Prostate cancer clinical trials in low- and middle-income countries
Sattam A Halaseh1, Amro Al-Karadsheh2, Deborah Mukherji3, Abdelrahman Alhjahaja4, Ala’a Farkouh5, Akram Al-Ibraheem6, Ibrahim Abu Gheida7, Sultan Al-Khateeb8, Humaid Al-Shamsi9 and Mohammed Shahait10
1Urology Department, Torbay Hospital, Torbay and South Devon NHS Foundation Trust, Newton Rd, Torquay TQ2 7AA, UK
2General Medicine, Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust, Greetwell Rd, Lincoln LN2 5QY, UK
3Hematology/Oncology Division, Department of Internal Medicine, American University of Beirut Medical Center, Riad El Solh, Beirut 1107 2020, Lebanon
4The Hashemite University, Damascus Hwy, Zarqa 453J+5C5, Jordan
5American Center for Reproductive Medicine, Cleveland Clinic, 10681 Carnegie Ave, Cleveland, OH 44106, USA
6Department of Nuclear Medicine and PET/CT, King Hussein Cancer Centre, Queen Rania Al Abdullah Street, Amman 11941, Jordan
7Cleveland Clinic Abu Dhabi LLC, Al Marayyah Island, PO Box 112412, Abu Dhabi, UAE
8King Faisal Specialist Hospital & Research Center, PO Box 3354, Riyadh 11211, Kingdom of Saudi Arabia
9Burjeel Cancer Institute/VPS Oncology UAE, 28th Street, Mohammed Bin Zayed City Abu Dhabi, PO Box 92510, UAE
10Surgery Department, Clemenceau Medical Center, Dubai Healthcare City Phase 2 - Al Jaddaf, Dubai, UAE
Abstract
Background: Prostate cancer is the second most common form of cancer and a leading cause of cancer-related death in men. In an era of evidence-based medicine, clinical trials play a critical role, and adherence to best practices is crucial in managing complicated and non-communicable diseases, such as prostate cancer. For this reason, extrapolating research conducted in high-income countries (HICs) to low-middle-income countries (LMICs) may lead to incorrect findings or treatment plans for patients in these areas. Unfortunately, clinical trials in LMICs face several challenges in terms of design, funding and recruitment. This study aimed to examine clinical trials on prostate cancer in LMICs, including the scope of these trials, the type of interventions being tested and funding sources.
Methods: A search of the Cochrane Library Controlled Trials Registry was conducted between January 2010 and June 2021 using keywords including: ‘prostate cancer’, ‘prostate adenocarcinoma’ and ‘prostate tumour’). The trials were classified into either HICs or LMICs based on the World Bank Atlas classification. A descriptive analysis was performed to determine the characteristics of the trials.
Results: A total of 3,455 clinical trials for prostate cancer have been conducted globally, with 542 (15.68%) conducted LMICs. Most of these trials (89%) were registered in upper-middle-income countries, with none being conducted in low-income countries. The majority of trials were prospective studies (98.1%), with 65.2% being randomised and 57% being phase III. Of the trials, 48.4% aimed to recruit fewer than 500 participants. The main source of funding was pharmaceutical companies in 78.1% of the cases, followed by institutional funds (16.1%) and public funds (5.8%). At the time of the search query, 74.6% of the trials were inactive, with 37% completed, 5% terminated due to insufficient funding and 75% terminated due to medical inefficacy or poor accrual. The majority of trials (88.2%) were interventional, with only 6% focusing on screening and prevention, and 2% designed for palliative care.
Conclusion: This study sheds light on the challenges faced in conducting clinical trials for prostate cancer in LMICs. The findings underline the need for improved support from international organisations and pharmaceutical companies to bridge the gaps in prostate cancer research and facilitate collaboration between researchers in LMICs and other countries.
Keywords: prostate cancer, developing countries, healthcare disparities, cost of illness, low middle-income countries
Correspondence to: Sattam A Halaseh and Mohammed Shahait
Email: halasehsattam1@outlook.com and mshahait@yahoo.com
Published: 13/11/2023
Received: 08/07/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Genitourinary malignancies such as prostate, renal, bladder, urethral, testicular and penile cancers represent almost 12.7% of newly diagnosed cancers in the world and almost 7.9% of cancer-related deaths globally. In 112 nations, prostate carcinoma was the most prevalent cancer diagnosed in men, followed by lung, colorectal and liver cancers [1]. In 2020, prostate cancer accounted for 375,000 deaths globally, with 1,400,000 new cases being diagnosed [2].
The regional incidence rates of prostate cancer range from 6.3% to 83.4% per 100,000 men, with the highest rates in Western Europe and Northern America and the lowest in Asia and Northern Europe. Regional patterns of death rates did not correspond to those of incidence, with the highest fatality rates in the Caribbean, sub-Saharan Africa and Micronesia/Polynesia. In 48 countries, including sub-Saharan Africa, the Caribbean, Central and South America (such as Ecuador, Chile and Venezuela) and Sweden, prostate cancer is the leading cause of cancer-related mortality among males [1].
In the era of evidence-based medicine, in which there is a growing emphasis on adherence to best practice guidelines, it is crucial to note that research conducted on a specific group of subjects may not be representative or applicable to other populations [3]. As a result, extrapolating from research undertaken by high-income countries (HICs) on complicated noncommunicable diseases, such as cancer or trauma, is challenging and may lead to impractical application in the context of limited resources [4], leading to inappropriate diagnostic approaches or treatment plans. This is crucial in establishing recommendations for best practices in low-middle-income country (LMIC) [5].
However, multilayer complexities hinder any efforts to design, fund and recruit clinical trials in LMIC countries [6]. As the management of locally advanced prostate cancer is evolving rapidly, there is limited data on the efficacy and feasibility of different novel therapies in LMICs, for example [5].
In this study, we sought to investigate the clinical trials of prostate cancer conducted in LMICs to better understand the scale of these trials, the type of intervention and funding sources. The results of this study may help international societies and pharmaceutical companies existing gaps in prostate cancer research and explore avenues for collaboration with researchers from LMIC.
Methods
A comprehensive search of the Cochrane Library Controlled Trials Registry was performed to include clinical trials conducted between January 2010 and June 2021. The search keywords included prostate cancer ‘prostate cancer’, ‘prostate adenocarcinoma’, and ‘prostate tumour’, and prostate tumor (Figure 1). All clinical trials on prostate cancer screening, diagnosis, management and follow-up were included. The authors conducted the search. The search query was performed on 20 March 2022.
The following data were extracted from each study: study year, country, date of study initiation, date of study completion, study type, primary purpose, time perspective, allocation, observational model, phase, intervention model, masking, type of intervention, control arm, sample size, active or inactive trial, reason for ending the trial, funding type and number of institutions (single or multicentre).
The World Bank Atlas classifies countries into low-income, lower-middle-income, upper-middle-income and high-income economies [7] (Table 1). For the analysis, the LMIC was used to represent countries with low- and middle-income economies. Descriptive statistics were used to analyse the results (Table 2).
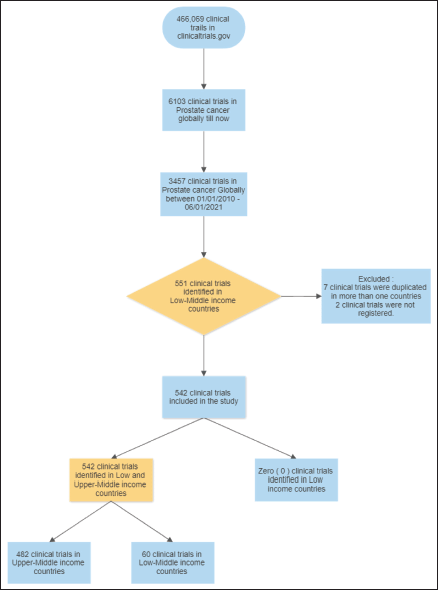
Figure 1. Evidence acquisition flow chart.
Search strategy
Topic: Prostate cancer clinical trials in low-middle income countries
Date range: 01/01/2010–06/01/2021
Language: English
Human only, globally
Search strategy terms
Prostate cancer
TS = ((Prostat* AND (cancer* OR carcinoma* OR adenocarc*)))
https://clinicaltrials.gov/search?cond=PROSTATE%20CANCER&start=2010-01-01_2021-06-01
Total number of clinical trials globally: 3,457
Total number of clinical trials in LMICs = 551
(Seven duplicated studies, two not registered).
Table 1. Prostate clinical trials distribution heat map in LMICs.
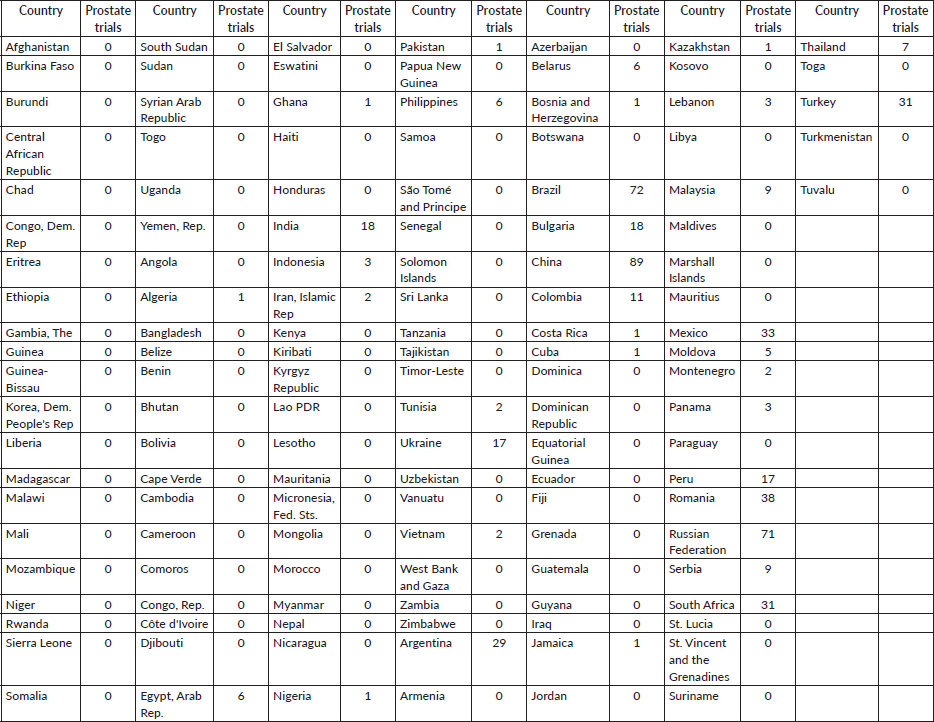
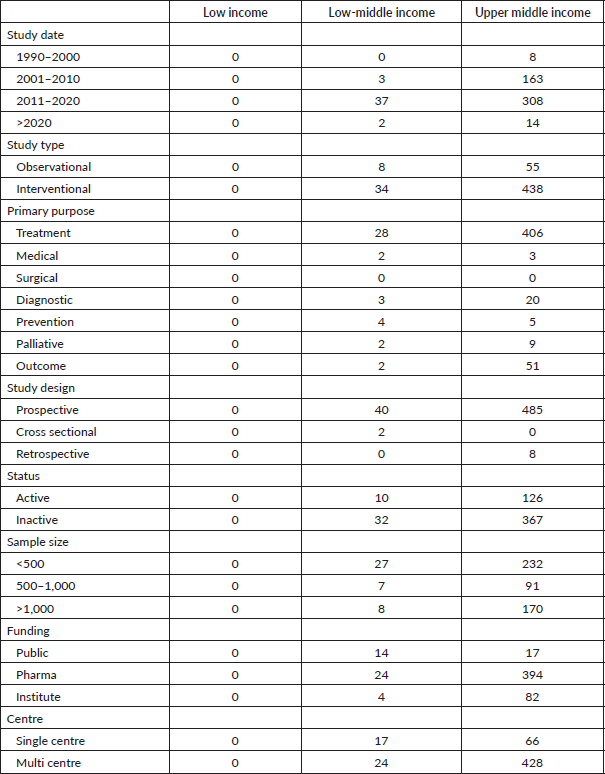
Table 2. Descriptive analysis of LMIC trials.
Results
A total of 3,455 prostate cancer trials have been conducted globally. 542 (15.68%) prostate clinical trials were identified in LMIC. Seven trials were duplicates and excluded from the upper-middle-income countries. Most of these trials were registered in upper-middle-income countries 489 (89%), and none were identified in low-income countries (Figure 1).
The majority of prostate cancer clinical trials in LMIC (67.47%) were conducted after 2010. Most of these trials were prospective (98.1%), randomised (65.2%), phase III (57%) and planned to recruit <500 subjects (48.4%). The primary funding source for these trials was pharmaceutical companies (78.1%), followed by institutional funds (16.1%) and public funds (5.8%). Most of the trials were multicentre trials (83.39%). Refer to Table 2 for further details.
At the time of search query, 74.6% were labelled inactive trials. 37% were completed trials, with 5% terminated to conclusion of the trial, 25% due to insufficient funding and 75% terminated due to medical inefficacy or poor accrual.
Prostate cancer clinical trials in LMIC were mainly interventional (88.2%), using medication. Only 6% of trials focused on screening and prevention, while (2%) of trials were designed for palliative intent.
Discussion
This study aimed to provide an in-depth understanding of the current status of prostate cancer clinical trials in LMICs. Of the total 3,455 global clinical trials for prostate cancer, only 542 were conducted in middle-income countries and none were conducted in low-income countries (Figure 2). Most of these trials were funded by pharmaceutical companies and involved prospective multicenter recruitment of fewer than 500 patients. Furthermore, most trials were inactive (74.6%).
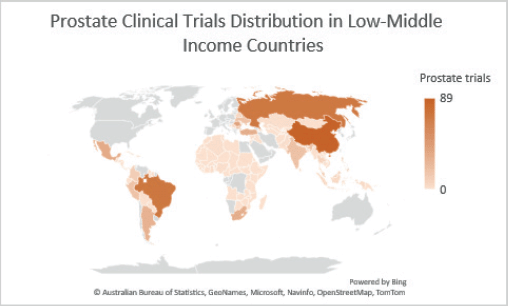
Figure 2. Prostate clinical trials distribution in LMICs.
Colour gradient
Grey colour: Indicates areas with the least activity, intensity or lowest data values.
Light orange: Represents areas with below-average values.
Orange: Signifies areas with median or average values.
Deep orange: Marks areas with the highest activity, intensity or peak data values.
Our study yielded findings consistent with prior research in the field, underscoring the insufficiency of clinical trials focused on prostate cancer in LMICs relative to the disease burden in these regions. This aligns with the results of a retrospective study that revealed a higher number of phase III trials related to oncology in HICs than the disease burden, as prostate cancer is the fourth most common cancer in several regions of LMICs, including Africa. Moreover, none of the prostate cancer clinical trials have been conducted in low-income countries, despite evidence suggesting that the incidence of urological diseases may be higher than that currently reported [3, 6, 8]. These findings highlight the urgent need for further clinical trials to address the growing burden of prostate cancer in LMICs.
Moreover, the evidence generated from trials conducted in HICs may not apply to LICs because of lack of resources, different treatment sequences, accessibility of care and different genomic make-up of the tumours [4, 9]. Cancer treatment toxicities and adverse effects, including adrenal hormone suppression and radiotherapy, are more pronounced in certain races. Additionally, there are differences in treatment responses across races [10].
Interestingly, several studies have also indicated that the availability of resources and funding is a crucial driver of clinical trial activity rather than the population's needs [3]. The high cost of oncological clinical trials is often more significant than the annual gross national income per capita, making it a significant challenge to conduct trials in these regions [8]. Our study also highlights the role of pharmaceutical companies in funding prostate cancer clinical trials, with almost three-quarters of the funding from these companies.
International and local institutions must intervene to counteract this large gap and prevent possible future bias in the results of clinical trials. Other research has emphasised the need for proper management of funding in LMICs, for example, the need to ensure that the work of the National Institution of Health is distributed more equitably to support clinical trials focused on a range of diseases beyond infectious diseases and regions other than South Africa [11].
Additionally, studies have found that clinicians in LMICs face a significantly higher workload than do those in other countries, making it almost impossible to find time to conduct clinical trials. Furthermore, trainees receive limited teaching regarding critical appraisal and research methodology [12, 13], further exacerbating the challenges of conducting clinical trials in LMICs. These findings underscore the need for increased investment in training programs for healthcare providers in LMICs to support their participation in clinical trials and other research initiatives [14].
An additional challenge in conducting clinical trials in LMIC is participant recruitment. A nationwide survey from Jordan, a middle-income country, included 3,196 participants and reported that less than 25% were willing to participate in clinical trials. Only 21.8% of the participants knew what the clinical trials were, with socioeconomic factors being a significant determinant of knowledge and willingness to participate [15]. This study highlights the importance of raising awareness and providing proper patient education regarding clinical trials in LMICs.
In addition to the challenges related to funding, training and recruitment, studies have also revealed disparities in the publication of clinical trials conducted in LMICs despite their positive value. Clinical trials in LMICs that demonstrate significantly prolonged survival with significantly lower funding are often published in low-impact journals. Clinical trials in HICs that cost hundreds of thousands of dollars and show only marginal prolongation of survival have been published in high-impact journals. This disparity in publication may contribute to the under-representation of LMICs in prostate cancer, creating what is often referred to as ‘publication prejudice’ [14].
Several journals have attempted to decrease the gap in this area by providing more opportunities for LMICs to present their results and data. For example, the American Society of Oncology's Journal of Global Oncology and Cancer has put more effort into decreasing the publication gap by providing more opportunities for LMICs to publish their research [16]. This is an essential step toward ensuring that clinical trials conducted in LMICs receive the recognition they deserve and contribute to the advancement of medical knowledge worldwide.
Conclusion
This study sheds light on the challenges faced in conducting clinical trials for prostate cancer in LMICs. The findings underline the need for improved support from international organisations and pharmaceutical companies in order to bridge the gaps in prostate cancer research and facilitate collaboration between researchers in LMICs and other countries.
Acknowledgments
We'd like to thank our mentor, Dr Mohammed Shahait, for all of the hard work and guidance they gave us this past year. Your helpful suggestions and advice were invaluable to me as I finished the project. For this, you have my undying gratitude.
Conflicts of interest
The authors declared that they have no competing interests.
Funding
The authors did not receive any external sources of funding.
Author contributions
Primary author: Sattam A. Halaseh, Mohammed Shahait and Amr Al-Karadsheh. All authors gave substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Cancer: key facts sheet (2018) [https://www.who.int/news-room/fact-sheets/detail/cancer]
3. Choksi AU, Hayden CS, and Rahman SN, et al (2023) The disparities in clinical trials addressing urologic conditions among lower-income countries Front Urol 2 1069265 https://doi.org/10.3389/fruro.2022.1069265
4. Mukherji D, Youssef B, and Dagher C, et al (2020) Management of patients with high-risk and advanced prostate cancer in the middle east: resource-stratified consensus recommendations World J Urol 38(3) 681–693 https://doi.org/10.1007/s00345-019-02872-x PMCID: 7064460
5. Herchenhorn D and Freire V (2022) Access of new systemic therapies for genito-urinary cancers in low-middle income countries Front Urol 2 1020215 https://doi.org/10.3389/fruro.2022.1020215
6. Metzler I, Bayne D, and Chang H, et al (2020) Challenges facing the urologist in low- and middle-income countries World J Urol 38(11) 2987–2994 https://doi.org/10.1007/s00345-020-03101-6 PMID: 32034500 PMCID: 8186537
7. Fantom NJ and Serajuddin U (2016) The World Bank's Classification of Countries by Income (English). Policy Research Working Paper No. WPS 7528 (Washington: World Bank Group) [http://documents.worldbank.org/curated/en/408581467988942234/The-World-Banks-classification-of-countries-by-income; https://documents.worldbank.org/en/publication/documents-reports/documentdetail/408581467988942234/the-world-banks-classification-of-countries-by-income]
8. Rubagumya F, Hopman WM, and Gyawali B, et al (2022) Participation of lower and upper middle-income countries in clinical trials led by high-income countries JAMA Netw Open 5(8) e2227252 https://doi.org/10.1001/jamanetworkopen.2022.27252 PMID: 35980637 PMCID: 9389348
9. Abdelsalam RA, Khalifeh I, and Box A, et al (2020) Molecular characterization of prostate cancer in middle eastern population highlights differences with western populations with prognostic implication J Cancer Res Clin Oncol 146(7) 1701–1709 https://doi.org/10.1007/s00432-020-03221-x PMID: 32350606
10. Abdelkarem OAI, Choudhury A, and Burnet NG, et al (2022) Effect of race and ethnicity on risk of radiotherapy toxicity and implications for radiogenomics Clin Oncol (R Coll Radiol) 34(10) 653–669 https://doi.org/10.1016/j.clon.2022.03.013 PMID: 35431121
11. Odedina FT, Shamley D, and Okoye I, et al (2020) Landscape of oncology clinical trials in Africa JCO Glob Oncol 6 932–941 https://doi.org/10.1200/JGO.19.00189 PMID: 32614728 PMCID: 7392757
12. Alemayehu C, Mitchell G, and Nikles J (2018) Barriers for conducting clinical trials in developing countries – a systematic review Int J Equity Health 17(1) 37 https://doi.org/10.1186/s12939-018-0748-6 PMID: 29566721 PMCID: 5863824
13. Grover S, Xu M, and Jhingran A, et al (2017) Clinical trials in low and middle-income countries – successes and challenges Gynecol Oncol Rep 19 5–9 https://doi.org/10.1016/j.gore.2016.11.007
14. Wells JC, Sharma S, and Del Paggio JC, et al (2021) An analysis of contemporary oncology randomized clinical trials from low/middle-income vs high-income countries JAMA Oncol 7(3) 379–385 https://doi.org/10.1001/jamaoncol.2020.7478 PMID: 33507236 PMCID: 7844695
15. Ahram M, Farkouh A, and Haddad M, et al (2020) Knowledge of attitudes to and participation in clinical trials in Jordan: a population-based survey East Mediterr Health J 26(5) 539–546 https://doi.org/10.26719/2020.26.5.539 PMID: 32538447
16. Cazap E, Sullivan R, and Foxall K (2020) New journal authorship criteria: how ecancer is supporting publications on cancer care in lower and middle income countries ecancer 14 ed106






