Association of serum cyclooxygenase-2 levels with hand-foot syndrome in patients receiving capecitabine: an exploratory analysis of D-TORCH study
Ghazal Tansir1*, Akhil Santhosh1*, Akash Kumar2, Hemavathi Baskarane1, Mohit Kumar Divakar1, Vishakha Hooda1, Arundhati J R Dev1, Chandra Prakash Prasad1, Ishaan Gupta3, Saran Kumar4, Pranay Tanwar5, Atul Sharma1, Sameer Bakhshi1 and Atul Batra1
1Department of Medical Oncology, Dr. B.R.A. IRCH, All India Institute of Medical Sciences, New Delhi 110029, India
2Department of Medical Oncology, National Cancer Institute, All India Institute of Medical Sciences, Haryana 124105, India
3Department of Biochemical Engineering and Biotechnology, Indian Institute of Technology Delhi, New Delhi 110016, India
4Kusuma School of Biological Sciences, Indian Institute of Technology Delhi, New Delhi 110016, India
5Department of Laboratory Oncology, Dr. B.R.A. IRCH, All India Institute of Medical Sciences, New Delhi 110029, India
*Shared first authorship.
Abstract
Background: The D-TORCH trial demonstrated superiority of 1% topical diclofenac over placebo in preventing capecitabine-induced hand-foot syndrome (HFS). We conducted an exploratory analysis of this study to assess the relationship between HFS and serum levels of the inflammatory marker, cyclooxygenase-2 (COX-2).
Methods: Serum COX-2 levels were measured in patients in the D-TORCH study's experimental and placebo arms at baseline and 12 weeks of capecitabine-based therapy or at the development of HFS (whichever occurred earlier) and in 20 age-matched healthy controls using a human COX-2 ELISA kit (E-EL-H5574).
Results: 233 (88.5%) patients of the D-TORCH cohort (n = 263) underwent serial COX-2 analysis. The population was female predominant (n = 165, 70.8) with a median age of 47 years (range: 19–73), including breast (n = 130, 55.8%) and gastrointestinal cancers (n = 103, 44.2%). 31 (13.3%) patients developed any-grade HFS, with 25 (10.7%) having grade 2 or worse HFS. Mean serum COX-2 levels at baseline and 12 weeks did not show a statistically significant difference (mean + standard deviation, 3.41 + 2.15 ng/ml versus 3.35 + 2.40 ng/ml, p = 0.69); however, baseline levels in patients were significantly higher than healthy controls (p < 0.001). No statistically significant difference was found between serial COX-2 levels by gender, use of topical diclofenac, type of malignancy or severity of HFS.
Conclusion: Serum COX-2 levels did not show a significant change with capecitabine-based therapy, regardless of the use of topical diclofenac possibly reflecting the predominant stromal production of the enzyme. This finding highlights the need to assess HFS-affected tissues for local COX-2 immuno-expression along with further blood-based biomarkers.
Keywords: hand-foot syndrome, cyclooxygenase, supportive care, biomarkers
Correspondence to: Atul Batra
Email: batraatul85@gmail.com
Published: 15/08/2025
Received: 19/02/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Capecitabine is an oral fluoropyrimidine with demonstrated efficacy in several cancers in the neoadjuvant, adjuvant and palliative settings [1–6]. Hand-foot syndrome (HFS) is a dose-limiting side effect that can affect over half the patients receiving capecitabine [7]. HFS manifests as palmoplantar numbness, tingling, erythema, edema, desquamation or ulceration of skin [8]. It is associated with pain, worsened quality of life, loss of fingerprint quality and superimposed infections [9].
Possible mechanisms of capecitabine-induced HFS include cyclooxygenase-2 (COX-2)-induced inflammation, accumulation of metabolites in palms and soles due to local concentration of thymidylate phosphorylase and genetic variants of ATP-binding cassette transporters [10,11]. COX-2 facilitates inflammation by metabolism of arachidonic acid into prostaglandin H, leading to the production of prostaglandin E2 [12]. Signs of inflammation have been demonstrated in HFS-affected tissues, such as white blood cell infiltration, vacuolar degeneration and vascular dilation, indicating the role of COX-2 in capecitabine-induced HFS [13, 14]. Thus, celecoxib was explored to prevent capecitabine-associated HFS in a randomised controlled trial demonstrating a significant reduction in the incidence of capecitabine-associated HFS among patients with colorectal cancer [7]. However, it is not routinely used due to concerns regarding cardiac and gastrointestinal adverse events.
We previously reported the D-TORCH study, a phase III randomised controlled trial that demonstrated superiority of topical 1% diclofenac gel versus placebo for preventing capecitabine-associated HFS. There was a significant reduction in Common Terminology Criteria for Adverse Events (CTCAEs) grade 1-3 (6.1% versus 18.1%) and grade 2 or higher HFS (3.8% versus 15%) with topical diclofenac compared to placebo [15, 16]. We present results of a planned assay of serum COX-2 at baseline and 12 weeks of therapy (or HFS, whichever was earlier) among patients of the D-TORCH study.
Methods
Study design
This investigator-initiated, phase III randomised, double-blind, placebo-controlled trial was conducted between January 2021 and February 2023 with its study protocol and primary results published earlier [15, 16]. It was prospectively registered with the Clinical Trials Registry of India (CTRI/202101/030592) and approved by the Institute Review Board (IECPG-82/27.01.2021).
Participants were randomised to receive topical 1% diclofenac or placebo for 12 weeks. The primary endpoint was grade 2 or higher HFS, and secondary endpoints included all-grade HFS, HFS-related dose changes of capecitabine and time to develop HFS.
Sample collection and laboratory assay
Five millilitres of blood from participants was collected at baseline and 12 weeks of therapy or the development of HFS, whichever occurred earlier. Additionally, 5 ml of blood was drawn at a single point from 20 healthy controls.
Serum was analysed for COX-2 levels using a human COX-2 ELISA kit (Cat # E-EL-H5574, Elabscience Biotechnology Inc., Houston, TX, USA) following the sandwich ELISA method. Pre-coated micro ELISA plate wells with antibodies against COX-2 were utilised. Serum samples were added to the wells and incubated for 90 minutes to facilitate antigen binding, followed by adding a biotinylated detection antibody and avidin-conjugated horseradish peroxidase, with each step accompanied by incubation and several washings.
A substrate solution was introduced to trigger a colorimetric reaction, which resulted in a blue color change in wells containing Human COX2. The reaction was halted with a stop solution, producing a yellow color. Optical density (OD) was measured at 450 nm using a spectrophotometer. The OD values were directly proportional to the COX-2 concentration and determined by plotting a standard curve. The kit has a detection sensitivity of 0.19 (ng/ml) and a detection range of 0.31–20 ng/ml.
Statistical analysis
Baseline clinical characteristics were presented using descriptive statistics, including median (inter-quartile range), mean, standard deviation (SD0 and frequencies. Paired samples t-test was used to compare mean (SD) COX-2 values at baseline and 12 weeks among the study cohort and various sub-groups. A p-value of < 0.05 was considered a priori to be statistically significant. Statistical analysis was done using GraphPad Prism version 10.3.1 for Windows, GraphPad Software, Boston, Massachusetts USA and SPSS version 29.0 (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp).
Results
As published, the D-TORCH study randomised 264 patients to topical diclofenac gel (n = 131) and placebo (n = 133) arms between February 2021 and January 2023 with 263 patients analysed for study outcomes [15]. In this full cohort, grade 2–3 HFS was observed in 3.8% participants in the diclofenac group and 15.0% in the placebo group (95% confidence interval (CI), 4.3–18.1; p = 0.003). Grade 1–3 HFS was lower in the diclofenac than in the placebo group (6.1% versus 18.1%; 95% CI, 4.1–19.6).
Among 263 patients in the D-TORCH study, 233 (88.5%) patients underwent paired assessment of serum COX-2 levels at baseline and 12 weeks of treatment. The clinical profile of the patients included in the entire D-TORCH cohort and this particular analysis is described in Table 1. The biomarker-assessment population had a median age of 47 years (range: 19–73) and female predominance (n = 165, 70.8%). The predominant subtype of disease was breast cancer (n = 130, 55.8%) and most patients had metastatic disease (n = 119, 51.1%).
Table 1. Clinical and demographic details of full study cohort and patients who underwent paired COX-2 level assay.
31 (13.3%) patients in the biomarker-assessment population (n = 233) developed HFS with >/= grade 2 HFS present in 25 (10.7%). Of these HFS-affected patients (n = 31), 8 (25.8%) and 23 (74.2%) were in the topical diclofenac and placebo arms, respectively. Mean serum COX-2 levels at baseline and 12 weeks were 3.41 ng/ml (SD: 2.15, range: 0.18–13.9) and 3.35 ng/ml (SD: 2.40, range: 0.18–12.04), respectively (p = 0.69, SD: 2.31, 95% CI: -0.23-0.35). Mean serum COX-2 levels in healthy controls (0.93 ng/ml, SD: 0.97, range: 0–2.6), were statistically significantly lower compared to baseline COX-2 levels in the overall patient cohort (p < 0.001, SD: 2.02, 95% CI:1.02–3.36) (Figure 1).
Subgroup analysis
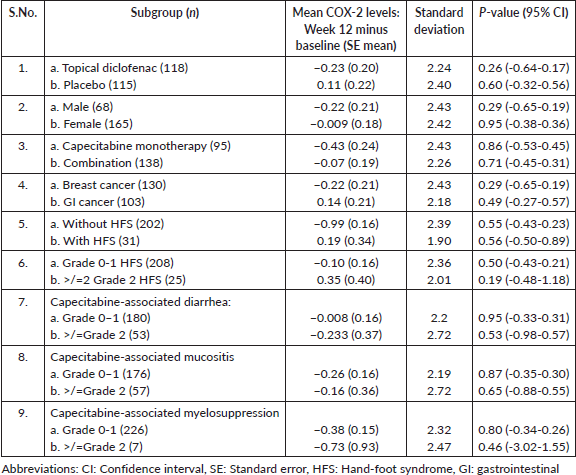
Difference between mean COX-2 levels at baseline and 12 weeks was not statistically significantly different among subgroups by gender, treatment arms, occurrence of HFS, severity of HFS (CTCAE grade 0–1 and grade >/=2 HFS, other toxicities (CTCAE grade 1 and >/= grade 2), type of chemotherapy administered (capecitabine monotherapy versus combination), type of primary malignancy (breast versus GI cancers). The difference between the mean baseline and 12-week COX-2 in each subgroup with standard deviation and p values is presented in Table 2.
Figure 1. Graphical representation of COX-2 concentrations in populations (a): All patients included in the study cohort (b): Normal (healthy control) population and patients with malignancy (c): Baseline and 12-week levels in patients without HFS (d): Baseline and 12-week levels in patients with HFS. Abbreviations: COX-2: Cycloooxygenase-2, conc: concentration, ng/ml: nanogram per milliliter, HFS: Hand foot syndrome
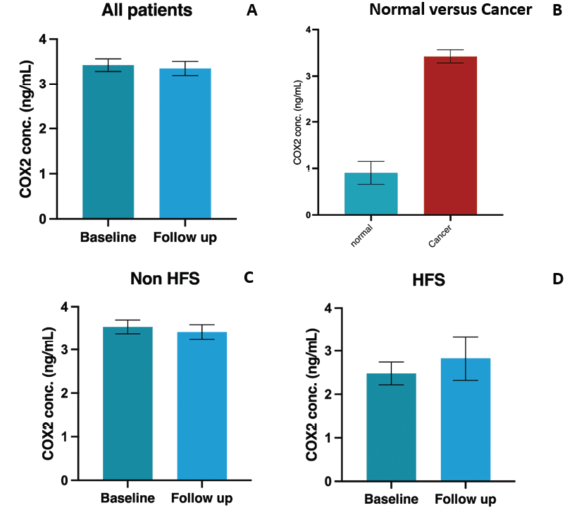
Table 2. COX-2 levels in various subgroups of the study cohort.
Discussion
We present the biomarker analysis among participants of the D-TORCH trial, which found no significant difference in serum COX-2 levels within 12 weeks of therapy. Limited studies have explored biomarkers for capecitabine-induced HFS, including the association of serum and red blood cell (RBC) folate levels with grade 2 or higher HFS [17]. Higher serum and RBC folate levels were found to be significantly correlated with the occurrence of grade 2 or worse HFS on multivariate analysis. However, only 1 in 150 individuals had true folate deficiency, reflected by levels of 283 nmol/l. Hence, true folate deficiency was present in only a small fraction of patients. It was, thus, opined by the authors that larger studies needed to be conducted to study the folate levels among different populations on capecitabine. It was also suggested that lower dietary folates in East Asian diets could prevent capecitabine-associated HFS by providing less substrate for the conversion of fluorouracil metabolites to thymidylate synthase. Serum dihydropyrimidine dehydrogenase levels were also found to be associated with grade 1 and 2 HFS among patients on celecoxib for prevention of capecitabine-induced HFS [7]. There are currently no available studies on the utility or cost-effectiveness of the use of these biomarkers in predicting the development of HFS, and this knowledge will be beneficial in choosing the appropriate test for further use. Currently, these tests are available for commercial use, but the cost can be a deterrent for routine use in resource-limited settings among those at risk of HFS.
High COX-2 immunoexpression was described in multiple myeloma [18], oropharyngeal cancers [19, 20] and malignant melanoma [21], also serving as an adverse prognostic factor in melanoma [22]. These studies utilised tissue COX-2 expression because the enzyme is predominantly secreted near the tumour stroma [23]. However, there is no literature assessing serum COX-2 and its association with outcomes or adverse events in cancer despite the enzyme being a part of systemic inflammation. Its role as a biomarker for treatment-related adverse events remains under-explored. Due to capecitabine-induced HFS being a potential inflammatory side effect and COX-2 inhibitors exhibiting benefit in its prevention, prospective serum COX-2 analysis was part of the D-TORCH study.
Baseline COX-2 levels in patients with cancer were significantly higher than controls, indicating the validity of the testing apparatus and the possibility of higher tumour-related inflammation in the patients. COX-2 levels were not significantly altered after 12 weeks of treatment with capecitabine, regardless of HFS and use of topical diclofenac. This persistent elevation could indicate active inflammation secondary to causes such as residual tumour and the use of chemotherapy. The use of topical diclofenac and the resulting reduction in HFS did not translate into a decrease in COX-2 levels, possibly due to the local rather than systemic action of the experimental drug.
Our study did not include tissue COX-2 assessment, due to which inferences on COX-2 expression in HFS are incomplete. Despite this limitation, ours is the first study to explore a potential biomarker of HFS in a commonly used drug. Future studies on HFS could be designed to test COX-2 immunoexpression on the palms or soles with paired serum assays. Other blood-based inflammatory biomarkers, such as prostaglandin E2 may also be assayed. We highlight the need for assaying tissue and blood-based inflammatory markers, including COX-2, that could serve as biomarkers to identify patients at high risk of developing capecitabine-related HFS.
Conclusion
Among participants of the D-TORCH study, delta serum COX-2 levels were not significantly different by treatment arms or occurrence of HFS, highlighting the need to study tissue expression of COX-2 as a biomarker for capecitabine-induced HFS.
Statements and declarations
The study has been cleared by the Institute Review Board (IECPG-82/27.01.2021) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The trial was registered prospectively under the Clinical Trials Registry of India (CTRI/2021/01/030592 (D-TORCH).
The persons included in the study gave their informed consent prior to inclusion.
The authors declare that they do not have any conflict of interest.
Funding
Supported in part by Alkem Laboratories Ltd and Indian Association of Supportive Care in Cancer.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ghazal Tansir and Akhil Santhosh. The first draft of the manuscript was written by Ghazal Tansir and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
1. Masuda N, Lee SJ, and Ohtani S, et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy N Engl J Med 376(22) 2147–2159 https://doi.org/10.1056/NEJMoa1612645
2. Parsons HA and Burstein HJ (2021) Adjuvant capecitabine in triple-negative breast cancer: new strategies for tailoring treatment recommendations JAMA 325(1) 36–38 https://doi.org/10.1001/jama.2020.23371
3. Geyer CE, Forster J, and Lindquist D, et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer N Engl J Med 355(26) 2733–2743 https://doi.org/10.1056/NEJMoa064320 PMID: 17192538</a>
4. Cassidy J, Clarke S, and Díaz-Rubio E, et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer J Clin Oncol 26(12) 2006–2012 https://doi.org/10.1200/JCO.2007.14.9898 PMID: 18421053</a>
5. Grothey A, Sobrero AF, and Shields AF, et al (2018) Duration of adjuvant chemotherapy for stage III colon cancer N Engl J Med 378(13) 1177–1188 https://doi.org/10.1056/NEJMoa1713709 PMID: 29590544</a> PMCID: 6426127</a>
6. Bang YJ, Kim YW, and Yang HK, et al (2012) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial Lancet 379(9813) 315–321 https://doi.org/10.1016/S0140-6736(11)61873-4 PMID: 22226517</a>
7. Zhang RX, Wu XJ, and Wan DS, et al (2012) Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial Ann Oncol 23(5) 1348–1353 https://doi.org/10.1093/annonc/mdr400
8. Janusch M, Fischer M, and Marsch WC, et al (2006) The hand-foot syndrome--a frequent secondary manifestation in antineoplastic chemotherapy Eur J Dermatol 16(5) 494–499 PMID: 17101468</a>
9. Zhao J, Zhang X, and Cui X, et al (2020) Loss of fingerprints as a side effect of capecitabine therapy: case report and literature review Oncol Res 28(1) 103–106 https://doi.org/10.3727/096504019X15605078731913
10. Aras E, Yucel KT, and Eki̇Nci̇Oglu AB, et al (2019) Capecitabine induced hand-foot syndrome: a systematic review of case reports Clin Exp Health Sci 9(2) 178–191 https://doi.org/10.33808/clinexphealthsci.469538
11. Milano G, Etienne-Grimaldi MC, and Mari M, et al (2004) Candidate mechanisms for capecitabine-related hand–foot syndrome Br J Clin Pharmacol 66(1) 88–95 https://doi.org/10.1111/j.1365-2125.2008.03159.x
12. Fitzpatrick FA (2004) Cyclooxygenase enzymes: regulation and function Curr Pharm Des 10(6) 577–588 https://doi.org/10.2174/1381612043453144 PMID: 14965321</a>
13. Nagore E, Insa A, and Sanmartín O (2000) Antineoplastic therapy—induced palmar plantar erythrodysesthesia (‘Hand-Foot’) syndrome: incidence, recognition and management Am J Clin Dermatol 1(4) 225–234 https://doi.org/10.2165/00128071-200001040-00004
14. Miller KK, Gorcey L, and McLellan BN (2014) Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management J Am Acad Dermatol 71(4) 787–794 https://doi.org/10.1016/j.jaad.2014.03.019 PMID: 24795111</a>
15. Santhosh A, Sharma A, and Bakhshi S, et al (2024) Topical diclofenac for prevention of capecitabine-associated hand-foot syndrome: a double-blind randomized controlled trial J Clin Oncol 42(15) 1821–1829 https://doi.org/10.1200/JCO.23.01730 PMID: 38412399</a>
16. Santhosh A, Kumar A, and Pramanik R, et al (2022) Randomized double-blind, placebo-controlled study of topical diclofenac in the prevention of hand-foot syndrome in patients receiving capecitabine (the D-TORCH study) Trials 23(1) 420 https://doi.org/10.1186/s13063-022-06353-2 PMID: 35590388</a> PMCID: 9117836</a>
17. Yap YS, Kwok LL, and Syn N, et al (2017) Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine–induced hand-foot syndrome JAMA Oncol 3(11) 1538–1545 https://doi.org/10.1001/jamaoncol.2017.1269 PMID: 28715540</a> PMCID: 5710192</a>
18. Pattnaik SA, Padhi S, and Panigrahi A, et al (2022) Cyclooxygenase 2 (Cox 2) expression in newly diagnosed plasma cell myeloma: a clinicopathological and immunohistochemical study on 73 cases from a single tertiary care center Indian J Hematol Blood Transfus 38(2) 235–245 https://doi.org/10.1007/s12288-021-01448-3 PMID: 35496959</a> PMCID: 9001773</a>
19. DeVoti JA, Israr M, and Lam F, et al (2022) Oropharyngeal tumor cells induce COX-2 expression in peripheral blood monocytes by secretion of IL-1α Front Immunol 13 1011772 https://doi.org/10.3389/fimmu.2022.1011772
20. Seyedmajidi M, Shafaee S, and Siadati S, et al (2014) Cyclo-oxygenase-2 expression in oral squamous cell carcinoma J Cancer Res Ther 10(4) 1024 https://doi.org/10.4103/0973-1482.138205
21. Tudor DV, Bâldea I, and Lupu M, et al (2020) COX-2 as a potential biomarker and therapeutic target in melanoma Cancer Biol Med 17(1) 20–31 https://doi.org/10.20892/j.issn.2095-3941.2019.0339 PMID: 32296574</a> PMCID: 7142851</a>
22. Soares CD, Borges CF, and Sena-Filho M, et al (2017) Prognostic significance of cyclooxygenase 2 and phosphorylated Akt1 overexpression in primary nonmetastatic and metastatic cutaneous melanomas Melanoma Res 27(5) 448–456 https://doi.org/10.1097/CMR.0000000000000368 PMID: 28604419</a>
23. Urban J, Kuźbicki Ł, and Szatkowski G, et al (2014) Stromal, rather than epithelial cyclooxygenase-2 (COX-2) expression is associated with overall survival of breast cancer patients BMC Cancer 14(1) 732 https://doi.org/10.1186/1471-2407-14-732 PMID: 25269624</a> PMCID: 4192334</a>






