cancer 121(6) 916–926 https://doi.org/10.1002/cncr.29150
18. Adli M, Shatri H, and Sutandyo N, et al (2020) Validity and reliability test of european organization for research and treatment of cancer quality of life questionnaire breast 23 in breast cancer patient on treatment Indones J Intern Med 7(4) 229–234
19. Imran M, Al-Wassia R, and Alkhayyat SS, et al (2019) Assessment of quality of life in breast cancer patients by using EORTC QLQ-C30 and BR-23 questionnaires: a tertiary care center survey in the western region of Saudi Arabia PLoS One 14(7) 1–13 https://doi.org/10.1371/journal.pone.0219093
20. Chen Q, Li S, and Wang M, et al (2018) Health-related quality of life among women breast cancer patients in eastern China Biomed Res Int 2018 1–12
21. Bobrie A, Jarlier M, and Moussion A, et al (2022) Sexual quality of life assessment in young women with breast cancer during adjuvant endocrine therapy and patient-reported supportive measures Support Care Cancer 30(4) 3633–3641 PMID: 35028721 PMCID: 8857103
22. Rahmah H, Afiyanti Y, and Rachmawati IN, et al (2020) I do not feel confident and uncomfortable discussing patients’ sexuality concerns: a thematic analysis of Indonesian nurses’ experiences in discussing sexuality with patients Nurs J Indones 23(1) 23–30
23. Wang F, Chen F, and Huo X, et al (2013) A neglected issue on sexual well-being following breast cancer diagnosis and treatment among chinese women PLoS One 8(9) 4
24. Meisel JL, Domchek SM, and Vonderheide RH, et al (2012) Quality of life in long-term survivors of metastatic breast cancer Clin Breast Cancer 12(2) 119–126 https://doi.org/10.1016/j.clbc.2012.01.010 PMID: 22444718
25. Aboshaiqah A, Al-Saedi TS, and Abu-Al-Ruyhaylah MM, et al (2016) Quality of life and satisfaction with care among palliative cancer patients in Saudi Arabia Palliat Support Care 14(6) 621–627 https://doi.org/10.1017/S1478951516000432 PMID: 27323905
26. Park H and Yoon HG (2013) Menopausal symptoms, sexual function, depression, and quality of life in Korean patients with breast cancer receiving chemotherapy Support Care Cancer 21(9) 2499-2507 PMID: 23616110
27. Marschner N, Trarbach T, and Rauh J, et al (2019) Quality of life in pre- and postmenopausal patients with early breast cancer: a comprehensive analysis from the prospective MaLife project Breast Cancer Res Treat 175(3) 701–712 https://doi.org/10.1007/s10549-019-05197-w PMID: 30868393 PMCID: 6534521
28. Haris I, Hutajulu SH, and Astari YK, et al (2023) Sexual dysfunction following breast cancer chemotherapy: a cross-sectional study in Yogyakarta, Indonesia Cureus 15(4) 1–11
29. Kuan WC, Kong YC, and Bustamam RS, et al (2022) Sexual wellbeing and supportive care needs after cancer in a multiethnic Asian setting: a qualitative study Sex Disabil 41 17–30 https://doi.org/10.1007/s11195-022-09772-w
30. Ussher JM, Perz J, and Gilbert E, et al (2013) Talking about sex after cancer: a discourse analytic study of health care professional accounts of sexual communication with patients Psychol Health 28(12) 1370–1390 https://doi.org/10.1080/08870446.2013.811242 PMID: 23805956
31. Carroll AJ, Baron SR, and Carroll RA (2016) Couple-based treatment for sexual problems following breast cancer: a review and synthesis of the literature Support Care Cancer 24(8) 3651–3659 PMID: 27154014
32. Chang O, Choi EK, and Kim IR, et al (2014) Association between socioeconomic status and altered appearance distress, body image, and quality of life among breast cancer patients Asian Pacific J Cancer Prev 15(20) 8607–8612 https://doi.org/10.7314/APJCP.2014.15.20.8607
33. Gupta H, Brar GK, and Jalota V (2022) Quality of life and its sociodemographic determinants in breast cancer patients Ind Psychiatry J 31(2) 313–317 https://doi.org/10.4103/ipj.ipj_6_21 PMID: 36419693 PMCID: 9678156
34. Telli S (2020) Examination of sexual quality of life and dyadic adjustment among women with mastectomy Eur J Breast Health 16(1) 48–54 https://doi.org/10.5152/ejbh.2019.4969 PMCID: 6939718
35. Prajoko YW and Supit T (2021) Sexual satisfaction of Indonesian women with breast cancer in Central Java, Indonesia Bali Med J 10(1) 53–57 https://doi.org/10.15562/bmj.v10i1.2119
36. Nguyen J, Popovic M, and Chow E, et al (2015) EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review J Comp Eff Res 4(2) 157–166 https://doi.org/10.2217/cer.14.76
37. Cardoso F, Rihani J, and Harmer V, et al (2023) Quality of life and treatment-related side effects in patients with HR + / HER2 − advanced breast cancer: findings from a multicountry survey 28(10) 856–865 PMID: 37523663 PMCID: 10546820
38. Folorunso SA, Abiodun OO, and Abdus-Salam AA, et al (2023) Evaluation of side effects and compliance to chemotherapy in breast cancer patients at a Nigerian tertiary hospital Ecancermedicalscience 17 1–10 https://doi.org/10.3332/ecancer.2023.1537
39. Ngo NTN, Nguyen HT, and Nguyen PTL, et al (2023) Health-related quality of life in breast cancer patients in low-and-middle-income countries in Asia: a systematic review Front Glob Women’s Heal 4(6) 1–9
40. Yusoff J, Ismail A, and Rizal M, et al (2022) Quality of life of women with breast cancer in a tertiary referral university hospital Health Qual Life Outcomes 20(15) 1–12 https://doi.org/10.1186/s12955-022-01921-1
41. Clegg-Lamptey JA, Vanderpuye V, and Dedey F (2019) Late presentation of breast cancer in lower- and middle-income countries late presentation of breast cancer in lower- and middle-income countries Curr Breast Cancer Rep 11 1–7 https://doi.org/10.1007/s12609-019-00312-8
42. Bichoo RA, Yadav SK, and Mishra A (2020) Fungating breast cancer: experience in low- and middle-income country Indian J Surg Oncol 11(6) 281–286 https://doi.org/10.1007/s13193-020-01040-7 PMID: 32523276 PMCID: 7260334
43. Turk KE and Yilmaz M (2018) The effect on quality of life and body image of mastectomy among breast cancer survivors Eur J Breast Heal 14(4) 205–210 https://doi.org/10.5152/ejbh.2018.3875
44. Wu TY, Chang TW, and Chang SM, et al (2019) Dynamic changes of body image and quality of life in breast cancer patients Cancer Manag Res 11 10563–10571 https://doi.org/10.2147/CMAR.S223314
45. Xie X, Li H, and Wang C, et al (2022) Effect of modified radical mastectomy combined with neo-adjuvant chemotherapy on postoperative recurrence rate, negative emotion, and life quality of patients with breast cancer Am J Transl Res 14(1) 460–467 PMID: 35173865 PMCID: 8829650
46. Panobianco MS and Guimarães PRB (2017) Adjuvant and neoadjuvant chemotherapy and the implications in the quality-of-life women with breast cancer Nurs J Indones 11 4732–4740
47. Norman SA, Localio AR, and Potashnik SL, et al (2009) Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms J Clin Oncol 27(3) 390–397 https://doi.org/10.1200/JCO.2008.17.9291 PMCID: 2645852
48. Boquiren VM, Hack TF, and Thomas RL, et al (2016) A longitudinal analysis of chronic arm morbidity following breast cancer surgery Breast Cancer Res Treat 157(3) 413–425 https://doi.org/10.1007/s10549-016-3834-8 PMID: 27194415
49. Shabrina A and Iskandarsyah A (2018) Case study on illness perception and treatment belief in breast cancer patient who undergo a traditional treatment Adv Soc Sci Educ Humanit Res 139 103–115
50. Lee YW, Chen TL, and Shih YR, et al (2014) Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer: a population-based study Cancer 120 1338–1344 https://doi.org/10.1002/cncr.28579 PMID: 24496917
51. Lund-Nielsen B, Adamsen L, and Kolmos HJ, et al (2011) The effect of honey-coated bandages compared with silver-coated bandages on treatment of malignant wounds-a randomized study Wound Repair Regen 19(6) 664–670 https://doi.org/10.1111/j.1524-475X.2011.00735.x PMID: 22092836
52. Lund-Nielsen B, Adamsen L, and Rorth M, et al (2011) Qualitative bacteriology in malignant wounds: a prospective, randomized, clinical study to compare the effect of honey and silver dressings Ostomy Wound Manag 57(7) 28–36
53. Christina YI, Nafisah W, and Atho’illah MF, et al (2021) Anti-breast cancer potential activity of Phaleria macrocarpa (Scheff.) Boerl. leaf extract through in silico studies J Pharm Pharmacogn Res 9(6) 824–845 https://doi.org/10.56499/jppres21.1092_9.6.824
54. Getu MA, Chen C, and Wang P, et al (2022) Quality of life and its influencing factors among breast cancer patients at Tikur Anbessa specialised hospital, Addis Ababa, Ethiopia BMC Cancer 22(1) 1–12 https://doi.org/10.1186/s12885-022-09921-6
55. Smedsland SK, Vandraas KF, and Falk RS, et al (2023) Sexual health in long-term breast cancer survivors: a comparison with female population controls from the HUNT study Breast Cancer Res Treat 201(3) 479–488 https://doi.org/10.1007/s10549-023-07021-y PMID: 37490170 PMCID: 10460729
56. Smail L, Jassim G, and Khan S, et al (2023) Quality of life of Emirati women with breast cancer Int J Environ Res Public Health 20(1) 1–16






