Possible mechanisms of action of clarithromycin and its clinical application as a repurposing drug for treating multiple myeloma
Nobuo Takemori1a, Hong-Kean Ooi2, Goro Imai3, Kazuo Hoshino4 and Masanao Saio5
1Department of Internal Medicine, Division of Hematology, Imai Hospital, Tanaka-cho 100, Ashikaga, Tochigi 326-0822, Japan
2Department of Veterinary Medicine, Azabu University, Fuchinobe 1-17-71, Sagamihara, Kanagawa 252-5201, Japan
3Department of Internal Medicine, Imai Hospital, Tanaka-cho 100, Ashikaga, Tochigi 326-0822, Japan
4Department of Surgery, Imai Hospital, Tanaka-cho 100, Ashikaga, Tochigi 326-0822, Japan
5Laboratory of Histopathology & Cytopathology, Department of Laboratory Sciences, Gunma University, Graduate School of Health Sciences, 39-22, 3-chome, Showa-machi, Maebashi, Gunma 371-8514, Japan
ahttps://orcid.org/0000-0001-9742-8385
Abstract
Clarithromycin (CAM), a semisynthetic macrolide antibiotic, is a widely used antibacterial drug. Recently, the efficacy of CAM as an add-on drug for treating multiple myeloma (MM) has been noted. Its effect on treating MM has been confirmed in combination chemotherapies that include CAM. However, a single treatment of CAM has no efficacy for treating MM. Many myeloma growth factors (MGFs) including interleukin (IL)-6 are known to be closely involved in the development of MM. CAM has been shown to suppress many MGFs, particularly IL-6. The possible mechanisms of action of CAM in treating MM have been suggested to include its immunomodulatory effect, autophagy inhibition, reversibility of drug resistance, steroid-sparing/enhancing effect and suppression of MGFs. In addition, MM is characterised by uncontrolled cell growth of monoclonal immunoglobulin (Ig)-producing neoplastic plasma cells. Large quantities of unfolded or misfolded Ig production may trigger considerable endoplasmic reticulum stress. Thus, MM is originally a fragile neoplasm particularly susceptible to autophagy-, proteasome- and histone deacetylase 6-inhibitors. Taken together, CAM plays an important role in MM treatments through its synergistic mechanisms.
In addition, CAM with its pleiotropic effects on cytokines including IL-6 and indirect antiviral effects might be worth a try for treating COVID-19.
Keywords: clarithromycin, effect, myeloma growth factors, multiple myeloma treatments, mechanisms of action, COVID-19
Correspondence to: Nobuo Takemori
Email: takemori_nobuo@circus.ocn.ne.jp
Published: 18/08/2020
Received: 30/03/2020
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background information on Clarithromycin (CAM)
Clarithromycin (CAM: Biaxin®) belongs to the 14-membered macrolide antibiotic family together with erythromycin and roxithromycin. The antibacterial effect of CAM is related to its capacity to inhibit protein synthesis in bacteria by binding to subunit 50S of the bacterial ribosome [1]. Kanoh and Rubin [2] reported that the 14- and 15-membered macrolides showed immunomodulatory properties but not the 16-membered ones. Following intestinal absorption, it has a fairly rapid first-pass metabolism in the liver. Its main metabolites are 14-(R)-hydroxy CAM, 14-(S)-hydroxy CAM and N-desmethyl CAM. 14-(R)-hydroxy CAM is an active metabolite that is also responsible for its anti-bacterial effect. CAM is acid stable and has a half-life of 5–7 hours with an oral dose of 500 mg administered every 12 hours. It is compatible with twice a day administration [3]. Ingestion of food increases CAM peak plasma concentration (Cmax) but does not affect the extent of CAM bioavailability. In non-fasting healthy human subjects, Cmax is estimated to be 3–4 μg/mL with an oral dose of 500 mg administered every 8–12 hours according to the database of the Food and Drug Administration (FDA) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050662s044s050,50698s026s030,050775s015s019lbl.pdf). Recently, the efficacy of CAM as an add-on drug for treating multiple myeloma (MM) has been reported [3]. In this paper, the significance of CAM as an add-on drug in MM treatments, its effects on myeloma growth factors (MGFs) [4–7], and its mechanisms of action leading to the suppression of MM cell proliferation were discussed.
Antineoplastic effects of 14-membered macrolides including CAM
The first experimental report suggesting the presence of the antineoplastic effect of macrolide (erythromycin) dates back to 1995. Hamada et al [8] experimentally demonstrated that the erythromycin induced cytotoxic macrophages in tumour-bearing mice and that these cytotoxic macrophages might work as one of the effector cells together with NK cells against the tumours transplanted in the mice. Clinically, Mikasa et al [9] first reported that long-term treatment with CAM significantly increased the median survival time of patients with advanced non-small cell lung cancer (NSCLC). Sakamoto et al [10] demonstrated that NK activity was significantly increased in patients with unresectable NSCLC after 1-month treatment with CAM. Furthermore, using Lewis lung carcinoma (LLC)-inoculated mice, they demonstrated that the administration of CAM after anticancer chemotherapy strongly inhibited tumour growth and significantly increased the NK activity. Recently, antineoplastic or immunomodulatory effects of CAM have again attracted attention in clinical fields. Several investigators reported that the CAM monotherapy was effective in treating hematologic malignancies including extranodal marginal zone B-cell lymphoma [11], mucosa-associated lymphoid tissue lymphoma [12, 13], follicular B-cell lymphoma [14, 15] and Hodgkin’s lymphoma [16].
According to the reports by Zhang et al [17], Klein and Bataillie [4], Klein [5], Hallek et al [18], Mahtouk et al [6] and Musolino et al [7], the following factors and cytokines are listed as MGFs: insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), macrophage inflammatory protein α (MIPα), growth/differentiation factor 15 (GDF15), pleiotrophin (PTN), brain-derived neurotrophic factor (BDNF), IL-6 family cytokines (IL-6, ciliary neurotrophic factor [CNTF], oncostatin M [OSM], leukaemia inhibitory factor [LIF], IL-11 and cardiotrophin-like cytokine factor 1 [CCLF1]), other cytokines (IL-1β, IL-10, IL-15, IL-16, IL-21, IL-22 and IL-23), TNF family members (B-cell activating factor [BAFF] and a proliferation-inducing ligand [APRIL]), transforming growth factor-β(TGF-β), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), epidermal growth factor (EGF) family members (amphiregulin [AREG], heparin-binding EGF-like growth factor [HB-EGF] and neuregulin1~4 [NRG1~4]), basic fibroblast growth factor (bFGF), Wnt family members (Wnt5A/10B/16), Jagged family (Jag1/2) and VEGF/PDGF family members. The important MGFs and other factors which are positively or negatively influenced by CAM are shown in Table 1 and briefly described as follows.
IL-1
IL-1 is a potent proinflammatory cytokine that functions as an endogenous pyrogen. IL-1 is mainly responsible for IL-6 production in the tumoural environment through prostaglandin E2. IL-1β gene and IL-1β protein, though less potent, are expressed in myeloma cells [19]. The bone marrow stromal cells (BMSCs) react with IL-1β to produce and secrete large quantities of IL-6, which in turn stimulate the survival and expansion of MM cells [7, 19, 20]. The suppression of IL-1β by CAM [21–30] (Table 1) might be related to MM cell reduction.
IL-2
IL-2 plays an essential role in key functions of the immune system, which includes immuno-tolerance and immunity. It is mainly produced by activated CD4+ T-cells and activated CD8+ T-cells and to a lesser extent by activated dendritic cells, NK/NKT cells and macrophages. IL-2 mediates its effects by binding to IL-2-receptors, which are expressed by lymphocytes. IL-2 is known to strongly stimulate NK-cell and T-cell growth which consequently augments the cytolytic action, leading to enhanced cytotoxicity. Anti-tumour properties of IL-2 are mediated by the cellular immune system, but IL-2 itself has no direct effect on tumours [31]. CAM has been reported to suppress IL-2 production [30, 32–34] (Table 1). The suppression of IL-2 by CAM seems to be disadvantageous from the view point of cellular immunity. However, the effect of reduced IL-2 on MM cells has not yet been fully elucidated.
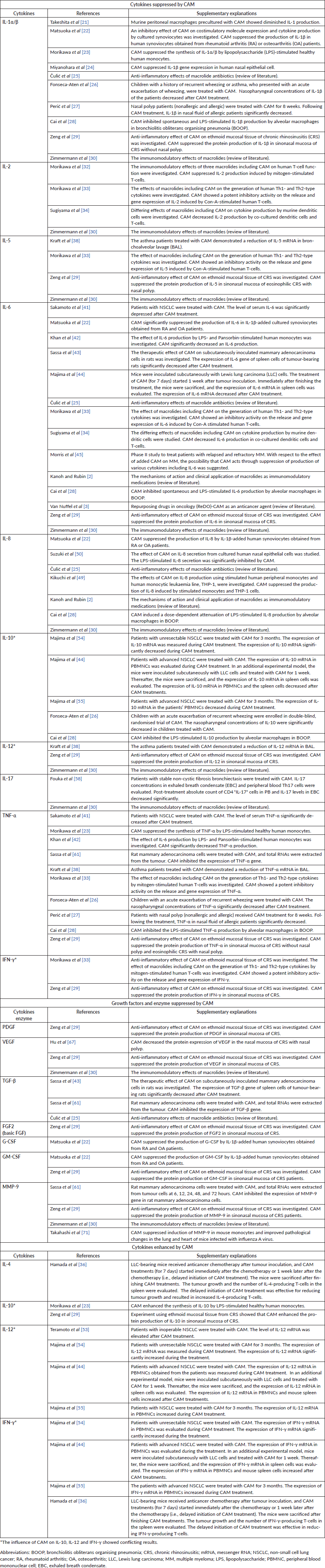
Table 1. Cytokines and enzyme, which are positively or negatively influenced by CAM, with respect to MM cell proliferation.
IL-4
IL-4 plays an essential role in promoting Th2 cell differentiation. It is primarily produced by Th2 cells, NKT cells, mast cells and eosinophils. It is also known that IL-4 induces B-cell class switching to IgE. The relationship between IL-4 and MM cell growth was demonstrated by Herrmann et al [35]. They showed that recombinant human IL-4 blocked endogenous IL-6 synthesis in a dose-dependent fashion and caused a significant reduction of plasma cell growth that could be reversed by exogenous recombinant human IL-6. Hamada et al [36] reported that CAM induced IL-4-producing T-cells in the spleen of tumour-bearing mice. Considering the suppressive effect of IL-4 on MM cell proliferation [35], it is probable that the activation of IL-4 by CAM might lead to MM cell reduction.
IL-5
IL-5 is produced by Th2 cells and mast cells and is known to stimulate B-cell growth and increase immunoglobulin secretion, primarily IgA. This cytokine is known to synergise with IL-6 to support myeloma cell proliferation [4, 37]. The suppression of IL-5 by CAM [29, 30, 33, 38] (Table 1) might result in the reduction of MM cell.
IL-6
IL-6 is a pleiotropic proinflammatory cytokine involved in acute inflammatory responses, immune reactions, haematopoiesis and inflammation. IL-6 is generated by monocytes, endothelial cells, macrophages and fibroblasts in response to diverse stimuli (TNF-α, IL-1 and IL-17) during systemic inflammation [7]. Among the MGFs, IL-6 represents the most important key cytokine for MM cell regulation [4–6, 18]. MM cells proliferate in response to IL-6 under a paracrine-regulated manner and proliferate in close contact with IL-6-producing BMSCs [4, 5, 39]. On the other hand, myeloma cells are also known to produce their own IL-6 under an autocrine manner [40]. IL-6 secreted from BMSCs binds to IL-6 receptor (IL-6R) to initiate IL-6 signalling. IL-6R, which is generated in MM cells, binds to signal transducer membrane protein (gp130), which then stimulates the Janus kinases/signal transducer-activator of transcription (JAK/STAT) and GTPase/mitogen-activated protein kinase (Ras/MAPK) pathways. Both JAK/STAT and Ras/MAPK pathways play an important role in MM proliferation and inhibition of apoptosis [7]. Since IL-6 is a potent proliferative factor in MM cells, the suppression of IL-6 by CAM [2, 3, 22, 25, 28, 29, 30, 33, 34, 41–45] (Table 1) will lead to the inhibition on the growth of MM cells.
IL-8 (CXCL8)
IL-8, a member of CXC chemokine family, was originally described as a neutrophil chemoattractant. IL-8 is known to promote cancer cell growth, survival, angiogenesis and metastasis in various human cancers. Herrero et al [46] demonstrated that IL-8 did not affect the growth of MM cells but protected them from death induced by serum starvation. Kline et al [47] reported that MM cells expressed IL-8 receptors (i.e., CXCR1 and CXCR2), BMSCs produced IL-8 in active MM and IL-8 production by BMSCs paralleled the MM disease activity. Pellegrino et al [48] demonstrated that IL-8 (CXCL8) and stromal cell-derived factor 1α (SDF-1α, a member of CXC chemokine which is also known as CXCL12) stimulate the proliferation and chemotaxis of human myeloma cells. Kikuchi et al [49] reported that CAM suppressed lipopolysaccharide (LPS)-induced IL-8 production by human monocytes through activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) transcription factors. Thus, the suppression of IL-8 by CAM [2, 22, 25, 28, 30, 49, 50] (Table 1) might be related to MM cell reduction.
IL-10
IL-10 is a cytokine with multiple and pleiotropic effects in immunoregulation and inflammation. IL-10 belongs to MGFs and represents a potent IL-6-unrelated MM cell proliferation factor but not a differentiation factor [51]. Gu et al [52] reported that IL-10 induced MM cell proliferation through an OSM autocrine loop. However, the studies on the influence of CAM on IL-10 showed conflicting results. Some investigators reported that CAM suppressed IL-10 production, secretion or regulation [26, 28, 44, 54, 55] (Table 1), whereas others [23, 29] (Table 1) showed the enhancement of IL-10 by CAM. These conflicting results might be attributed to different experimental conditions. Thus, it is very difficult to draw a definite conclusion on the effect of CAM on IL-10, pending further studies.
IL-12
IL-12 is a cytokine that exerts potent antitumor activity through a combination of immunomodulatory and antiangiogenic mechanism. According to Airoldi et al [56], IL-12 mediates the development and maintenance of Th1 cells and induces IFN-γ production by Th1 and NK cells. They demonstrated that IL-12Rβ2 was expressed in primary MM cells but downregulated as compared with normal polyclonal plasmablastic cells and plasma cells. They showed that IL-12 reduced the proangiogenic activity of primary MM cells in vitro and significantly decreased the tumourgenicity of the NCI-H929 MM cells transplanted in SCID/NOD mice by inhibiting cell proliferation and angiogenesis. However, the study of CAM effect on IL-12 seems to show conflicting results. Some investigators reported that CAM enhanced IL-12 production or gene expression [44, 53–55], whereas others reported the suppressive effect of CAM on IL-12 [29, 30, 38] (Table 1). The contradictory results might be caused by the difference in the experimental settings. Further detailed studies are needed to define the actual effect of CAM on IL-12.
IL-17
IL-17, a member of MGFs, is a proinflammatory cytokine produced by Th-17 cells. It is closely related to TGF-β, nerve growth factor, bone morphogenic protein and platelet-derived growth factor (PDGF) with similar structural motifs. IL-17 induces the expression of a number of chemokines and cytokines including IL-6, TGF-β, GCSF, GM-CSF, MMP and intercellular adhesion molecule-1 in a variety of cell types, including BMSCs [57]. Prabhala et al [57] demonstrated that Th17 cells and serum IL-17 increased in MM, and IL-17 promoted MM cell growth both in vitro and vivo through IL-17 receptors and inhibited Th1 responses [57]. The suppressive effect of CAM on IL-17 [30, 58] (Table 1) might be manifested as a reduction of MM cells.
TNF-α
TNF-α, a member of TNF superfamily, is a major proinflammatory cytokine involved in the innate immune response. TNF-α is known to be produced mainly by activated macrophages. According to Jourdan et al [59], TNF-α is a survival and proliferation factor for human MM cells, though less potent than IL-6. Its survival activity is not affected by anti-IL-6 or anti-gp130 monoclonal antibodies, and it induces MM cells into the cell cycle to promote long-term MM cell growth. Hideshima et al [60] investigated the role of TNF-α using human IM-9 MM cells and demonstrated that TNF-α secreted from MM cells induced only a modest increase of cell proliferation, as well as MAPK and NF-κB activation. They also showed that TNF-α upregulated MAPK and NF-κB and markedly increased IL-6 secretion from BMSCs. Furthermore, they demonstrated that TNF-α induced the adhesion molecules on both MM cells (VLA-4, LFA-1 and MUC-1) and BMSCs (ICAM-1 and VCAM-1), which resulted in increased adhesion between MM cells and BMSCs. An increase in adhesion activity, in turn, induces IL-6 secretion from BMSCs, leading to MM cell proliferation. Therefore, the important role of TNF-α in MM is to augment paracrine IL-6-mediated MM cell growth. Thus, the suppression of TNF-α by CAM [23, 26–29, 33, 38, 41, 42, 61] (Table 1) might cause MM cell reduction.
IFN-γ
IFN-γ is crucial for immunity against intracellular pathogens and tumour control. Cells from the innate immune system (e.g., NK cells, NKT cells, macrophages and myelomonocytic cells) and those from the adaptive immune system (e.g., Th1 cells, cytotoxic T lymphocytes and B cells) are known to produce IFN-γ [62, 63]. Antitumour potential of INF-γ is well known. Portier et al [64] experimentally demonstrated that IFN-γ inhibited IL-6-dependent MM cell growth and downregulated IL-6R. According to Klein and Bataille [4], IFN-γ downregulates the expression of IL-6R on MM cells and completely inhibits the IL-6-mediated MM cell proliferation. In this respect, some investigators reported that CAM suppressed IFN-γ production or gene expression [29, 33], whereas others [36, 44, 54, 55] (Table 1) presented conflicting results. The conflicting results might have arisen by the use of different materials and methods. Thus, we still cannot draw a definite conclusion about the effect of CAM on IFN-γ.
VEGF/PDGF family
The VEGF/PDGF family belongs to MGFs. VEGF is a multifunctional cytokine that plays an important role in triggering tumour cell migration and angiogenesis. It also possesses a modest proliferative effect on myeloma cells. The production of VEGF in the bone marrow environment is upregulated by myeloma cell adhesion to BMSCs and by IL-6. Moreover, VEGF reciprocally enhances the secretion of IL-6 from BMSCs, suggesting the existence of paracrine interaction between MM cells and BMSCs [65]. PDGF plays a significant role in blood vessel formation, mitogenesis and chemotaxis. PDGF is produced mainly by megakaryocytes and alpha granules of platelets and is released by platelets on activation. It is also produced by other cells such as smooth muscle cells, activated macrophages and endothelial cells. It was shown that PDGF receptor (PDGFR)-α and β were frequently expressed on MM cells of newly diagnosed MM patients, and high PDGFR-β expression at diagnosis was associated with advanced stage of the disease [66]. PDGF-β/PDGFR-β kinase axis plays an important role in angiogenesis and proliferation in MM. The suppressive effect of CAM on VEGF/PDGF [29, 30, 67] (Table 1) might thus be led to MM cell reduction.
TGF-β
TGF-β, a member of MGFs, is a multifunctional cytokine belonging to the TGF superfamily and has pleiotropic biological effect in haematopoiesis and tumourigenesis. It is produced by a large variety of cells including epithelial cells, fibroblasts, eosinophils, macrophages and Treg cell [62]. Urashima et al [68] reported that MM cells secreted more TGF-β1 than splenic B-cells or CD40L pretreated B-cells, and BMSCs of MM produced more TGF-β1 than normal BMSCs [53]. According to Dong and Blobe [69], TGF-β is secreted at higher levels from both MM cells and BMSCs in MM and the increased production of TGF-β correlates well with increased IL-6 and VEGF secretion by BMSCs. Furthermore, neutralising antibody to TGF-β can block increased IL-6 and VEGF secretion, supporting TGF-β as the major inducer for the secretion of IL-6 and VEGF from BMSCs. The suppression of TGF-β by CAM [25, 43, 61] (Table 1) might cause MM cell reduction.
FGF2 (Basic FGF)
FGF2 (also called basic FGF) is a growth factor with angiogenetic properties and represents one of the MGFs [6]. FGF2 is known to be a significant mediator supporting MM cell expansion and survival. According to Mahtouk et al [6], BMSCs are the main source of FGF2, and they demonstrated that FGF2 gene was expressed in MM cells, but its expression was lower than that of BMSCs. The suppression of FGF2 by CAM [29] (Table 1) was noted.
G-CSF
G-CSF, a member of MGFs, is a potent growth factor for MM cells as well as a hematopoietic growth factor with structural homology to IL-6. G-CSF receptor shares some homology with gp130. Both G-CSF and IL-6 induce the activation of nuclear factor for IL-6 (NF-IL-6), which is a transcription factor involved in the synthesis of IL-6 [18]. The suppressive effect of CAM on G-CSF [22] (Table 1) was documented.
GM-CSF
GM-CSF, a member of MGFs, is a hematopoietic growth factor which has a broad impact on haematopoiesis; it stimulates the development and differentiation of committed stem cells to neutrophils, eosinophils and monocytes. According to Zhang et al [17], GM-CSF is a strong stimulator of in vitro myeloma cell proliferation by potentiating the response of myeloma cells to IL-6. The suppression of GM-CSF by CAM [22, 29] (Table 1) might indirectly lead to MM cell reduction.
Matrix metalloproteinases (MMPs)
MMPs play an important role in cell growth, invasion, angiogenesis, metastasis and bone degradation, which are all important events in the pathogenesis of cancer. Van Valckenborgh et al [70] showed the multifunctional role of several MMPs in the development of MM, especially MMP-9, which is closely related to angiogenesis. The suppressive effect of CAM on MMP-9 [29, 30, 61, 71] (Table 1) might be related to MM cell reduction through the downregulation of angiogenesis.
Taken together, CAM can suppress MMP-9 and the following MGFs: IL-1, IL-2, IL-5, IL-6, IL-8, IL-17, TNF-α, PDGF, VEGF, TGF-β, FGF2, G-CSF and GM-CSF (Table 1). Of these MGFs, IL-6 seems to be a crucial factor for MM cell proliferation. Thus, it is probable that the suppression of these MGFs by CAM will cause MM cell reduction. On the contrary, CAM enhanced IL-4 activity [36] (Table 1). However, IL-10, IL-12 and IFN-γ, which are positively or negatively involved in MM cell proliferation, were reported to show the conflicting patterns of response to CAM (Table 1). Thus, it is difficult to draw a definite conclusion on the actual effect of CAM on these cytokines. The effects of CAM on other MGFs (IL-16, IL-22, IL-23, IGF1, HGF, MIPα, GDF-15, PTN, BDNF, CNTF, OSM, LIF, CCLF1, BAFF, APRIL, AREG, NRG, HB-EGF, Wnt family members, Jagged family members and other suppressive cytokines against MM [e.g., IL-27]) have not yet been investigated.
CAM-monotherapy for MM
The first report on the use of CAM in MM treatment dates back to 1997. Durie et al [72] pioneered a unique trial of CAM monotherapy to treat active MM. In their trial, 500 mg of CAM was administered to the patients twice a day approximately for 1 year as the longest follow-up period. They reported that greater than 50% response rate was obtained. However, several responding patients in their study were on concurrent antimyeloma agents that include steroids. Moreau et al [73] reported that no response was achieved in 35 MM patients receiving CAM, at 500 mg, twice a day for 4–20 weeks (median 8 weeks) without chemotherapy or steroids; MM progressed in 80% of cases, requiring other therapeutic approaches. Musto et al [74] reported that among 38 evaluable patients with relapsed or refractory MM (RRMM), who were treated with CAM, at 500 mg, twice a day for 12 weeks, an objective response or minor response (MR) was obtained only in one and two patients, respectively. On the other hand, Stewart et al [75] reported no significant response among 20 evaluable MM patients at various phases of the disease treated with CAM alone. CAM monotherapy seems to have no effect on treating MM.
Low-dose thalidomide and low-dose Dex combined with CAM (Biaxin®) (BLTd) for MM treatment
On contrary to the low efficacy of CAM-monotherapy, CAM has added value when combined with thalidomide or its analogues and Dex. Coleman et al [76] pioneered low-dose thalidomide and low-dose Dex combined with CAM (BLTd) to treat previously untreated or treated MM as well as Waldenström’s macroglobulinemia and succeeded in achieving a high overall response rate (ORR) of 93%. Niesvizky et al [77] carried out a prospective, randomised trial which compared the safety and efficacy of standard pulsed Dex to those of low-dose thalidomide and low-dose Dex (LTd). A total of 23 advanced MM patients were randomised to either pulsed D or LTd. The patients given pulsed Dex displayed almost similar drops in paraprotein at 8 weeks of treatment as the LTd patients (median: 45% versus 52%). Nine patients (D=3, LTd=6) showed a <50% response (median 26%, SD 15.9) to induction therapy after 8 weeks. Then, Niesvizky et al [77] added CAM to the pulsed Dex or LTd treatments, and they showed that the addition of CAM produced further tumour mass reduction at 16 and 20 weeks of treatment. Overall response rate (>50% response in paraprotein) for pulsed Dex and LTd patients was 80%. They suggested that the addition of CAM contributes to tumour mass reduction by potentiating Dex’s effect. Morris et al [45] also reported ORR of 96% in RRMM patients when they received BLTd.
Lenalidomide (Len: Revlimid®) and Dex/low-dose Dex combined with CAM (BiRD/BiRd) or pomalidomide (Pom) and low-dose Dex combined with CAM (Clapd) for MM treatments
Len is one of the immunomodulatory drugs (IMiDs) with a structural and functional analogue of thalidomide [78, 79]. In 2008, Niesvizky et al [80] first reported the BiRD regimen for the treatment of naive symptomatic MM. The addition of CAM allowed a significant reduction of Dex, and an objective response rate of 90.3% was achieved. A combined stringent and conventional CR rate of 38.9% was achieved, and 73.6% of the patients achieved at least a 90% decrease in M-protein levels. Gay et al [81] performed a case-matched study and compared the efficacy and toxicity of BiRd versus Len combined with low-dose Dex (Rd) for newly diagnosed MM. BiRd regimen, compared to Rd regimen, showed increased CR rates (45.8% versus 13.8%), very good partial response (VGPR) rates (73.6% versus 33.3%) and a longer progression-free survival (PFS) (median: 48.3 months versus 27.5 months). The superiority of BiRd regimen was thus suggested. Rossi et al [82] reported that 72 previously untreated MM patients who were treated with BiRd achieved ORR of 93% (VGPR or better of 68%), and median progression-free survival (PFS) was 49 months. They demonstrated that BiRd was highly effective.
Pomalidomide (Pom) is a distinct immunomodulatory agent similar to Len. Mark et al [83] reported that Clapd proved to be effective for treating relapsed or refractory MM (RRMM). In their study, 117 patients with heavily pretreated RRMM including prior Len-treated RRMM were analysed. ORR (≧PR) and clinical benefit response rates (≧MR) were 60% and 67%, respectively. They concluded that Clapd is a highly effective and tolerable regimen for heavily treated RRMM.
Steroid-enhancing/sparing effect of CAM
The role of CAM in BiRd, BLTd and Clapd regimen has been attributed to its steroid-enhancing/sparing effect [3, 76, 77, 80, 81, 82, 84]. It is well known that CAM is a potent CYP3A4 inhibitor [3, 84]. CAM added in BiRd, BLTD and Clapd regimen slows the hepatic clearance of Dex, leading to the augmentation of Dex responsiveness to MM cells [3]. Fost et al [85] investigated the inhibitory effect of CAM on the elimination of methyl-prednisolone (methyl-PSL) and prednisolone (PSL) in six patients with asthma. In their study, the patients received a single oral dose of methyl-PSL (40 mg/1.73 m2) or PSL (40 mg/1.73 m2) before and post administration of 7 days of CAM (500 mg twice a day). Pre- and post-CAM pharmacokinetic profiles of the two steroids showed a 65% reduction in clearance of methyl-PSL but no significant reduction in clearance of PSL. The interactions of CAM with methyl-PSL and PSL were found to be different. To the best of the authors’ knowledge, studies directly evaluating the effect of CAM on Dex metabolism have not been reported.
Reversibility of drug resistance by CAM
Juge-Morineau et al [86] demonstrated that the gp130 family cytokines, such as IL-6, LIF and OSM, inhibit the antiproliferative effect of Dex on human MM cells. Yang and Lin [87] reviewed the mechanisms of resistance against Dex in MM. According to them, IL-6 induces drug resistance against Dex by stimulating JAK/STAT to BCL-XL/MCL1 transduction cascade and PI3K/AKT transduction cascade, consequently leading to the upregulation of antiapoptotic signalling [88, 89, 90]. It is noteworthy that IGF-1, a member of MGFs, is also involved in the development of drug resistance against current standard-of-care agents for MM [91]. MM cells express IGF-I receptor, and its stimulation activates two distinct signal transduction cascades (PI3K/Akt and MEK/ERK cascades), leading to the proliferation of MM cells as well as protection against apoptosis [91, 92]. As far as we know, no studies demonstrating the effect of CAM on IGF-1 have been done. Since CAM can potently suppress IL-6, the reversibility of drug resistance including steroid resistance by CAM in MM treatments seems more important than the CYP3A4-related steroid-sparing/enhancing effect thus far reported.
Specific character of MM as a monoclonal immunoglobulin (Ig)-producing neoplasm
Since MM is characterised by the uncontrolled cell growth of monoclonal immunoglobulin-producing neoplastic plasma cells, the large quantities of unfolded or misfolded Ig production itself trigger endoplasmic reticulum stress (ER stress) which is followed by the unfolded protein response (UPR) [93, 94, 95, 96]. According to Obeng et al [95], MM cells are inherently sensitive to proteasome inhibitors because of their large volume of Ig production which requires the expression of physiologic UPR genes. If the ER stress is severe or prolonged, UPR activation leads to cell-cyclic arrest and the induction of apoptosis of MM cells. Thus, it is probable that MM is a specific neoplasm susceptible to proteasome-, autophagy- and histone deacetylase 6 (HDAC6)-inhibitors. In this study, the bone marrow of MM patients obtained before chemotherapy showed that some of the myeloma cells were already under ER stress at the electron microscopic level (Figure 1).
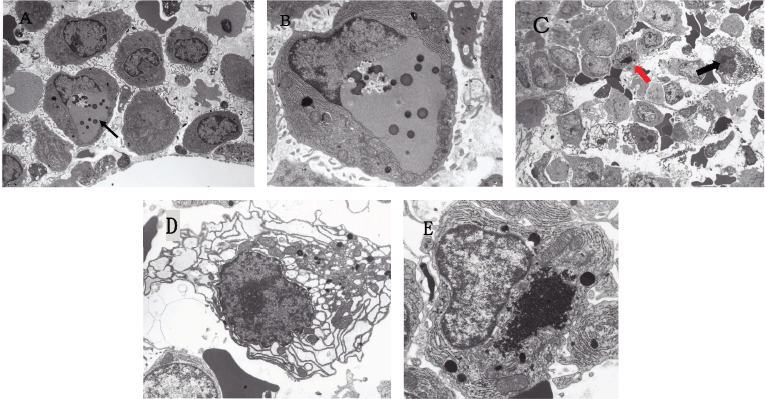
Figure 1. Electron micrographs of MM cells reflecting ER stress. (A) Low power view of the bone marrow (IgA-κ type MM) obtained before the initiation of chemotherapy. An arrow indicates a myeloma cell with an autophagosome. Original magnification ×2,000. (B) Enlarged view of a myeloma cell indicated by an arrow in (A) showing a well-developed autophagosome formed in perinuclear space. Several dense bodies (lysosomes) are scattered in the autophagosome. Original magnification ×5,000. (C) Low power view of the bone marrow (Bence Jones-λ type MM) obtained before the initiation of chemotherapy. A black arrow indicates a myeloma cell showing dilated RER, and a red arrow indicates a myeloma cell with aggresome. Original magnification ×1,000. (D) Enlarged view of a myeloma cell indicated by a black arrow in (C) showing remarkably dilated RER in the cytoplasm, suggesting RER-stress. Original magnification ×4,000. (E) Enlarged view of a myeloma cell indicated by a red arrow in (C) showing the well-developed aggresome formation in the cytoplasm. Original magnification ×4,000.
Role of CAM as a potent autophagy inhibitor
It is well known that CAM is a potent and continuous inhibitor of autophagy. Nakamura et al [97] analysed the direct effect of CAM on MM cells in vitro. They demonstrated that CAM attenuated autophagy by blocking the late phase of the autophagic process, probably after the fusion of autophagosomes with lysosomes at clinically relevant concentrations (6–50 μg/mL). In other words, CAM halts the autophagy process and induces the inhibition of MM cell growth. Thus, CAM can serve as a potential adjuvant for MM treatment modalities, where autophagy is used by the tumour as an escape mechanism from apoptosis.
Synergistic effect of CAM with proteasome inhibitor or histone deacetylase 6 (HDAC6) inhibitor
Bortezomib (Bor, Velcade®), the first-in-class proteasome inhibitor of the 26S proteasome, was initially approved for the treatment of patients with RRMM as a single agent [98]. Bor is now widely used in combination regimens: Bor, thalidomide and low-dose Dex (VTd) [99], Bor and low-dose Dex (Vd) [100], Bor, Len and low-dose Dex (VRd) [101], Bor, cyclophosphamide and low dose Dex (VCd) [102], Bor, melphalan and prednisolone (VMP) [103], daratumumab, Bor and low-dose Dex (DVd) [104] or pomalidomide, Bor and low-dose Dex (PVd) [105] in RRMM and newly diagnosed MM. The effect of Bor was reported due to its blocking of the ubiquitin-proteasome system, leading to the accumulation of unfolded or misfolded protein in the ER in myeloma cells. This results in ER stress followed by a coordinated cellular response known as UPR [93, 94, 95]. UPR is known to induce activation of the chaperone protein GRP-78 (Bip: binding immunoglobulin protein) to maintain ER integrity, and it also upregulates the transcription factor CHOP (i.e., C/EBP homologous protein) to mediate cell death when ER stress is beyond the tolerance of the cell adaptation [95]. The combined inhibition of the ubiquitin (Ub)-proteasome system by Bor and autophagy-lysosome system by CAM synergistically activates UPR, resulting in MM cell apoptosis. Moriya et al [93] experimentally demonstrated that combined treatment of CAM and Bor resulted in increased cytotoxicity as compared to the use of Bor alone. The effectiveness of this combined treatment with CAM, Len and Bor was recently demonstrated at the clinical level [106]. The apoptosis-inducing effect was further enhanced when vorinostat (HDAC6 inhibitor), which is known to inhibit aggresome formation, was added to Bor and CAM [94] (Figure 2). However, no clinical studies have been done using this combination.
Carfilzomib (Kyprolis®) is a selective and irreversible epoxyketone proteasome inhibitor of the 20S proteasome. Recently, carfilzomib, Len and low-dose Dex (KRd) [107] combined with CAM (KRd-CAM) were also found to be effective for treating refractory MM (unpublished data).
Safety of long-term administration of CAM
Antibacterial treatment with CAM is usually recommended for a duration of 1–2 weeks. Safety of the long-term administration of CAM has been proved [2, 108]. However, there remains a concern regarding the development of resistance of Streptococcus pneumoniae against CAM due to the long-term administration of CAM. Recently, concerns have been raised on the long-term effect regarding the risk of cardiovascular events and mortality in both patients with stable coronary heart disease and patients without heart disease, following the short-term use of CAM (e.g., daily for 2 weeks) [3].
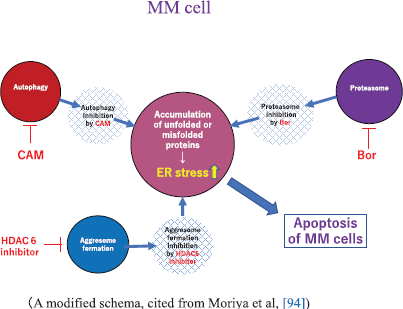
Figure 2. A proposed schema showing apoptosis of MM cells by autophagy, proteasome and aggresome-inhibitors. A modified schema, cited from Moriya et al, [94]. Abbreviations: Bor, bortezomib; CAM, clarithromycin; ER, endoplasmic reticulum; HDAC6, histone deacetylase 6; MM, multiple myeloma.
Adverse effects of CAM
The frequent and common adverse effects of CAM are diarrhoea, nausea, abnormal taste, dyspepsia, abdominal pain/discomfort, headache, insomnia, tooth discolouration, smell loss and taste loss. These adverse reactions are usually mild in intensity and resolve after the discontinuation of treatment. All other adverse reactions are uncommon or rare [3]. However, in post-marketing surveillance, allergic reactions ranging from urticaria and mild skin eruptions to rare cases of anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrosis, liver dysfunction, somnolence and confusion have occurred [https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050662s044s050,50698s026s030,050775s015s019lbl.pdf]. In particular, CAM is also known to prolong QT interval. Rarely, it can be fatal, leading to fatal ventricular arrhythmias, including ventricular tachycardia and torsades de pointes [109]. Thus, CAM therapy should not be given to the patients with a history of QT prolongation and arrhythmia. Since CAM inhibits CYP3A4, the coadministration of CAM with some drugs metabolised by CYP3A4 should be carefully done.
Indirect effects of CAM against virus infections
Recently, the World Health Organisation (WHO) has declared the new coronavirus (SARS-CoV-2) outbreak a pandemic. Several repurposing drugs (e.g., remdesivir, favipiravir, lopinavir·ritonavir, chloroquine, nafamostat, ciclesonide, tocilizumab and ivermectin) possibly effective for treating the disease (COVID-19) caused by SARS-CoV-2 have been tested. However, no definite drugs for treating COVID-19 have yet been established after the declaration by the WHO of the pandemic. Recently, the FDA of the United States of America authorised the emergency use of remdesivir for treating COVID-19.
In general, antibiotics are known to be ineffective against virus infection. Shinahara et al [110] treated 195 influenza-A-infected children with oseltamivir (OSV) and zanamivir (ZNV) with or without CAM. They demonstrated that the combination of CAM plus OSV or ZNV boosted and restored the production of mucosal secretory IgA (S-IgA) and systemic IgG. Furthermore, they reported that CAM supplementation reduced the reinfection rate in the subsequent year in the patients treated with OSV and ZNV. They suggested the possibility that CAM enhanced influenza virus-specific S-IgA production through the induction of IgA class switching recombination.
It is interesting to note that hydroxychloroquine treatment combined with azithromycin, 15-membered macrolide, reinforced viral load reduction/disappearance in COVID-19 patients [111]. Just recently, Millán-Oñate et al [112] first reported that a 34-year-old Columbian man with COVID-19 pneumonia was successfully recovered after receiving chloroquine and CAM. Emerging data suggest that many patients with COVID-19 may die due to an excessive response of their immune system, characterised by the abnormal release of circulating cytokines including IL-1β, IL-6, IL-12, IL-18, TNF-α, TGF-β, GM-CSF, IFN-γ and various chemokines. This phenomenon is referred to as cytokine release syndrome (CRS), and a crucial role seems to be played by IL-6. CRS plays a major role in the deterioration of COVID-19 patients with pneumonia to acute respiratory distress syndrome, cumulating in systemic inflammation and multiorgan failure [113]. In addition, clarithromycin is also known to be effective for treating organising pneumonia [114]. Cai et al [28] demonstrated that spontaneous and lipopolysaccharide-stimulated alveolar macrophages from patients with BOOP upregulated the production of TNF-α, soluble TNF receptor 1/2, IL-1β, IL-6, IL-8, IL-10 and chemokine ligand 18. They suggested that CAM significantly attenuated or inhibited the production of these cytokines. Considering the pleiotropic immunomodulative effects of CAM, the suppressive effects of CAM on cytokines related to CRS, particularly on IL-6, should be noted. The administration of CAM as a single agent or combined with other drugs might be worth a try for treating COVID-19. One trial of CAM against COVID-19 has already started in Greece in May, 2020 (https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001882-36/GR).
Conclusion
We reviewed the current preclinical, clinical and experimental evidence for supporting the efficacy of CAM for treating MM, especially when it is combined with IMiDs, steroids or proteasome inhibitors. IMiDs, steroids, proteasome inhibitors and CAM might synergistically play an important role in inducing apoptosis of MM cells. In addition, the reversibility of drug resistance by CAM to chemotherapeutic drugs including steroids seems to be more important than steroid-sparing/enhancing effect thus far reported in MM treatments. Since multiple myeloma is characterised by the uncontrolled cell growth of monoclonal Ig-producing neoplastic plasma cells, the large quantities of unfolded or misfolded immunoglobulin production itself may trigger ER stress. Thus, MM is a specific neoplasm particularly susceptible to proteasome, autophagy and HDAC6 inhibitors. The development of CAM analogues specific for more potent immunomodulatory effects may provide a novel approach to MM treatment in the future.
In addition, considering the pleiotropic effects of CAM on cytokines including IL-6 and its indirect antiviral effects, CAM might be worth a try for treating COVID-19.
Abbreviations
• AP-1: activator protein
• APRIL: a proliferation-inducing ligand
• AREG: amphiregulin
• BAFF: B-cell activating factor
• bFGF: basic fibroblast growth factor
• EGF: epidermal growth factor
• BCL-xL: B-cell lymphoma-extralarge
• Bip: chaperone protein GRP-78
• BiRd: Len and low-dose Dex combined with CAM
• BDNF: brain-derived neurotrophic factor
• BMSCs: bone marrow stromal cells
• BLTd: low-dose thalidomide and low-dose DE combined with CAM
• BOOP: bronchiolitis obliterans organising pneumonia
• Boor: bortezomib
• CAM: clarithromycin
• CCLF1: cardiotrophin-like cytokine factor 1
• CHOP: C/EBP homologous protein
• Clapd: Pom and low-dose Dex combined with CAM
• CNTF: ciliary neurotrophic factor
• CR: complete response
• Dex: dexamethasone
• ER: endoplasmic reticulum
• G-CSF: granulocyte colony-stimulating factor
• GDF15: growth/differentiation factor15
• GM-CSF: granulocyte macrophage colony-stimulating factor
• gp130: glycoprotein 130
• HB-EGF: heparin-binding EGF-like growth factor
• HDAC6: histone deacetylase 6
• HGF: hepatocyte growth factor
• Ig: immunoglobulin
• IGF-1: insulinlike growth factor-1
• ICAM-1: intercellular adhesion molecule-1
• IL: interleukin
• IL-6R: IL-6-receptor
• IMiDs: immunomodulatory drugs
• IFN-γ: interferon-γ
• JAK/STAT: Janus kinases/signal transducer-activator of transcription
• KRd : Kyprolis®, Len and low-dose Dex
• Len: lenalidomide
• LFA-1: lymphocyte function-associated antigen-1
• LIF: leukaemia inhibitory factor
• LTD: Len, low-dose thalidomide and Dex
• MCL-1: myeloid cell leukaemia sequence-1
• MGF: myeloma growth factor
• MIPα: macrophage inflammatory protein α
• MM: multiple myeloma
• MMP: matrix metalloproteinase
• MR: minor response
• MUC-1: muchin-1
• NF-IL-6: nuclear factor for IL-6
• NF-κB: nuclear factor-κB
• NRG1~4: neureglin1~4
• NK: natural killer
• NKT: natural killer T
• ORR: overall response rate
• OSM: oncostatin M
• PFS: progression-free survival
• PI3K/AKT: phosphatidylinositol-3 kinase/AKT kinase
• PDGF: platelet-derived growth factor
• Pom: pomalidomide
• PR: partial response
• PSL: prednisolone
• PTN: pleiotrophin
• Ras/MAPK: GTPase/mitogen-activated protein kinase
• Rd: Len combined with low-dose dex
• RRMM: relapsed or refractory multiple myeloma
• SDF-1: stromal cell-derived factor 1
• S-IgA: secretory immunoglobulin A
• TGF-β: transforming growth factor-β
• TNF-α: tumour necrosis factor-α
• UPR: unfolded protein response
• VEGF: vascular endothelial growth factor
• VCAM-1: vascular cell adhesion molecule-1
• VGPR: very good partial response
• VLA: very late activating antigen
• Wnt5A/10B/16: Wnt family members
Competing interests
All of the authors declare that they have no conflicts of interest.
Funding
This study was not supported by any grant.
References
1. Douthwaite S and Champneys WS (2001) Structures of ketolides and macrolides determine their mode of interaction with the ribosomal target site J Antimicrobe Chemother 48 Topic. T1 1–8 https://doi.org/10.1093/jac/48.suppl_2.1
2. Kanoh S and Rubin BK (2010) Mechanisms of action and clinical application of macrolides as immunomodulatory medications Clin Microbiol Rev 23 590–615 https://doi.org/10.1128/CMR.00078-09 PMID: 20610825 PMCID: 2901655
3. Van Nuffel AMT, Gartung A, and Sulciner ML, et al (2015) Repurposing drugs in oncology (ReDO)---clarithromycin as an anti-cancer agent Ecancermedicalscience 9 513 https://doi.org/10.3332/ecancer.2015.513
4. Klein B and Bataille R (1992) Cytokine network in human multiple myeloma Hematol Oncol Clin North Am 6 273–284 https://doi.org/10.1016/S0889-8588(18)30344-7 PMID: 1582974
5. Klein B (1995) Cytokine, cytokine receptors, transduction signals, and oncogenes in human multiple myeloma Semin Hematol 32 4–19 PMID: 7878477
6. Mahtouk K (2010) Growth factors in multiple myeloma: a comprehensive analysis of their expression in tumor cells and bone marrow environment using Affymetrix microarrays BMC Cancer 10 198 https://doi.org/10.1186/1471-2407-10-198 PMID: 20465808 PMCID: 2882921
7. Musolino C, Allegra A, and Innao V, et al (2017) Inflammatory and anti-inflammatory equilibrium, proliferative and antiproliferative balance: the role of cytokines in multiple myeloma Mediators Inflamm 2017 1852517 https://doi.org/10.1155/2017/1852517 PMID: 29089667 PMCID: 5635476
8. Hamada K, Kita E, and Sawaki M, et al (1995) Antitumor effect of erythromycin in mice Chemotherapy 41 59–69 https://doi.org/10.1159/000239325 PMID: 7875024
9. Mikasa K, Sawaki M, and Kita E, et al (1997) Significant survival benefit to patients with advanced non-small-cell lung cancer from treatment with clarithromycin Chemotherapy 43 288–296 https://doi.org/10.1159/000239580 PMID: 9209786
10. Sakamoto M, Mikasa K, and Hamada K, et al (1998) Effect of clarithromycin treatment of natural killer cell activity in patients with advanced non-small cell lung cancer Gan to Kagaku Ryoho 25 2259–2266
11. Govi S, Dognini GP, and Licata G, et al (2010) Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas: final results of a single-centre phase II trial Br J Amatol 150 226–229
12. Ishimatsu Y, Mukae H, and Matsumoto K, et al (2010) Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin Chest 138 730–733 https://doi.org/10.1378/chest.09-2358 PMID: 20822996
13. Ferreri AJM, Sassone M, and Kiesewetter B, et al (2015) High-dose clarithromycin is an active monotherapy for patients with relapsed/refractory extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT): the HD-K phase II trial Ann Oncol 26 1760–176514 https://doi.org/10.1093/annonc/mdv214 PMID: 25935794
14. Ohe M and Hashino S (2011) A case of follicular B-cell lymphoma treated using clarithromycin Korean J Hematol 46 203–206 https://doi.org/10.5045/kjh.2011.46.3.203 PMID: 22065978 PMCID: 3208206
15. Kiesewetter B, Dolak W, and Mayerhoefer ME, et al (2018) Successful clarithromycin monotherapy in a patient with primary follicular lymphoma of the duodenum Case Rep Oncol 11, 239–245 https://doi.org/10.1159/000488388
16. Takemori N, Kaneko H, and Nakamura M, et al (2012) Complete remission of methotrexate-related Epstein-Barr-virus-associated Hodgkin-like lymphoma following withdrawal of MTX coupled with clarithromycin administration Case Rep Hematol 2012 658745 https://doi.org/10.1155/2012/658745
17. Zhang XG, Bataille R, and Jourdan M, et al (1990) Granulocyte -macrophage colony-stimulating factor synergizes with interleukin-6 in supporting the proliferation of human myeloma cells Blood 76 2599–2605 https://doi.org/10.1182/blood.V76.12.2599.2599 PMID: 2265252
18. Hallek M, Bergsagel PL, and Anderson KC (1998) Multiple myeloma: increasing evidence for a multistep transformation process Blood 91 3–21 https://doi.org/10.1182/blood.V91.1.3 PMID: 9414264 PMCID: 3901996
19. Costes V, Portier M, and Lu ZY, et al (1998) Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production Br J Haematol 103 1152–1160 https://doi.org/10.1046/j.1365-2141.1998.01101.x
20. Xiong Y, Donovan KA, and Kline MP, et al (2006) Identification of two groups of smoldering multiple myeloma patients who are either high or low producers of interleukin-1 J Interferon Cytokine Res 26 83–95 https://doi.org/10.1089/jir.2006.26.83 PMID: 16487028
21. Takeshita K, Yamagishi I, and Harada M, et al (1989) Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages Drugs Exp Clin Res 15 527–533 PMID: 2534682
22. Matsuoka N, Eguchi K, and Kawakami A, et al (1996) Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells Clin Exp Immunol 104 501–508 https://doi.org/10.1046/j.1365-2249.1996.46752.x PMID: 9099936 PMCID: 2200457
23. Morikawa K, Watabe H, and Morikawa S, et al (1996) Modulatory effect of antibiotics on cytokine production by human monocytes in vitro Antimicrob Agents Chemother 40 1366–1370 https://doi.org/10.1128/AAC.40.6.1366 PMID: 8726002 PMCID: 163332
24. Miyanohara T, Ushikai M, and Matsune S, et al (2000) Effects of clarithromycin on cultured human nasal epithelial cells and fibroblasts Laryngoscope 110 126–131 https://doi.org/10.1097/00005537-200001000-00023 PMID: 10646728
25. Čulić O, Eraković V, and Parnham MJ (2001) Anti-inflammatory effects of macrolide antibiotics Eur J Pharmacol 429 209–229 https://doi.org/10.1016/S0014-2999(01)01321-8 PMID: 11698042
26. Fonseca-Aten M, Okada PJ, and Bowlware KL, et al (2006) Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double-blind, randomized, placebo-controlled trial Ann Allergy Asthma Immunol 97 457–463 https://doi.org/10.1016/S1081-1206(10)60935-0
27. Perić A, Vojvodić D, and Jakovljević V, et al (2012) Effects of long-term low-dose treatment by clarithromycin on Th1 cytokine levels in nasal discharge of patients with nasal polyposis Med Flum 48 63–7128
28. Cai M, Bonella F, and Dai H, et al (2013) Macrolides inhibit cytokine production by alveolar macrophages in bronchiolitis obliterans organizing pneumonia Immunobiology 218 930–937 https://doi.org/10.1016/j.imbio.2012.10.014
29. Zeng M, Li Z-Y, and Ma J, et al (2015) Clarithromycin and dexamethasone show similar anti-inflammatory effects on distinct phenotypic chronic rhinosinusitis: an explant model study BMC Immunol 16 37 https://doi.org/10.1186/s12865-015-0096-x PMID: 26047816 PMCID: 4456709
30. Zimmermann P, Ziesenitz VC, and Curtis N, et al (2018) The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms Front Immunol 9 302 https://doi.org/10.3389/fimmu.2018.00302
31. Harrison SJ and Cook G (2005) Immunotherapy in multiple myeloma—possibility or probability? Br J Haematol 130 344–362 https://doi.org/10.1111/j.1365-2141.2005.05534.x PMID: 16042684
32. Morikawa K, Oseko F, and Morikawa S, et al (1994) Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro Antimicrob Agents Chemother 38 2643–2647 https://doi.org/10.1128/AAC.38.11.2643 PMID: 7532933 PMCID: 188255
33. Morikawa K, Zhang J, and Nonaka M, et al (2002) Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production Int J Antimicrob Agents 19 53–59 https://doi.org/10.1016/S0924-8579(01)00457-5 PMID: 11814768
34. Sugiyama K, Shirai R, and Mukae H, et al (2007) Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells Clin Exp Immunol 147 540–546 https://doi.org/10.1111/j.1365-2249.2007.03299.x PMID: 17302905 PMCID: 1810497
35. Herrmann F, Andreeff M, and Gruss HJ, et al (1991) Interleukin-4 inhibits growth of multiple myelomas by suppressing interleukin-6 expression Blood 78 2070–2074 https://doi.org/10.1182/blood.V78.8.2070.2070 PMID: 1912585
36. Hamada K, Mikasa K, and Yunou Y, et al (2000) Adjuvant effect of clarithromycin on chemotherapy for murine lung cancer Chemotherapy 46 49–61 https://doi.org/10.1159/000007256
37. Anderson KC, Jones RM, and Morimoto C, et al (1989) Response patterns of purified myeloma cells to hematopoietic growth factors Blood 73 1915–1924 https://doi.org/10.1182/blood.V73.7.1915.1915 PMID: 2713508
38. Kraft M, Cassell GH, and Pak J, et al (2002) Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin Chest 121 1782–1788 https://doi.org/10.1378/chest.121.6.1782 PMID: 12065339
39. Uchiyama H, Barut BA, and Mohrbacher AF, et al (1993) Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion Blood 82 3712–3720 https://doi.org/10.1182/blood.V82.12.3712.3712 PMID: 8260708
40. Gerlo S, Haegeman G, and Vanden Berghe W (2008) Transcriptional regulation of autocrine IL-6 expression in multiple myeloma cells Cell Signal 20 1489–1496 https://doi.org/10.1016/j.cellsig.2008.04.004 PMID: 18502099
41. Sakamoto M, Mikasa K, and Hamada K, et al (1996) Long-term clarithromycin treatment for cancer cachexia of inoperable non-small cell lung cancer patients Jpn J Chemother 44 879–882
42. Khan AA, Slifer TR, and Araujo FG, et al (1999) Effect of clarithromycin and azithromycin on production of cytokines by human monocytes Int J Antimicrob Agents 11 121–132 https://doi.org/10.1016/S0924-8579(98)00091-0 PMID: 10221415
43. Sassa K, Mizushima Y, and Fujishita T, et al (1999) Therapeutic effect of clarithromycin on a transplanted tumor in rats Antimicrob Agents Chemother 43 67–72 https://doi.org/10.1128/AAC.43.1.67
44. Majima T, Mikasa K, and Sakamoto M, et al (2000) Clarithromycin regulates cytokine mRNA in non-small cell lung cancer Jpn J Chemother 48 780–785
45. Morris TCM, Kettle PJ, and Drake M, et al (2008) Clarithromycin with low dose dexamethasone and thalidomide is effective therapy in relapsed/refractory myeloma Br J Haematol 143 349–354 https://doi.org/10.1111/j.1365-2141.2008.07360.x PMID: 18759764
46. Herrero AB, García-Gómez A, and Garayoa M, et al (2016) Effects of IL-8 up-regulation on cell survival and osteoclastogenesis in multiple myeloma Am J Pathol 186 2171–2182 https://doi.org/10.1016/j.ajpath.2016.04.003 PMID: 27301357
47. Kline M, Donovan K, and Wellik L, et al (2007) Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression Leuk Res 31 591–598 https://doi.org/10.1016/j.leukres.2006.06.012
48. Pellegrino A, Ria R, and Di Pietro G, et al (2005) Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells Br J Haematol 129 248–256 https://doi.org/10.1111/j.1365-2141.2005.05443.x PMID: 15813853
49. Kikuchi T, Hagiwara K, and Honda Y, et al (2002) Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors J Antimicrob Chemother 49 745–755 https://doi.org/10.1093/jac/dkf008 PMID: 12003967
50. Suzuki H, Hagiwara K, and Honda Y, et al (1997) Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells Laryngoscope 107 1661–1666 https://doi.org/10.1097/00005537-199712000-00016 PMID: 9396683
51. Lu ZY, Zhang XG, and Rodriguez C, et al (1995) Interleukin-10 is a proliferation factor but not a differentiation factor for human myeloma cells Blood 85 2521–2527 https://doi.org/10.1182/blood.V85.9.2521.bloodjournal8592521 PMID: 7727780
52. Gu Z-J, Costes V, and Lu ZY, et al (1996) Interleukin-10 is a growth factor for human myeloma cells by induction of an oncostatin M autocrine loop Blood 88 3972–3986 https://doi.org/10.1182/blood.V88.10.3972.bloodjournal88103972 PMID: 8916964
53. Teramoto S et al (1997) Expression of IL-12 mRNA in peripheral blood mononuclear cells from inoperable non-small cell lung cancer patients treated with clarithromycin Jpn J Chemother 45 144–147
54. Majima T, Mikasa K, and Sakamoto M, et al (1999) Changes of cytokine mRNA in peripheral blood mononuclear cells from unresectable non-small cell lung cancer patients before and after clarithromycin therapy Jpn J Chemother 47 345–348
55. Majima T, Mikasa K, and Sakamoto M, et al (2000b) Administration of clarithromycin (CAM) to non-small cell lung cancer Jpn J Antibiot 53(Suppl A) 52–55
56. Airoldi I, Cocco C, and Giuliani N, et al (2008) Constitutive expression of IL-12Rβ2 on human multiple myeloma cells delineates a novel therapeutic target Blood 112 750–759 https://doi.org/10.1182/blood-2008-02-139378 PMID: 18474725 PMCID: 5070715
57. Prabhala RH, Pelluru D, and Fulciniti M, et al (2010) Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma Blood 115 5385–5392 https://doi.org/10.1182/blood-2009-10-246660 PMID: 20395418 PMCID: 2902136
58. Fouka E, Lamprianidou E, and Arvanitidis K, et al (2014) Low-dose clarithromycin therapy modulates Th17 response in non-cystic fibrosis bronchiectasis patients Lung 192 849–855 https://doi.org/10.1007/s00408-014-9619-0 PMID: 25016929
59. Jourdan M, Tarte K, and Legouffe E, et al (1999) Tumor necrosis factor is a survival and proliferation factor for human myeloma cells Eur Cytokine Netw 10 65–70 PMID: 10210775 PMCID: 2025696
60. Hideshima T, Chauhan D, and Schlossman R, et al (2001) The role of tumor necrosis factor α in the pathophysiology of human multiple myeloma: therapeutic applications Oncogene 20 4519–4527 https://doi.org/10.1038/sj.onc.1204623 PMID: 11494147
61. Sassa K, Mizushima Y, and Kobayashi M (1999b) Differential modulatory effects of clarithromycin on the production of cytokines by a tumor Antimicrob Agents Chemother 43 2787–2789 https://doi.org/10.1128/AAC.43.11.2787
62. Akdis M, Burgler S, and Crameri R, et al (2011) Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases J Allergy Clin Immunol 127 701–721 https://doi.org/10.1016/j.jaci.2010.11.050
63. Akdis M, Aab A, and Altunbulakli C, et al (2016) Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases J Allergy Clin Immunol 138 984–1010 https://doi.org/10.1016/j.jaci.2016.06.033 PMID: 27577879
64. Portier M, Zhang XG, and Caron E, et al (1993) γ-Interferon in multiple myeloma: inhibition of interleukin-6 (IL-6)-dependent myeloma cell growth and downregulation of IL-6-receptor expression in vitro Blood 81 3076–3082 https://doi.org/10.1182/blood.V81.11.3076.3076 PMID: 8499642
65. Kalita LK and Saikia B (2016) Recent advances in molecular pathogenesis of multiple myeloma—role of cytokine: review of literature Int J Med Health Res 2 43–45
66. Bilalis A, Pouliou E, and Roussou M, et al (2017) Increased expression of platelet derived growth factor receptor β on trephine biopsies correlates with advanced myeloma JBUON 22 1032–1037
67. Hu S, You X, and Chen C, et al (2014) The expression of VEGF and the regulation of clarithromycin on it in chronic rhinosinusitis with nasal polys Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 28 303–305 PMID: 25185282
68. Urashima M, Ogata A, and Chauhan D, et al (1996) Transforming growth factor-β1: differential effects on multiple myeloma versus normal B cells Blood 87 1928–1938 https://doi.org/10.1182/blood.V87.5.1928.1928 PMID: 8634441
69. Dong M and Blobe GC (2006) Role of transforming growth factor-β in hematologic malignancies Blood 107 4589–4596 https://doi.org/10.1182/blood-2005-10-4169 PMID: 16484590 PMCID: 1895802
70. Van Valckenborgh E, Croucher PI, and De Raeve H, et al (2004) Multifunctional role of matrix metalloproteinases in multiple myeloma—a study in the 5T2MM mouse model Am J Pathol 165 869–878 https://doi.org/10.1016/S0002-9440(10)63349-4 PMID: 15331411 PMCID: 1618595
71. Takahashi E, Indalao IL, and Sawabuchi T, et al (2018) Clarithromycin suppresses induction of monocyte chemoattractant protein-1 and matrix metalloproteinase-9 and improves pathological changes in the lungs and heart of mice infected with influenza A virus Comp Immunol Microbiol Infect Dis 56 6–13 https://doi.org/10.1016/j.cimid.2017.11.002 PMID: 29406285
72. Durie BGM, et al (1997) Clarithromycin (Biaxin®) as primary treatment for myeloma Blood 90(Suppl. 1 Part 1) 579A
73. Moreau P, Huynh A, and Facon T, et al (1999) Lack of efficacy of clarithromycin in advanced multiple myeloma Leukemia 13 490–491 https://doi.org/10.1038/sj.leu.2401332 PMID: 10086745
74. Musto P, Falcone A, and Sanpaolo G, et al (2002) Inefficacy of clarithromycin in advanced multiple myeloma: a definitive report Haematologica 87 658–659 PMID: 12031924
75. Stewart AK, Trudel S, and Al-Berouti BM, et al (1999) Lack of response to short-term use of clarithromycin (Biaxin) in multiple myeloma Blood 93 4441–4449 https://doi.org/10.1182/blood.V93.12.4441 PMID: 10391696
76. Coleman M, Leonard J, and Lyons L, et al (2002) BLT-D (Clarithromycin [Biaxin], low-dose thalidomide, and dexamethasone) for the treatment of myeloma and Waldenström’s macroglobulinemia Leuk Lymphoma 43 1777–1782 https://doi.org/10.1080/1042819021000006303
77. Niesvizky R et al (2003) Dexamethasone alone, or in combination with low-dose thalidomide as induction therapy for advanced multiple myeloma, and the effect of the addition of clarithromycin (BiaxinTM) on response rate. Interim results of a prospective, sequential, randomized trial Blood 102 237a Ember 16 https://doi.org/10.1182/blood-2002-09-2725
78. Knight R (2005) IMiDs: A novel class of immunomodulators Semen Oncol 32(Suppl. 5) S24–S30 https://doi.org/10.1053/j.seminoncol.2005.06.018
79. Vallet S, Palumbo A, and Raje N, et al (2008) Thalidomide and lenalidomide: mechanism-based potential drug combinations Leuk Lymphoma 49 1238–1245 https://doi.org/10.1080/10428190802005191 PMID: 18452080
80. Niesvizky R, Jayabalan DS, and Christos PJ, et al (2008) BiRD (Biaxin[clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete-and overall-response rates in treatment-naive symptomatic multiple myeloma Blood 111 1101–1109 https://doi.org/10.1182/blood-2007-05-090258
81. Gay F, Rajkumar SV, and Coleman M, et al (2010) Clarithromycin (Biaxin)-lenalidomide-low-dose dexamethasone (BiRd) versus lenalidomide-low-dose dexamethasone (Rd) for newly diagnosed myeloma Am J Hematol 85 664–669 https://doi.org/10.1002/ajh.21777 PMID: 20645430 PMCID: 3956597
82. Rossi A, Mark T, and Jayabalan D, et al (2013) BiRd (clarithromycin, lenalidomide, dexamethasone): an update on long-term lenalidomide therapy in previously untreated patients with multiple myeloma Blood 121 1982–1985 https://doi.org/10.1182/blood-2012-08-448563 PMID: 23299315 PMCID: 3596960
83. Mark TM, Boyer A, and Yadlapati S, et al (2015) Clapd (clarithromycin, pomalidomide, dexamethasone) therapy in relapsed or refractory multiple myeloma overcomes negative prognostic impact of adverse cytogenetics and prior resistance to lenalidomide and bortezomib Blood 126 https://doi.org/10.1182/blood.V126.23.4232.4232
84. Ghosh N, Tucker N, and Zahurak M, et al (2014) Clarithromycin overcomes resistance to lenalidomide and dexamethasone in multiple myeloma Am J Hematol 89 E116–E120. https://doi.org/10.1002/ajh.23733 PMID: 24723438 PMCID: 4390045
85. Fost DA, Leung DY, and Martin RJ, et al (1999) Inhibition of methylprednisolone elimination in the presence of clarithromycin therapy J Allergy Clin Immunol 103 1031–1035 https://doi.org/10.1016/S0091-6749(99)70175-2 PMID: 10359882
86. Juge-Morineau N, François S, and Puthier D, et al (1995) The gp 130 family cytokines IL-6, LIF and OSM but not IL-11 can reverse the anti-proliferative effect of dexamethasone on human myeloma cells Br J Haematol 90 707–710 https://doi.org/10.1111/j.1365-2141.1995.tb05605.x PMID: 7647014
87. Yang W-C and Lin S-F (2015) Mechanisms of drug resistance in relapse and refractory multiple myeloma Biomed Res Int 2015 https://doi.org/10.1155/2015/341430
88. Catlett-Falcone R, Landowski TH, and Oshiro MM, et al (1999) Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells Immunity 10 105–115 https://doi.org/10.1016/S1074-7613(00)80011-4 PMID: 10023775
89. Dalton WS and Jove R (1999) Drug resistance in multiple myeloma: approaches to circumvention Semen Oncol 26(Suppl 13) 23–27
90. Wei L-H, Kuo ML, and Chen CA, et al (2001) The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway Oncogene 20 5799–5809 https://doi.org/10.1038/sj.onc.1204733 PMID: 11593385
91. Bieghs L, Johnsen HE, and Maes K, et al (2016) The insulin-like growth factor system in multiple myeloma: diagnostic and therapeutic potential Oncotarget 7 48732–48752 https://doi.org/10.18632/oncotarget.8982 PMID: 27129151 PMCID: 5217049
92. Ge NL and Rudikoff S (2000) Insulin-like growth factor I am a dual effector of multiple myeloma cell growth Blood 96 2856–2861 https://doi.org/10.1182/blood.V96.8.2856 PMID: 11023522
93. Moriya S, Che XF, and Komatsu SI, et al (2013) Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells Int J Oncol 42 1541–1550 https://doi.org/10.3892/ijo.2013.1870 PMID: 23546223 PMCID: 3661227
94. Moriya S, Komatsu S, and Yamasaki K, et al (2015) Targeting the integrated networks of aggresome formation, proteasome, and autophagy potentiates ER stress-mediated cell death in multiple myeloma cells Int J Oncol 46 474–486 https://doi.org/10.3892/ijo.2014.2773 PMCID: 4277245
95. Obeng EA, Carlson LM, and Gutman DM, et al (2006) Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells Blood 107 4907–4916 https://doi.org/10.1182/blood-2005-08-3531 PMID: 16507771 PMCID: 1895817
96. Verfaillie T, Salazar M, and Velasco G, et al (2010) Linking ER stress to autophagy: potential implications for cancer therapy Int J Cell Biol 2010 930509 https://doi.org/10.1155/2010/930509 PMID: 20145727 PMCID: 2817393
97. Nakamura M, Kikukawa Y, and Takeya M, et al (2010) Clarithromycin attenuates autophagy in myeloma cells Int J Oncol 37 815–820 PMID: 20811702
98. Richardson PG, Barlogie B, and Berenson J, et al (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma N Engl J Med 348 2609–2617 https://doi.org/10.1056/NEJMoa030288 PMID: 12826635
99. Wang LM, Weber DM, and Delasalle KB, et al (2004) VTD (velcade, thalidomide, dexamethasone) as primary therapy for newly-diagnosed multiple myeloma Blood 104 210 https://doi.org/10.1182/blood.V104.11.210.210
100. Harousseau JL, Attal M, and Avet-Loiseau H, et al (2010) Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial J Clin Oncol 28 4621–4629 https://doi.org/10.1200/JCO.2009.27.9158 PMID: 20823406
101. Richardson PG, Weller E, and Lonial S, et al (2010) Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma Blood 116 679–686 https://doi.org/10.1182/blood-2010-02-268862 PMID: 20385792 PMCID: 3324254
102. Zepeda VHJ, Duggan P, and Neri PE, et al (2014) Cyclophosphamide, bortezomib and dexamethasone (CyBORD) is a feasible and active regimen for non-transplant eligible multiple myeloma patients Blood 124 5751 https://doi.org/10.1182/blood.V124.21.5751.5751
103. Herbaux C, Joao CM, and Plocque A, et al (2014) Subcutaneous bortezomib, melphalan and prednisone in elderly newly diagnosed multiple myeloma patients Blood 124, 3481. https://doi.org/10.1182/blood.V124.21.3481.3481
104. Palumbo A, Chanan-Khan A, and Weisel K, et al (2016) Daratumumab, bortezomib, and dexamethasone for multiple myeloma N Engl J Med 375 754–766 https://doi.org/10.1056/NEJMoa1606038 PMID: 27557302
105. Paludo J, Mikhael JR, and LaPlant BR, et al (2017) Pomalidomide, bortezomib, and dexamethasone for patients with relapsed lenalidomide-refractory multiple myeloma Blood 130 1198–1204 https://doi.org/10.1182/blood-2017-05-782961 PMID: 28684537 PMCID: 5606008
106. Takemori N, Imai G, and Hoshino K, et al (2018) A novel combination of bortezomib, lenalidomide, and clarithromycin produced stringent complete response in refractory multiple myeloma complicated with diabetes mellitus—clinical significance and possible mechanisms: a case report J Med Case Rep 12 40 https://doi.org/10.1186/s13256-017-1550-6
107. Dimopoulos MA, Stewart AK, and Masszi T, et al (2017) Carfilzomib-lenalidomide-dexamethasone vs lenalidomide-dexamethasone in relapsed multiple myeloma by previous treatment Blood Cancer J 7 e554 https://doi.org/10.1038/bcj.2017.31 PMID: 28430175 PMCID: 5436074
108. Kadota J, Mukae H, and Ishii H, et al (2003) Long-term efficacy and safety of clarithromycin treatment in patients with diffuse pan bronchiolitis Respir Med 97 844–850 https://doi.org/10.1016/S0954-6111(03)00042-8 PMID: 12854636
109. Owens RC and Nolin TD (2006) Antimicrobial-associated QT interval prolongation: pointes of interest Clin Infect Dis 43, 1603-1611 https://doi.org/10.1086/508873 PMID: 17109296
110. Shinahara W, Takahashi E, and Sawabuchi T, et al (2013) Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis PLoS One 8 e70060 https://doi.org/10.1371/journal.pone.0070060 PMID: 23875018 PMCID: 3714257
111. Gautret P, Lagier J-C, and Parola P, et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Int J Antimicrob Agents 56(1) 105949 https://doi.org/10.1016/j.ijantimicag.2020.105949 PMCID: 7102549
112. Millán-Oñate J, Millan W, and Mendoza LA, et al (2020) Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin Ann Clin Microbiol Antimicrob 19 16 https://doi.org/10.1186/s12941-020-00358-y PMID: 32331519 PMCID: 7180682
113. Sun X, Wang T, and Cai D, et al (2020) Cytokine storm intervention in the early stages of COVID-19 pneumonia Cytokine Growth Factor Rev 53 38–42 https://doi.org/10.1016/j.cytogfr.2020.04.002 PMID: 32360420 PMCID: 7182527
114. Pathak V, Kuhn JM, and Durham C, et al (2014) Macrolide use leads to clinical and radiological improvement in patients with cryptogenic organizing pneumonia Ann Am Thorac Soc 11 87–91 https://doi.org/10.1513/AnnalsATS.201308-261CR PMID: 24460438






