Expert insights: 10 key questions on managing common infections in cancer care in India
Lingaraj Nayak1,2, Gaurav Salunke2,3, Trupti Gilada4, Sukhada Savarkar2,5, Bindiya Salunke2,5,Sanjay Biswas2,3, Vanita Noronha1,2, Atul Kulkarni2,5, Manju Sengar1,2, Akshay Baheti2,6, Pradnya Samant2,3, Anant Gokarn2,7, Anuradha Mehta1,2, Chetan Dhamne2,8 and Keerthna Batyala1,2
1Department of Medical Oncology, Tata Memorial Hospital, Mumbai, India
2Homi Bhabha National Institute(HBNI), Mumbai, India
3Department of Microbiology, Tata Memorial Hospital, Mumbai, India
4Department of Infectious Diseases, Unison Medicare and Research Centre, Mumbai, India
5Department of Anaesthesiology and Critical Care, Tata Memorial Hospital, Mumbai, India
6Department of Radiodiagnosis, Tata Memorial Hospital, Mumbai, Mumbai, India
7Bone Marrow Transplant Unit, ACTREC, Tata Memorial Hospital, Mumbai, India
8Department of Paediatric Oncology, Tata Memorial Hospital, Mumbai, India
Abstract
Cancer patients are at a heightened risk of infections due to immunosuppression from chemotherapy, radiotherapy and the malignancy itself, contributing to increased morbidity and mortality. Effective infection management in this vulnerable population requires a systematic and timely approach to diagnosis and treatment. This review addresses ten critical questions concerning the management of infections in cancer patients, synthesising insights from clinical guidelines, expert opinions and current evidence.
The review begins by discussing the optimal diagnostic workup for neutropenic patients, including investigations, risk stratification and treatment approaches for various neutropenia-specific syndromes. It further explores the principles of antibiotic escalation and de-escalation for gram-negative infections, emphasising the need for tailored therapeutic strategies. Advances in microbiological diagnostics, such as early detection methods and understanding resistance mechanisms in gram-negative organisms and Clostridioides difficile infections, are analysed in dedicated sections. The role of radiological investigations, which remain the cornerstone for diagnosing infections in immunocompromised patients, has been addressed. Catheter-related blood stream infection and the role of surveillance culture are explored in the final section. By addressing these critical questions, this review provides oncology clinicians with practical, evidence-based guidance for preventing, diagnosing and managing infections in cancer patients. The insights presented aim to enhance clinical outcomes and ensure patient safety in this high-risk population.
Keywords: cancer, infections, MDR, immunosuppression, sepsis
Correspondence to: Lingaraj Nayak
Email: lingarajnayak86@gmail.com
Published: 19/08/2025
Received: 13/01/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Patients with cancer are at an increased risk of developing infections due to a combination of factors, including immunosuppression from chemotherapy, radiation therapy and the underlying malignancy itself. Infections in this population can lead to significant morbidity and mortality, often complicating the course of cancer treatment. Understanding the types of infections based on newer methods of diagnosis is essential for timely diagnosis and management. This paper aims to address ten key questions related to the management of infections in patients with active malignancy, providing an overview based on current evidence and expert insights.
Method
Study design
This is a question-and-answer-based review paper synthesising information from clinical guidelines and expert opinions on infections in cancer patients.
Selection of questions
The development of this manuscript involved a systematic process to identify and curate 10 key questions addressing the management of common infections in cancer care. The following steps were undertaken to prepare the manuscript.
Identification of clinical gaps
Based on the literature review, gaps in clinical knowledge and areas requiring practical guidance were identified. Emphasis was placed on questions that reflect real-world dilemmas faced by oncology clinicians, such as infection prevention, diagnosis and treatment in immunocompromised patients.
Consultation with experts
An expert panel of oncologists, infectious disease specialists, microbiologist, intensivists and healthcare professionals actively involved in cancer care was convened. Each author was asked to provide a list of commonly encountered and challenging questions related to infections in their clinical practice. Based on priority and clinical significance, 10 important questions were selected for this consensus document.
Question No- I:- What is the optimal work-up in patients with neutropenia and suspected infection and how do you risk stratify?
Answer
History taking
I.1. a Include the type of chemotherapy, prior infections and their resistance patterns and comorbidities.
Clinical examination
I.2. a Focus on areas commonly affected in neutropenic patients: oral mucosa, perianal area, skin, catheter insertion sites, GI and lung focus.
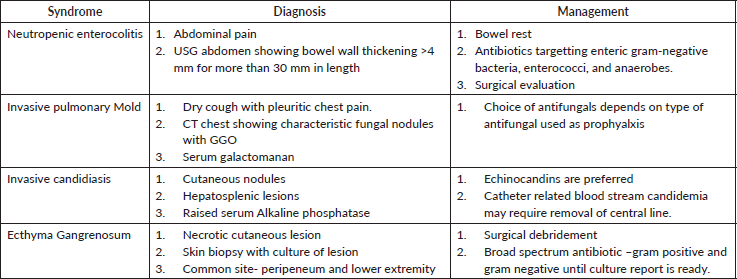
I.2. b Identification of neutropenic-specific syndrome(Table 1) is important in tailoring the investigation and management early.
Table 1. Neutropenia specific syndromes.
Investigations
I.3. a A minimum of one set of blood cultures should be sent, including samples from a peripheral line and existing indwelling intravenous lines.
I.3. b If a focus of infection is suspected, bacteriological cultures from the specific site will aid in tailoring antibiotic decisions after 24–48 hours.
I.3. c The roles of CRP and procalcitonin in neutropenic patients remain areas of ongoing research. Routine use of these biomarkers for prognostic and predictive purposes is not recommended.
I.3. d A non-contrast CT scan of the chest is strongly recommended as a baseline investigation in neutropenic patients with clinical findings or non-response to first-line antibiotics after 48 hours.
Risk stratification
Assessment of the risk for complications of severe infection should be undertaken at the presentation of fever. Risk stratification helps determine the type of empirical antibiotic therapy (oral versus intravenous), treatment venue (inpatient versus outpatient) and duration of antibiotic therapy. Key considerations include the anticipated duration and degree of neutropenia, the chemotherapy regimen used, type of cancer (hematolymphoid or solid tumour), disease remission status and significant medical comorbidities.
I.4. a High-risk patients
Patients with any of the following factors require initial hospitalisation for empirical antibiotic therapy:
• Host factors
a. Age > 60 years
b. Comorbidities such as uncontrolled diabetes, cardiac or renal conditions or obstructive lung disease
c. History of ICU admission or multidrug-resistant (MDR) sepsis
d. In paediatrics, down syndrome and other immune deficiency.
• Disease and Therapy
e. Acute leukemia patients on induction chemotherapy
f. Expected prolonged neutropenia (>7 days)
g. High-risk regimens (e.g., OGS-2012, EFT-2001, DCF regimen, high-dose cytarabine consolidation, Cladribine for hairy cell leukemia, BEP, TIP, VbIP, EMACO/EP regimen, COG protocol for Ewing's Sarcoma, MAP Protocol for OGS, VIP Protocol for GCT)
h. Profound neutropenia (absolute neutrophil count [ANC] < 100 cells/mm³)
• Comorbidity/Focus of infection
i. Hypotension (BP < 100/60 mmHg or poor pulse volume)
j. Hypoxia (SpO2 < 94%)
k. Specific foci of infection (e.g., pneumonia, catheter-related infections, chest tubes, nephrostomy tubes/DJ stents)
l. Features suggestive of enterocolitis
m. Depressed level of consciousness
n. Evidence of hepatic or renal insufficiency
I.4. b Low-risk patients
Patients without any high-risk features after careful selection may be candidates for outpatient empirical antibiotic therapy with daily follow-up.
Question No – II :- What is escalation and de-escalation approach for gram-negative infection?
Answer:-
II.1. Definitions
- Antibiotic escalation: Refers to the addition of another antibiotic or switching to a broader-spectrum antibiotic whenever there is clinical deterioration or laboratory evidence suggesting inadequacy of the initial coverage [3].
- Antibiotic de-escalation: Refers to deliberate narrowing or stopping of antibiotic therapy based on clinical improvement and laboratory evidence, if a non-infectious syndrome is suspected [2] including identification of the causative organism and its sensitivity.
II.2. Steps for good practice in antibiotic use
II.2.a. Initial empirical therapy
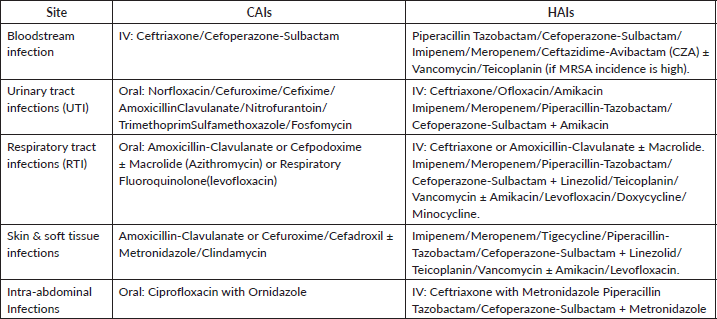
- Suspected infection/sepsis should prompt initiation of empirical broad-spectrum antibiotics based on the site of infection (e.g., blood, respiratory, intra-abdominal, soft tissue or urinary tract) and the origin of the sample (Outpatient Department (OPD) versus inpatient department (IPD)) and hospital antibiogram [1, 2].
- Table 2 gives the overlook about the emperical antibiotic therapy based on site of infection and origin of sample.
II.2.b. Sample collection
- Collect appropriate samples for culture and sensitivity before initiating antibiotics.
Table 2. Empirical antibiotic therapy based on site of infection [24, 25].
II.3. Rapid diagnostic tests
- Utilise rapid tests like gram stains to guide the initial choice of empirical therapy.
II.4 Regular reassessment:
- Reassess antibiotic therapy regularly to ensure appropriateness of dose, route, indication and patient improvement.
II.4 a Review after 48–72 hours
- Review clinical and microbiological data to guide further antibiotic management.
II.4. b Continuing empirical therapy
- Continue empirical treatment if the pathogen is sensitive to the drug, culture is negative and the patient responds clinically.
II.4. c Antibiotic escalation
- Consider escalation if:
i. Persistent or worsening infection leading to hypoxia, hypotension or organ dysfunction despite empirical therapy.
ii. New evidence suggests inadequately covered pathogens (clinical, microbiological or imaging evidence).
iii. Persistence of fever is not an indication for antibiotic escalation if patient is clinically stable.
II.4. d Antibiotic de-escalation:
- Consider de-escalation if:
iv. Patient shows clinical stability or improvement in infection markers.
v. Microbiological evidence identifies a pathogen susceptible to narrower-spectrum agents.
vi. A non-infectious cause of the clinical condition is diagnosed.
vii. Redundant gram-negative or anaerobic coverage is present.
viii. Patient stabilises clinically and can be switched from intravenous to oral antibiotics.
- Avoid prolonged therapy: The duration of antibiotics should be driven by the site of infection, blood culture and clinical status.
II.4. e. Antibiotic selection
- Community-acquired infections (CAIs)
- For patients from the community (not hospitalised) with minimal risk of multidrug-resistant pathogens, broad-spectrum antibiotics are typically unnecessary.
- Hospital-acquired infections (HAIs)
- For hospitalised patients, there is a higher probability of healthcare-associated or nosocomial infections caused by resistant or multidrug-resistant pathogens such as extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, MRSA, Pseudomonas and Acinetobacter. Consider broader-spectrum antibiotics such as:
- Carbapenems
- Beta-lactam/beta-lactamase inhibitors (BL + BLI)
- Vancomycin/Teicoplanin
- Polymyxins
- Ceftazidime-Avibactam (CZA) with Aztreonam
II.4. f. Empirical treatment recommendations
- For CAIs
- Initiate empirical treatment based on common pathogens. If no improvement is seen within 48 hours, escalate to antibiotics typically used for HAIs until microbiological evidence is available (Table 2).
- For HAIs
- If no improvement occurs with initial empirical treatment, consider the possibility of more resistant organisms such as Pseudomonas spp., Acinetobacter spp. or Vancomycin resistant Staphylococcus aureus/Vancomycin resistant Enterococci (VRSA/VRE) and adjust treatment accordingly.
Question No – III :- Methods of susceptibility testing for gram-negative infections and their limitation.
Answer :- For determining the most appropriate course of treatment for gram-negative infections, antimicrobial susceptibility testing is crucial. Several methods are used, each with special advantages and disadvantages (Table 3).
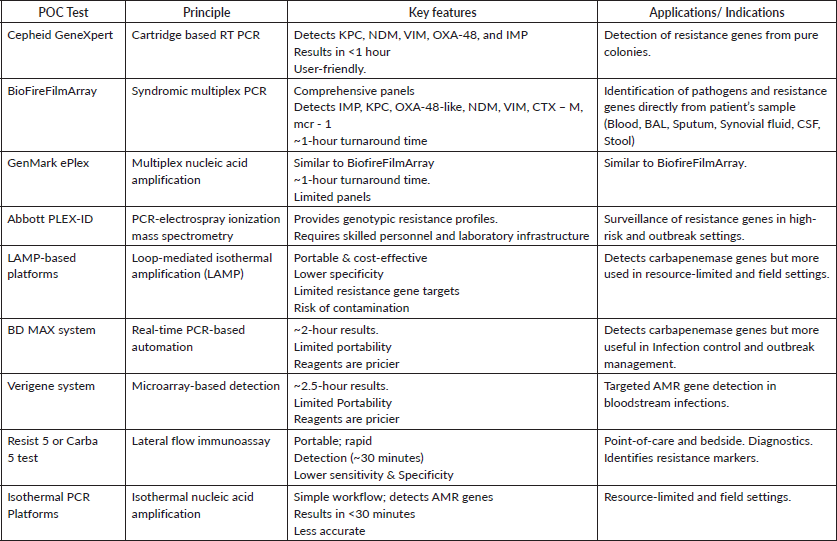
Question No – IV:- What are the indications and methods of doing ‘point of care’ resistant gene testing in gram-negative infections?
Answer:-Point-of-care (POC) resistance gene testing has shown considerable promise in the management of gram-negative infections, providing swift identification of antimicrobial resistance (AMR) genes. Research indicates its significance in enhancing therapeutic outcomes and refining antimicrobial stewardship. Table 4 depicts the various indication where point of care testing is indicated. Overview of POC tests for resistance gene detection has been briefed in Table 5.
Overview of POC tests for resistance gene detection has been briefed in Table 5.
Innovations in technology have made POC platforms like Cepheid GeneXpert and BioFireFilmArray reliable for rapid testing. According to Charnot-Katsikas et al [4], these systems have high sensitivity (90%–95%) and specificity (95%–99%), detecting important resistance genes in 1–2 hours as opposed to the 24–72 hours needed for culture-based techniques. This short turnaround time enables prompt tailored therapy and avoids unnecessary broad-spectrum antibiotic use by 25%–30% [5]. They are especially beneficial in ICUs, where infections with multidrug-resistant (MDR) organisms like Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter are common. Moreover, rapid detection of ESBL and carbapenemase genes facilitates managing antimicrobial resistance more effectively.
While advantages exist, challenges like high costs limit its accessibility in resource-limited environments. Additionally, POC testing focuses only on the detection of resistant genotypes and does not provide any assessment of phenotypic patterns, thus requiring additional confirmation for comprehensive data.
Table 3. Various methods for susceptibility testing from older to newer methods.
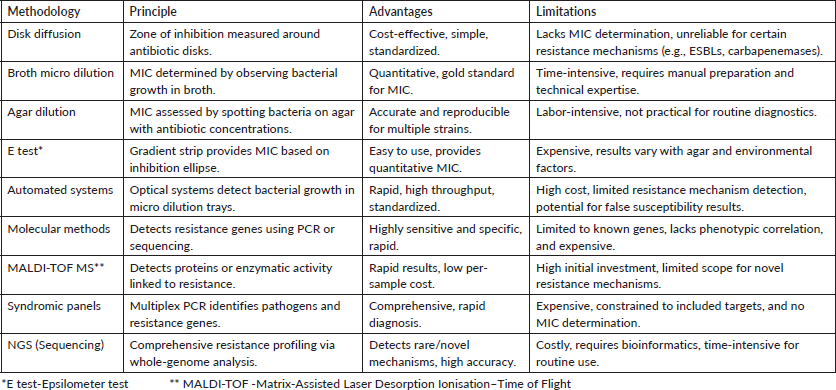
Table 4. Indications of point-of-care testing for infections.
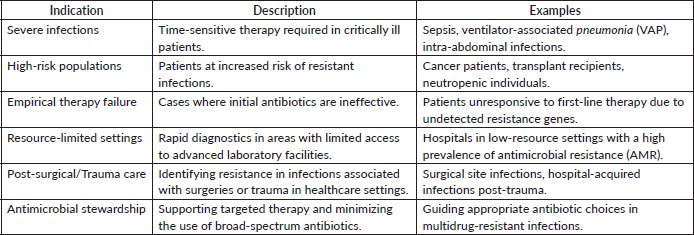
Table 5. Overview of POC tests for resistance gene detection, highlighting their technology, features and clinical applications.
In summary, evidence supports the use of POC resistance gene testing as a groundbreaking tool in managing gram-negative infections. Table 4 provides an overview of POC tests for resistance gene detection, highlighting their technology, features and clinical applications. Its incorporation into routine clinical workflows will significantly enhance infection management in high-risk and resource-limited settings, facilitating rapid, precise and economically viable outcomes.
Question No – V:- How will the decision of antibiotic choice be affected based on results of resistant gene testing?
Answer:- The information from antibiotic resistance gene testing is crucial for prompt initiation of the most effective targeted therapy and to avoid empiric use of broad-spectrum antibiotics. The choice of antibiotic is based on the site of infection, susceptibility data (if available) and host factors like organ dysfunction and so on. In resistant gram-negative infections, a combination therapy is recommended instead of monotherapy.
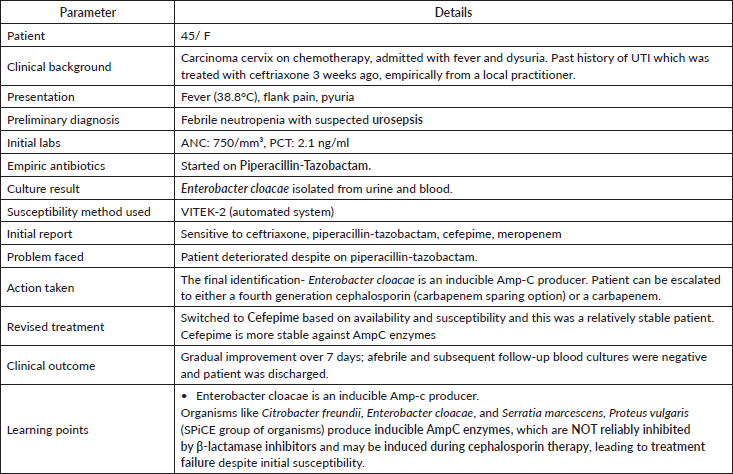
Case studies based on real world scenarios where point-of-care testing can help in the selection of appropriate antibiotics early leading to better outcome has been explained in appendix-A.
Table 6. Resistance genes and preferred antibiotics.
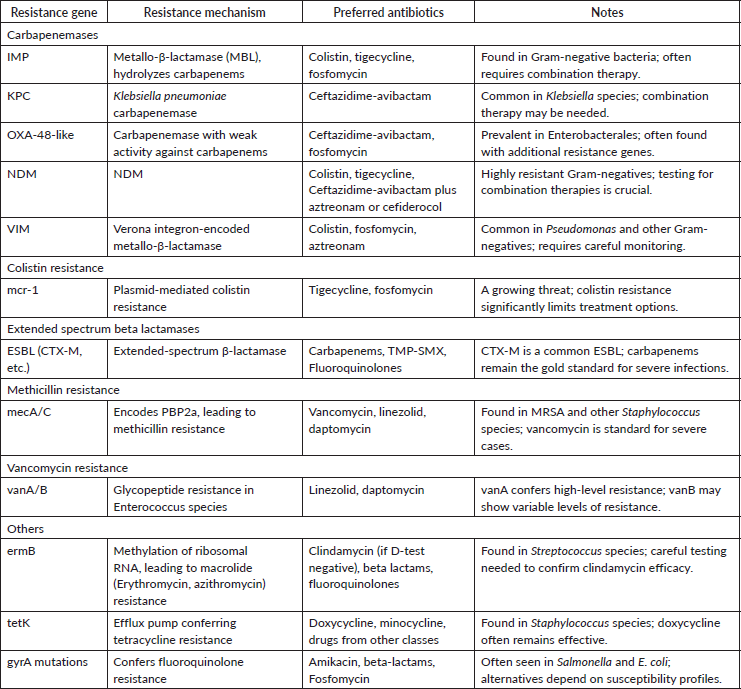
Advances in treatment strategies for multidrug-resistant pathogens: focus on New Delhi metallo-β lactamase (NDM) and carbapenemase-producing organisms
1. Ceftazidime-Avibactam and Aztreonam for NDM-Producing Organisms- The combination of ceftazidime-avibactam and aztreonam has shown efficacy against infections caused by NDM-producing Enterobacterales. This regimen capitalises on avibactam’s inhibition of serine β-lactamases, enabling aztreonam to remain effective against metallo-β-lactamases like NDM. Clinical studies have reported favorable outcomes with this combination, positioning it as a viable treatment option for such resistant infections [6].
2. Treatment options for carbapenemase-producing organisms
Colistin and tigecycline have traditionally been first-line agents for treating infections caused by carbapenemase-producing organisms. However, their efficacy remains uncertain, particularly when used in combination with other agents. More recently, several new agents have been approved for clinical use or are nearing late-stage clinical development. These include ceftazidime-avibactam +/- aztreonam, ceftolozane-tazobactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam, plazomicin, eravacycline and cefiderocol. Additionally, fosfomycin has been redeveloped in a new intravenous formulation. Data on the clinical efficacy of these newer agents specific to infections caused by carbapenem-resistant pathogens are emerging, with early evidence favoring these new treatments over previously available options [7, 8].
3. Colistin minimum inhibitory concentration (MIC) variability by contemporary methods
Determining the MIC of colistin remains challenging due to variability among testing methods, particularly with automated systems, which may fail to reliably detect colistin resistance. Broth microdilution, the recommended reference method for colistin susceptibility testing, ensures accurate and consistent results, but it is labor-intensive. The diverse mechanisms and genes involved in colistin resistance hinder the rapid detection of resistance through molecular biology techniques. Combining newer phenotypic and genotypic testing methods is essential for a comprehensive understanding of colistin resistance, especially for strains carrying mcr genes [9].
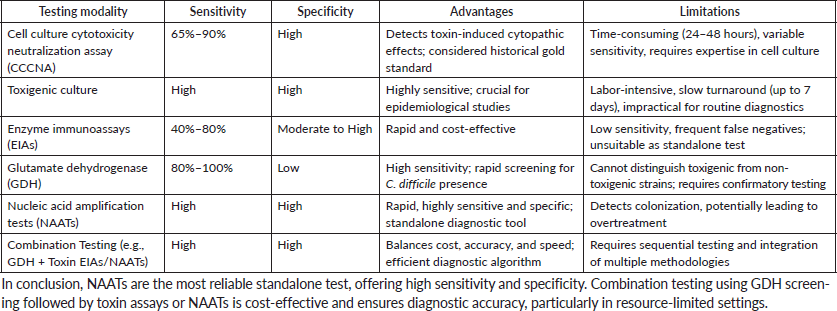
Question No –VI:-What is the best modality for Clostridioides difficile test? How do you compare between various methods?
Answer:- Peterson and Robicsek [10] conducted a prospective study in 2009 to examine the role of susceptibility testing in guiding clinical decision-making for C. difficile infections. Taking into consideration the balance between sensitivity, specificity and clinical outcomes, they recommended algorithms, combining both molecular and immunoassay methods to enhance diagnostic accuracy. Clinical guidelines [11] recommend an algorithm-based approach, by integrating molecular methods, such as NAATs, along with toxin detection assays for susceptibility testing. The objective was to improve isolation measures and infection control while minimising unnecessary antibiotic usage by separating colonisation from active infection.
The data collectively support the need for novel and integrated susceptibility testing methods to improve diagnosis accuracy and clinical management of C. difficile infections. Table 7 depicts Various methods for C difficile detection.
Table 7. Various methods for C difficile detection.
Question No- VII :-Indications and utility of radiological investigations in immunocompromised patients for selection of antibiotics/antifungals or antiviral?
Answer:- The American College of Radiology Appropriateness Criteria, the Guideline for Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients by the Society of Clinical Oncology for imaging and the COG Diagnostic Imaging Committee/SPR Oncology Committee White Paper label the following studies as appropriate for initial evaluation of patients with febrile neutropenia [12–14].
a. Chest radiograph (in patients with respiratory signs or symptoms – strong recommendation).
b. CT chest without or with IV contrast, for patients with prolonged (>96 hours) febrile neutropenia when there is a concern for invasive fungal disease (strong recommendation).
c. CT paranasal sinuses without or with IV contrast to not be performed without localising signs or symptoms (weak recommendation) as abnormal findings are commonly seen incidentally as well and cannot differentiate invasive from non-invasive fungal disease.
d. FDG PET/CT (moderate recommendation)
Specific signs like the halo sign (central nodular consolidation surrounded by ground glass opacity) and the air crescent sign (intracavitary nodular soft tissue with a crescentic collection of air that separates it from the wall of the cavity) help in the diagnosis of angioinvasive fungal infection [15]. Ground glass opacities are more common in atypical bacterial or viral infections or Pneumocystis jirovecii, while lobar consolidation and effusions are more common with bacterial infection [16]. Mixed infections can, however, often complicate the picture in clinical practice and correlation with clinical and laboratory findings is always necessary.
Question No- VIII :-What is catheter-related blood stream infection and how to treat CRBSI?
Answer:- CRBSI can be diagnosed if there is bloodstream infection (BSI) in a patient with an intravascular device who has more than one positive blood culture obtained from a peripheral vein, has clinical features suggestive of infection and no other apparent known source for the BSI.
VIII.1. Recommendations for diagnosis
For definitive diagnosis of CRBSI, paired blood samples, drawn from the catheter hub and a peripheral vein, should be cultured before initiation of antimicrobial therapy. If a blood sample cannot be taken from a peripheral vein, it is recommended that ≥2 blood samples should be drawn through different catheter lumens. If the catheter is already removed for suspected CRBSI; then the catheter tip cultures should be performed [17].
One of the following should be present for diagnosis of CRBSI:
VIII 1.1. Isolation of the same organism from a quantitative blood culture drawn through the catheter hub and from a peripheral vein with a ratio of 3:1colony-forming units (cfu)/ml of blood (catheter versus peripheral blood).
VIII 1.2. Differential time to positivity: positive blood culture obtained at least 2 hours earlier in the catheter culture than the peripheral culture.
VIII 1.3. If 2 quantitative blood cultures are drawn from 2 catheter lumens; the colony count for the blood sample drawn through one lumen should beat least 3-fold greater than the colony count for the sample obtained from the second lumen.
VIII 1.4. If catheter removed: Same organism should be isolated from the peripheral blood culture and catheter tip culture. Catheter tip culture should have a positive result either by semi quantitative (growth of >15 colony forming units (cfu) from a 5-cm segment of the catheter tip) or by quantitative (growth of > 102cfu by sonication method) method.
VIII 2. Recommendations for catheter management:
Once CRBSI is confirmed, the catheter should be removed if it was left in place and it should be reinserted at a new site, unless the organism is coagulase-negative staphylococcus or sensitive enterococcus and patient has shown improvement after starting antibiotics. In case of long-term catheters which are inserted in view of limited access; treatment can be attempted without catheter removal, with use of both systemic and antimicrobial lock therapy in case of uncomplicated CRBSI. If antibiotic lock therapy cannot be used, systemic antibiotics should be administered through the suspected lumen of the catheter. The catheter should be removed if blood cultures remain positive in spite of 72 hours of antimicrobial therapy.
VIII 3.0 -Recommendations for treatment of CRBSI
VIII 3.1. Empirical antibiotic therapy should be started promptly (preferably after obtaining blood cultures) when CRBSI is suspected. The local antibiogram should be considered while treating CRBSI. In general, coverage for both common gram-positive and gram-negative organisms is necessary [17].
VIII 3.2. Since staphylococci and enterococci are commonest organisms responsible for CRBSI; Vancomycin/Teicoplanin should be started as empirical therapy.
VIII 3.5. For carbapenem-resistant gram-negative organisms, treatment options include ceftazidime-avibactam plus aztreonam or polymyxins/colistin in combination with another agent demonstrating susceptible MIC [18, 19].
VIII 3.6. Empiric antifungal coverage should be given for patients with following risk factors: presence of femoral catheters, patients on total parenteral nutrition, prolonged use of broad-spectrum antibiotics or colonisation due to Candida species at multiple sites [20]. Echinocandins are favoured over azoles for empiric therapy as some candida species are resistant to azoles.
VIII 3.9. Usually, 10–14 days of antimicrobial therapy are required for treating uncomplicated CRBSI. For uncomplicated candidemia, antibiotic therapy should continue for 14 days after obtaining a negative blood culture.
VIII 3.10. 4–6 weeks of antibiotic/antifungal therapy should be administered to patients in whom fungaemia or bacteraemia persists even after 72 hours of catheter removal and to patients who are found to have infective endocarditis [21, 22, 17].
VIII 3.11. Teicoplanin has comparable efficacy to vancomycin, with the added advantage of lower nephrotoxicity [23]. Therefore, it can be a suitable alternative to vancomycin for first-line treatment for gram-positive infections. At our centre, teicoplanin is started as the first-line agent for suspected CRBSI.
Question No- IX. What are the choices and duration of antifungals in invasive pulmonary aspergillosis (IPA)?
Answer:- Guidelines recommend considering empiric antifungal therapy in neutropenic fever when it is prolonged beyond 4–5 days [26]. However, efforts should be made to diagnose invasive fungal infections definitely when patients are on azole prophylaxis. There is a recent trend of increasing non-aspergillousmolds in severely immunocompromised patients which can change the choice and duration of antifungals. The initial choice for IPA is voriconazole which is based on RCT comparing voriconazole against amphotericin B deoxycholate demonstrated improved 12-week survival in the voriconazole treatment arm (71% versus 58%) [27]. When patients are on azole prophylaxis, the choice is between echinocandin or amphotericin-B based on type of organism and sensitivity. In patients with severe established fungal pneumonia with profound neutropenia, combination of echinocandin and voriconazole may be considered despite limited retrospective literature.
The duration of antifungals in IPA is usually 6–12 weeks based on expert recommendation. However, the duration also depends on severity, resolution and ongoing immunosuppression or neutropenia. Serial imaging can be considered; however, transient radiological worsening can be seen during recovery of neutrophil counts.
Question No- X. What is the role of surveillance stool culture in guiding antibiotic decision?
Answer:-
The use of stool surveillance cultures to guide antibiotic selection has been proposed and practiced since late 1980s; however, robust literature confirming its utility is lacking [28]. However, robust literature confirming its utility is lacking. Data propounding its use is mainly retrospective in nature and limited in sample size. The largest data from India involving 313 allogeneic transplants in 299 patients showed a 56% incidence of MDR isolates in stool between 2014–2015 [29]. MDR isolation in stool was associated with higher incidence of 100 day mortality and higher incidence of MDR positivity in blood culture. However, a prospective study of 79 pediatric patients from PGIMER, India did not show an impact of MDR stool isolation on mortality or MDRO sepsis. Selection of antibiotics based on stool surveillance did not show any improvement in outcomes in this study [30]. Large retrospective data of 190 allogeneic transplant patients has shown that selecting first line antbitotics on basis of stool culture led to earlier defervescence of fever; however, there was no difference in infection-related mortality [31]. Similarly, a retrospective study of 317 transplant patients for benign diseases from 3 large pediatric bone marrow transplant centres from India did not any correlation between colonisation in rectal swab and clinical outcome. Antibiotic susceptibility testing didn’t correlate with in vivo clinical response [32].
Currently, there is limited evidence to recommend the use of stool surveillance culture to select first-line antibiotics. Hence, routine use of surveillance cultures or rectal swab for selection of antibiotics in immunocompromised patients is not recommended.
Acknowledgments
Meenakshi Ganeshan, samruddhi sakpal, EBM -2025 organising committee.
Conflicts of interest
No conflicts of interests.
Funding
No finances involved.
Authors' contributions
Concept-MS, AK, VN, LN
Selection of questions- LN, GS, TG
Answers to the questions-LN, PS, AK, SS, BS, SB, AM, KB
Proofreading- SS, LN, BS, CD.
References
1. Zimmer AJ and Freifeld AG (2019) Optimal management of neutropenic fever in patients with cancer J Oncol Pract 15(1) 19–24 https://doi.org/10.1200/JOP.18.00269 PMID: 30629902
2. Evans L, Rhodes A, and Alhazzani W, et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021 Intensive Care Med 47(11) 1181–1247 https://doi.org/10.1007/s00134-021-06506-y PMID: 34599691 PMCID: 8486643
3. Lakbar I, De Waele JJ, and Tabah A, et al (2020) Antimicrobial de-escalation in the ICU: from recommendations to level of evidence Adv Ther 37(7) 3083–3096 https://doi.org/10.1007/s12325-020-01390-2 PMID: 32462606 PMCID: 7252418
4. Charnot-Katsikas A., Tesic V., Love N., Hill B., Bethel C., Boonlayangoor S., et al. (2017). Use of the accelerate pheno system for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J. Clin. Microbiol. 56, e01166–17. 10.1128/JCM.01166-17
5. Pulcini C and et al 2023 Meta-analysis of POC testing in optimizing antibiotic use Lancet Infect Dis 23(1) 15–23
6. Tamma PD, Heil EL, and Justo JA, et al (2024) Infectious diseases society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections Clin Infect Dis ciae403 https://doi.org/10.1093/cid/ciae403
7. Sangiorgio G, Calvo M, and Stefani S (2024) Aztreonam and avibactam combination therapy for metallo-β-lactamase-producing gram-negative bacteria: a comprehensive review Clin Microbiol Infect 31 971–978. https://doi.org/10.1016/j.cmi.2024.11.006
8. Doi Y (2019) Treatment options for carbapenem-resistant gram-negative bacterial infections Clin Infect Dis 69 S565–S575 https://doi.org/10.1093/cid/ciz830 PMID: 31724043 PMCID: 6853760
9. Bardet L and Rolain JM (2019) Development of new tools to detect colistin-resistance among enterobacteriaceae strains Can J Infect Dis Med Microbiol 2018 3095249 PMID: 30631384 PMCID: 6305056
10. Peterson LR and Robicsek A (2009) Does my patient have clostridium difficile infection? Ann Intern Med 151(3) 176–179 https://doi.org/10.7326/0003-4819-151-3-200908040-00005 PMID: 19652187
11. NHS Clinical Guidelines (2013) Recommendations for the Diagnosis and Management of Clostridium difficile Infection (National Health Service)
12. Expert Panel on Pediatric Imaging, Westra SJ, and Karmazyn BK, et al (2016) ACR appropriateness criteria fever without source or unknown origin-child J Am Coll Radiol 13 922–930 https://doi.org/10.1016/j.jacr.2016.04.028 PMID: 27374781
13. Lehrnbecher T, Robinson PD, and Ammann RA, et al (2023) Guideline for the management of fever and neutropenia in pediatric patients with cancer and hematopoietic cell transplantation recipients: 2023 update J Clin Oncol 41 1774–1785 https://doi.org/10.1200/JCO.22.02224 PMID: 36689694 PMCID: 10022858
14. Chan SS, Coblentz A, and Bhatia A, et al (2023) Imaging of pediatric hematopoietic stem cell transplant recipients: a COG diagnostic imaging committee/SPR oncology committee white paper Pediatr Blood Cancer 70 Suppl 4 e30013 https://doi.org/10.1002/pbc.30013
15. Orlowski HLP, McWilliams S, and Mellnick VM, et al (2017) Imaging spectrum of invasive fungal and fungal-like infections Radiographics 37 1119–1134 https://doi.org/10.1148/rg.2017160110 PMID: 28622118
16. Dueck NP, Epstein S, and Franquet T, et al (2021) Atypical pneumonia: definition, causes, and imaging features Radiographics 41 720–741 https://doi.org/10.1148/rg.2021200131 PMID: 33835878
17. Mermel LA, Allon M, and Bouza E, et al (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America Clin Infect Dis 49(1) 1–45 Erratum in (2010) Clin Infect Dis 50(7) 1079 Erratum in (2010) Clin Infect Dis 50(3) 457 https://doi.org/10.1086/599376
18. Centers for Disease Control and Prevention (CDC) (2011) Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009 MMWR Morb Mortal Wkly Rep 60(8) 243–248 PMID: 21368740
19. Pandit P, Sahni AK, and Grover N, et al (2021) Catheter-related blood stream infections: prevalence, risk factors and antimicrobial resistance pattern Med J Armed Forces India 77(1) 38–45 https://doi.org/10.1016/j.mjafi.2019.07.002 PMID: 33487864 PMCID: 7809495
20. Patil HV, Patil VC, and Ramteerthkar MN, et al (2011) Central venous catheter related bloodstream infections in the intensive care unit Indian J Crit Care Med 15 213–223 https://doi.org/10.4103/0972-5229.92074
21. Grady NPO, Alexander M, and Burns LA (2011) Guidelines for the prevention of intravascular catheter-related infections Clin Infect Dis 52 e162–e193 https://doi.org/10.1093/cid/cir257
22. Parameswaran R, Sherchan JB, and Varma DM, et al (2011) Intravascular catheter-related infections in an Indian tertiary care hospital J Infect Dev Ctries 5 452–458 https://doi.org/10.3855/jidc.1261 PMID: 21727644
23. Bugano DD, Cavalcanti AB, and Goncalves AR, et al (2011) Cochrane meta-analysis: teicoplanin versus vancomycin for proven or suspected infection Einstein (Sao Paulo) 9(3) 265–282 https://doi.org/10.1590/s1679-45082011ao2020 PMID: 26761092
24. ICMR Guidelines for Treatment Guidelines for Antimicrobial Use in Common Syndromes [https://www.icmr.gov.in/guidelines]
25. Guidance on Diagnosis and Management of Carbapenem Resistant Gram-Negative Infections [https://www.icmr.gov.in/guidelines]
26. Patterson TF, Thompson GR 3rd, and Denning DW, et al (2016) Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America Clin Infect Dis 63(4) e1–e60 https://doi.org/10.1093/cid/ciw326 PMID: 27365388 PMCID: 4967602
27. Herbrecht R, Denning DW, and Patterson TF, et al (2002) Voriconazole versus amphotericin b for primary therapy of invasive aspergillosis N Engl J Med 347(6) 408–415 https://doi.org/10.1056/NEJMoa020191 PMID: 12167683
28. Wingard JR, Dick J, and Charache P, et al (1986) Antibiotic-resistant bacteria in surveillance stool cultures of patients with prolonged neutropenia Antimicrob Agents Chemother 30(3) 435–439 https://doi.org/10.1128/AAC.30.3.435
29. Korula A, Perumalla S, and Devasia AJ, et al (2020) Drug-resistant organisms are common in fecal surveillance cultures, predict bacteremia and correlate with poorer outcomes in patients undergoing allogeneic stem cell transplants Transpl Infect Dis 22(3) e13273 https://doi.org/10.1111/tid.13273 PMID: 32107829
30. Gundluru SB, Roy PS, and Biswal M, et al (2023) Isolation of multidrug-resistant organisms in surveillance stool culture at diagnosis fails to predict mortality or subsequent sepsis due to multidrug-resistant organisms in children with acute leukemia: a single-center, prospective, observational study Indian J Pediatr 93 295–298 https://doi.org/10.1007/s12098-023-04683-w
31. Paul D, Gokarn AG, and Bhat V, et al (2016) Impact of surveillance stool culture guided selection of antibiotics in allogeneic hematopoietic stem cell transplant patients Blood 128(22) 3389 https://doi.org/10.1182/blood.V128.22.3389.3389
32. Dhanya R, Agarwal RK, and Ramprakash S, et al (2022) Do weekly surveillance cultures contribute to antibiotic stewardship and correlate with outcome of HSCT in children? A multicenter real-world experience of 5 years from the Indian subcontinent Transplant Cell Ther 28(3) 170.e1-170.e7 https://doi.org/10.1016/j.jtct.2021.12.008
Appendix-A
Clinical scenario 1: Indications for point-of-care resistant gene testing in GNB.
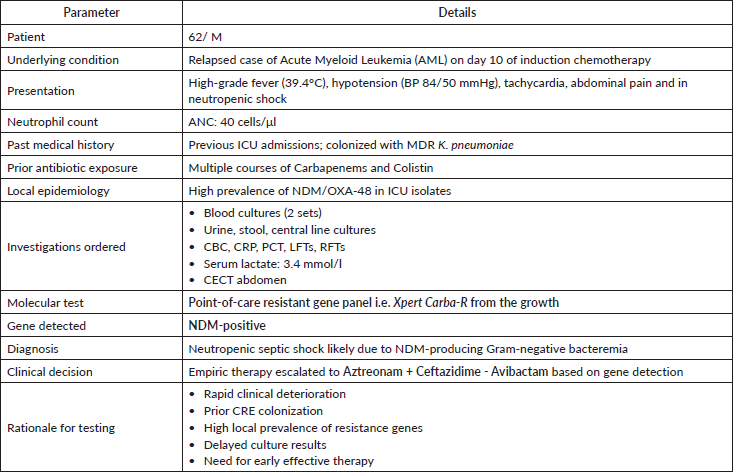
Clinical scenario 2: Susceptibility testing in gram-negative infections.