Targeted literature review of the global burden of gastric cancer
Montserrat Casamayor1, Robert Morlock2, Hiroshi Maeda2 and Jaffer Ajani3
1IQVIA, C/Provença 392-3ª, 08025 Barcelona, Spain
2Astellas Pharma Global Development, Inc., 1 Astellas Way, Northbrook, IL 60062 USA
3University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030 USA
Correspondence to: Robert Morlock. Email: robert.morlock.contractor@astellas.com
Abstract
Gastric cancer (GC) and gastroesophageal junction cancers (GEJCs) are the third leading cause of cancer-related death worldwide. Although several studies have evaluated the epidemiology and management of GC and GEJC, to our knowledge, no global estimates of the economic burden of GC and GEJC have yet been reported. This targeted literature review was conducted to summarise the epidemiology and management of GC and GEJC and to estimate its global economic and humanistic burden.
The incidence of GC and GEJC is highest in Eastern Asia, several South and Central American countries and Central and Eastern Europe and lowest in North America and Africa. Prognosis is generally poor; the global 5-year survival rate is 5%–10% in advanced stages. Patients with GC and GEJC have more severe symptoms compared with patients with other cancers, and health-related quality of life (HRQoL) worsens as the disease progresses. Given the rapid progression of GC and GEJC at advanced stages, chemotherapy, despite its toxicity, improves HRQoL compared with best supportive care.
The costs of GC/GEJC are generally higher than for other cancers; in the US, the average annual cost per patient between 1998 and 2003 was 46,501 USD, compared with 29,609 USD and 35,672 USD for colorectal and lung cancer, respectively. Based on the 2012 incidence data and average costs per patient, estimates of the annual financial burden of GC and GEJC revealed great regional differences. Japan and Iran had the highest (8,492 million USD) and lowest (27 million USD) costs for 2017, respectively, while the estimate for the US was 3,171 million USD. The overall annual cost of GC and GEJC estimated for 2017 in a geographic area including Europe (France, Germany, Italy, Spain and the UK), Asia (Iran, Japan and China), North America (Canada and the US) and Australia was 20.6 billion USD.
Keywords: gastric cancer, gastroesophageal junction, humanistic, economic, burden, global
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 26/11/2018; Received: 06/06/2018
Background
Gastric cancer (GC) is an aggressive cancer that develops in the stomach and represents one of the leading overall causes of deaths worldwide, ranking fifth in cancer incidence and third in cancer-related deaths [1]. GC can develop in any part of the stomach; however, the majority of GCs are located in the pyloric area. Gastroesophageal junction cancers (GEJCs) develop in the gastroesophageal junction (GEJ) and can be classified as GC or oesophageal cancer depending on their extension in the stomach [2]. The American Joint Committee on Cancer considers all GEJCs to be oesophageal unless they arise in the stomach >5 cm from the GEJ [2]; however, GEJCs are often referred to as GC in the literature.
It is estimated that 80%–90% of GCs are adenocarcinomas and can be classified based on their histological characteristics as differentiated (intestinal) or undifferentiated (diffuse) type (Lauren classification) [3]. The differentiated type has a relative occurrence of approximately 54%, is more common in elderly males, and develops slowly, whereas the diffuse type is more common in females of a younger age and has a worse prognosis [4]. Similar to GC, most GEJCs (90%) are also adenocarcinomas while the remainder are classified as either squamous cell carcinomas or unspecified carcinomas [5].
Worldwide, the incidence of GC varies widely. The highest age standardised-incidence rate (ASIR) per 100,000 individuals is observed in Eastern Asia, particularly in China, Mongolia, Japan and Republic of Korea [6] (35.4 males; 13.8 females), Central and Eastern Europe (20.3 males; 8.9 females) and South America (14.2 males; 7.0 females), particularly concentrated in the Pacific regions of South America [7, 8]. The lowest incidence is observed in North America (5.5 males; 2.8 females) and Africa (4.5 males; 3.2 females) [7]. The global incidence has declined over the last decades, and estimates up to the year 2030 represent a projected 2.3% annual decrease [6].
Early stages of GC typically present with minimal or no symptoms; therefore, GC is frequently diagnosed at advanced stages, resulting in poor prognosis [9]. This is particularly evident in Western countries where, due to the low incidence of GC, awareness is low and screening programmes are not established [9]. In contrast, in some Asian countries (i.e. Republic of Korea and Japan) where the incidence is higher and where mass screening programmes are cost-effective and widely available, early detection occurs more frequently [9–11]. This evidence suggests that early diagnosis represents an important strategy to improve outcome and survival.
Several genetic factors, as well as age, sex, family history, radiation exposure, Helicobacter pylori (H. pylori) infection and smoking, were all shown to be associated with an increased risk of GC [6, 12–14] and it is likely that differences in the geographical distribution of risk factors may be partly responsible for the observed variation in incidence [6]. Among the potential prognostic and therapeutic biomarkers for GC, HER2 is the only biomarker currently screened in clinical practice and is used to identify patients who will respond to trastuzumab; overexpression of HER2 occurs in 9%–38% of GC and appears to impact gastric carcinogenesis [15]. Another potential therapeutic biomarker is PD-L1/2 (ligands of programmed death receptor 1 [PD-1]). Since its expression is elevated in Epstein-Barr virus (EBV)-positive tumours, PD-L1/2 antagonists may be effective in the treatment of EBV-related GCs. However, the validity of PD-L1/2 as a predictive biomarker has not been fully established [15]. Exploratory biomarkers include Claudin 18.2 [16], tumour mutation burden, including microsatellite instability [15], and gamma interferon [17]. Further understanding and identification of predictive biomarkers for GC will help improve outcomes.
Despite the increased awareness of GC and the growing effort to develop new therapies, and although several studies have investigated the economic cost of GC [18–45], to our knowledge, no global estimates of the economic burden of GC and GEJC had been reported at the time we began writing this manuscript. This work summarises the current landscape of GC and GEJC described in the literature, provides an overview of its epidemiology and management and estimates its global economic and humanistic burden.
Methods
To evaluate the burden associated with GC and GEJC, four targeted literature reviews were conducted to identify the following outcomes in 1) Epidemiology: incidence, prevalence, morbidity, mortality, trend of incidence and trend of prevalence; 2) Management: current management and availability of national/international treatment guidelines; 3) Humanistic burden: patients’ HRQoL, disease- versus treatment-related impact on HRQoL and changes of humanistic burden after treatment implementation and 4) Economic burden: direct/indirect cost in different regions, cost trends after treatment implementation, health resource utilisation and management of treatment.
Literature searches
Databases that were searched include PubMed, Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment [HTA] and National Health Service Economic Evaluation Database), Cost-Effectiveness Analysis Registry, EconLit and IQVIA proprietary HTA Accelerator. Proceedings of international scientific meetings (2014–2017) included American Society of Clinical Oncology, American Society of Clinical Oncology-Gastrointestinal Cancers Symposium, European Society for Medical Oncology, European Cancer Organisation, International Gastric Cancer Congress, Gastrointestinal Cancers Symposium and International Society for Pharmacoeconomics and Outcomes Research. Websites of the national cancer associations and Google searches were used to identify the most recent epidemiology data and guidelines. The search strategy applied to all databases included disease-specific keywords (e.g. ‘stomach neoplasms’, ‘gastroesophageal’ and ‘tumour’) and keywords associated with epidemiology endpoints (e.g. ‘incidence’, ‘prevalence’ and ‘mortality’), management endpoints (e.g. ‘guidelines’ and ‘real world’), humanistic endpoints (e.g. ‘quality of life’, ‘health status’ and ‘humanistic burden’) and economic endpoints (e.g. ‘cost’, ‘healthcare cost’ and ‘cost of illness’). The search was limited to studies published within the last 15 years. No geographic limits were applied; however, the priority was given to studies that focused on more than one country and on key geographic areas. No language restriction was applied to the search but only studies with an abstract written in English and full text in English, German, French, Italian or Spanish were eligible for inclusion.
Study eligibility criteria
The reviews were conducted in accordance with the guidelines from the Centre for Reviews and Dissemination [46] and NICE guidance [47] and were based on the PICOS (P: population, I: intervention, C: comparator, O: outcomes and S: study design) criteria. The population of interest was adults with GC or GEJC regardless of stage, histology or biomarker. No restrictions with respect to intervention or comparator were applied.
Study selection
All retrieved articles were reviewed by a researcher, and those considered irrelevant were removed. The remaining articles were further assessed to identify those studies that met the eligibility criteria. A quality check was conducted on a sample of the selected articles/abstracts by a second researcher, and a full-text review was conducted to determine relevance to the eligibility criteria. A quality assessment was conducted on all selected studies by one reviewer, and a second reviewer performed a further quality assessment on a sample of all the studies.
Based on the data available in the reviewed articles, we calculated the annual cost of GC and GEJC in different geographic regions and estimated an annual global financial cost expected in 2017.
Results
The literature search identified 9,101 records through the selected databases and 638 records using other resources (congresses, websites and proprietary HTA accelerator database). After duplicates were removed, 8,141 records were screened and 7,836 were excluded based on the eligibility criteria. A full-text analysis was conducted on the remaining 305 articles and resulted in the exclusion of 210 articles that did not meet the eligibility criteria. A total of 95 references were included in the qualitative analysis. Epidemiology outcomes were reported in 19 records, clinical management in 16, humanistic outcomes in 27 and economic outcomes in 17. More than one outcome was reported in 16 articles (Figure 1).
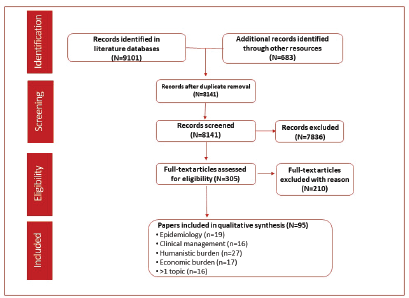
Figure 1. PRISMA flow diagram of studies identified through the predefined search strategy.
Epidemiology
In 2015, GC ranked fifth for incidence in both sexes after breast, lung, colorectal and prostate cancer (Figure 2a), and third for cancer-related mortality in both sexes after lung and colorectal cancers (Figure 2b) [1]. In 2012, the global ASIR was 12.1 per 100,000 individuals, ranging between 10.6 and 12.7 per 100,000 for more and less developed regions, respectively [7, 48]. The highest ASIR (per 100,000) of GC was observed in Eastern Asia (overall, 24.2; China, 22.7; Mongolia, 32.5; Japan, 29.9; Democratic People’s Republic of Korea, 14.3; and Republic of Korea, 41.8), Central and Eastern Europe (13.5) (range 10.3─24.2) and South America (10.3), particularly concentrated in the Pacific regions of South America (i.e. Colombia, Ecuador, Peru and Chile) [7, 8]. The lowest incidence was observed in North America (4), Africa (3.8) and the Eastern Mediterranean region (5.5) [7, 49]. Regardless of the geographic region, incidence was 2–3 times higher in men than in women, and higher in black and Latino populations compared with non-Hispanic white populations [13].
Mortality associated with GC was 2–3 times higher in men than women [1]; the highest age-standardised mortality rates (per 100,000) were reported in East Asia (16.5), Central and Eastern Europe (10.9) and South America (8.5), while the lowest rate occurred in North America (2.1) [6, 12]. Over the last two decades, a steady decline in mortality rates in patients with GC was observed in both developed and developing countries [1, 6, 50, 51].
Adenocarcinomas represent the most common histological type of GC (80%–90%), and the ‘differentiated’ type is the most frequently observed [3, 4].
In 2012, among all GCs reported worldwide, 27% were cardia and 73% were non-cardia located; the pyloric area was the most reported location for non-cardia GC [52]. The majority of both cardia (59%) and non-cardia (63%) GC cases occurred in Eastern/Southeastern Asia and more than 50% of both cardia and non-cardia GC cases were reported in China [52].
Our search indicates that the prognosis of GC was poor in all geographical areas; the global overall 5-year survival rate was 20%–30% and 5%–10% in the advanced stage (stages III and IV, respectively) [53–55].
Management
For resectable GCs, the guideline recommendations from the European Society for Medical Oncology [3], the Spanish Society of Medical Oncology [56] and the Japanese Gastric Cancer Association [57] all include endoscopic resection at early stages. For localised GC, a multidisciplinary approach including gastrectomy and postsurgical (adjuvant) [3, 10, 56–58] or perisurgical (before and after) [3, 10, 56, 58] chemotherapy with [3, 10, 56, 58] or without [57] radiation, is recommended. For patients with stage II or III GC undergoing tumour resection/lymphadenectomy D2, the Italian Association of Medical Oncology recommends adjuvant chemotherapy (monochemotherapy with fluoropyrimidine or chemotherapy regimens based on capecitabine or oxaliplatin) [58]; whereas perisurgical chemotherapy is recommended by the European Society for Medical Oncology guidelines for stage IV or higher resectable GC [3] and by the Italian Association of Medical Oncology guidelines for locally advanced GC (T3 or clinical N ) [58] and fluoropyrimidine or platinum derivatives are recommended for perisurgical chemotherapy [34, 58]. FLOT (i.e. Fluorouracil, leucovorin, oxaliplatin and docetaxel) represents a new promising perioperative regimen option for patients with locally advanced, resectable GC [59]. Post-surgery chemotherapy with S-1 monotherapy (tegafur, gimeracil and oteracil potassium), capecitabine plus oxaliplatin or S-1 plus oxaliplatin is recommended by the Japanese Gastric Cancer Association guidelines [57].
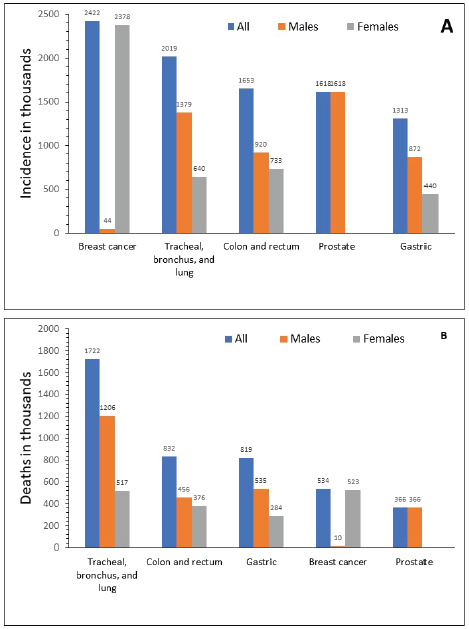
Figure 2. Global incidence (a) and mortality (b) for different types of cancer in 2015. Source: Fitzmaurice 2017 [1].
Six guidelines (Europe, Italy, Japan, Spain, UK and the US) provide recommendations for first-line therapy for advanced and metastatic GC and GEJC [3, 47, 56–58, 60]. Chemotherapy is the recommended first-line therapy for advanced GC and GEJC but there is no consensus among the available guidelines on the specific regimen that should be used (Table S1 and S2 in Supplement). The US [60] and Japanese [57] guidelines recommend doublet over triplet chemotherapy to reduce toxicity and indicate that triplet therapy be used only in fit patients who have frequent access to toxicity screenings. The US guidelines recommend a regimen with a 5-Fluorouracil (5-FU) or capecitabine and either cisplatin or oxaliplatin, whereas the Japanese guidelines recommend a regimen of S-1 and cisplatin as the first choice, followed by the combination of capecitabine and cisplatin. The European guidelines [3] recommend both doublet and triplet protocols, and favour EOX (epirubicin, oxaliplatin and capecitabine) over ECF (epirubicin, cisplatin and fluorouracil) and capecitabine over infused 5-FU due to better overall survival. For the management of HER2-positive GC and GEJC, all guidelines recommend adding trastuzumab to first-line chemotherapy regimens, which commonly include cisplatin and a fluoropyrimidine [56, 58, 60]. Second lines of chemotherapy include the same regimens for GC and GEJC and are generally based on the patients’ condition. The preferred regimen for second-line treatment is ramucirumab plus paclitaxel, or ramucirumab monotherapy if the patient is not eligible for paclitaxel (Table S2 in Supplement) [3, 10, 56–58]. A PD-1 inhibitor has recently been approved as third-line or higher therapy for GC/GEJC patients in the US [61].
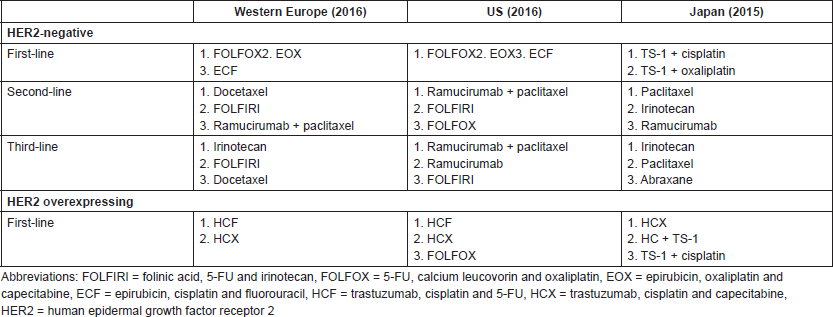
Evaluation of the reports of advanced stage GC and GEJC management in clinical practice shows a lack of consensus on the choice of chemotherapy regimens. In 2016, the most commonly prescribed first-line chemotherapy regimens for HER2-negative or unknown status of GC or GEJC were FOLFOX (5-FU, calcium leucovorin and oxaliplatin), EOX and ECF in the US and in Europe, while TS-1 plus cisplatin and TS-1 plus oxaliplatin were mostly used in Japan [62]. Differences among guidelines have also been observed for HER2 overexpressing tumours. In the US, the most common first-line regimens in 2016 were trastuzumab plus cisplatin plus 5-FU, trastuzumab plus cisplatin plus capecitabine, and FOLFOX, whereas in Western Europe they were trastuzumab plus cisplatin plus 5-FU, and trastuzumab plus cisplatin plus capecitabine. In Japan, the most commonly used regimens in 2015 were trastuzumab plus cisplatin plus capecitabine, trastuzumab plus cisplatin plus TS-1, and TS-1 plus cisplatin (Table S3 in Supplement) [62].
Humanistic burden of GC and GEJC
No large longitudinal epidemiological studies assessing the humanistic burden of GC from diagnosis to death were identified in our search. Among the identified studies, five were systematic literature reviews [63–67], nine were economic analyses [18, 20, 24, 26, 33, 37, 41, 42, 68], six were randomised controlled trials [69–73], one was an observational study [74], seven were prospective cohort or case-control studies [39, 75–80], seven were cross-sectional survey studies [81–87], four were retrospective studies [88–91] and one included a retrospective analysis and a prospective cross-sectional survey [92]. Only four studies used tools specific to GC or GEJC (GC module [STO22] and oesophago-gastric [OG25] modules of European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire [EORTC-QLQ]) to assess HRQoL [78, 80, 86, 87]. Among all studies reporting humanistic data (n = 40), EORTC-QLQ-C30 was the most frequently used cancer-specific tool to assess HRQoL (n = 13) [39, 64, 69–72, 74–78, 87, 91]. Symptoms associated with GC and GEJC worsen and change in nature as the disease progresses. Symptoms such as anaemia, loss of appetite, dysphagia, dyspepsia, reflux and insomnia are often reported at any stage, whereas weight loss, abdominal pain, vomiting, gastric obstruction and bleeding are commonly associated with advanced stages. In addition to symptoms that are attributed to the disease, other symptoms are chemotherapy-related [63–65] (Table 1).
Assessments based on the EORTC-QLQ-C30 revealed that patients with GC have worse general well-being, functional difficulties and symptoms than patients with colon and rectal cancer [75] and worse emotional and cognitive functioning than patients with oesophageal cancer [77]. Moreover, patients with GC have worse nausea, vomiting and constipation but comparable fatigue, pain, dyspnoea, sleep disturbance, appetite loss, diarrhoea and financial difficulty versus patients with oesophageal cancer [77]. Assessments conducted at different disease stages show that physical, emotional and social functioning, global HRQoL, fatigue, appetite, weight loss, dyspnoea and constipation worsen as the disease progresses [77].
Assessments of the impact of surgery on HRQoL showed that the negative impact of total or subtotal gastrectomy on various functional scales was completely recovered between 3–6 months after surgery while gastrointestinal symptoms (dietary restrictions, loss of appetite and diarrhoea) persisted longer [64, 87].
Table 1. Common symptoms associated with advanced GC and chemotherapy.
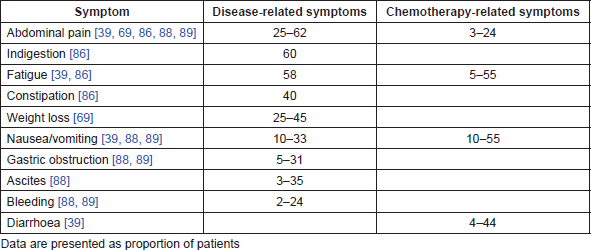
Several studies reported the impact of palliative chemotherapy on the HRQoL of patients with advanced GC [69–72, 77]. As GC is associated with rapid disease progression at the advanced stages, the delay of deterioration observed with chemotherapy accounts for the improvement in HRQoL when compared with best supportive care (BSC). At the end of a 16-week chemotherapy treatment, 55% of patients with advanced GC reported a large or moderate improvement in the global HRQoL score compared with baseline, while 19% reported a decrease [72]. Increased scores were also observed in 40%, 60% and 30% of patients in the physical, emotional and social functioning scales, respectively.
Most studies reported the impact of specific chemotherapy regimens on HRQoL. The addition of docetaxel to cisplatin plus 5-FU prolonged the time to 5% worsening of global HRQoL deterioration, 5% physical and social functioning deterioration, 10%, 20% and 30% nausea, vomiting and pain deterioration, respectively, and 30% appetite loss deterioration [69]. Leucovorin plus 5-FU (LV5FU2) plus irinotecan was associated with improved global health compared with LV5FU2 monotherapy or with cisplatin [70].
One study compared the accuracy of the oesophageal cancer-specific scale, EORTC-QLQ-OG25, with the GC-specific scale, EORTC-QLQ-STO22, for the assessment of the HRQoL in patients who underwent partial and total gastrectomy and found that the OG25 was more sensitive than the STO22. In this study, differences between the total and partial gastrectomy groups in weight loss, odynophagia, choking when swallowing and difficulty eating were identified only with the OG25 scale [80], and the OG25 scores for body image, dysphagia, odynophagia, pain and discomfort, anxiety and weight loss were worse after total compared with partial gastrectomy [80].
A study assessing patient satisfaction with different types of surgery and therapies showed that subtotal gastrectomy was associated with higher satisfaction than total gastrectomy and, among different therapies, patient satisfaction was higher for radiotherapy alone than for chemotherapy alone or combination chemotherapy/radiotherapy [84]. Moreover, patients involved in decision making reported higher treatment satisfaction and would or did choose the same treatment again [84]. In patients with advanced GC, the most important objectives of treatment were improving survival (54.6%), avoiding disease progression (34.6%) and having no limitations in daily routine (27.3%) [83].
Economic burden of GC and GEJC
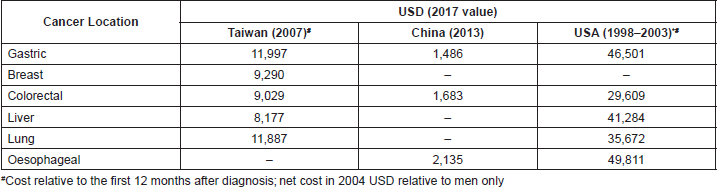
A total of 28 studies reported costs associated with the management of GC or GEJC [18–45]. A review of the economic data available on GC management shows that, even though GC is a major clinical and financial burden, only a few evaluations of the costs of GC management and cost-effectiveness were available, most of which were conducted in Asia [18–21, 25, 27, 28, 31, 35, 38, 44, 88, 89, 93, 94]. Moreover, few studies were conducted according to high-quality standards and the reported data are not always clear [30]. The cost of GC management per patient is generally higher than the cost for other cancers [27, 28, 36]. The overall healthcare cost of GC compared with other cancers in China [28], Taiwan [27] and the US [36] is reported below (Table 2).
Table 2. Total annual healthcare cost of GC per patient for different cancers in different regions.
In Japan, the ASIR of GC has been decreasing in the last 30 years; however, an increase in the number of new GC patients has been reported during the same period due to the rapid population ageing [95]. The direct cost of GC management in Japan has increased between 1996 and 2008 and is predicted to continue to increase until 2020, whereas the total cost is expected to decrease (when considering constant mortality rate) [21]. In Portugal, the total cost of hospitalisation per patient for GC and oesophageal cancer between 2000 and 2010 was €6,422 and €5,049, respectively [32]. The average financial burden per patient was not reported in any of the examined studies. Therefore, using the data provided in the reviewed studies, we estimated a financial burden per patient associated with GC and GEJC in Western countries, Japan, Iran and China (Figure 3).
Furthermore, we estimated the annual financial burden associated with GC for different countries based on the 2012 incidence data and the average healthcare cost per patient (Table 3). Overall, these data reveal a disparity in the cost of GC and GEJC among different regions.
Among studies conducted in the US, one of the studies reported an average overall cost of USD 96,571 for a GC patient in the 18 months after diagnosis, which was estimated to be more than 10 times the cost of gender-matched controls [23], whereas the costs of GC per patient ≥65 years old was USD 44,203 for men and USD 41,899 for women over a 5-year period and increased with the tumour stage [36] (Figure 4). Among Medicare-enrolled patients with advanced GC who received first-line treatment, the average total follow-up cost from diagnosis of the advanced or metastatic stage was USD 70,808 per patient [43]. The estimated healthcare costs for GC patients were significantly higher in the US than in Iran, where the average cost per patient was USD 3,940 and USD 2,596 in private and public centres, respectively [25]. Moreover, the cost increased from USD 2,707 for stage I to USD 4,608 for stage IV in private centres, and from USD 2,191 for stage I to USD 2,877 for stage IV in public centres [25].
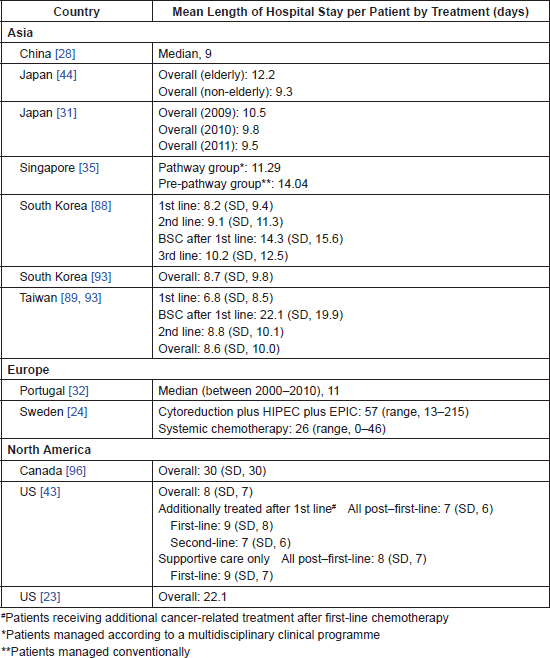
Several studies examined health resource utilisation. Length of hospital stay in different countries is summarised in Table 4.
The economic burden of GC and GEJC associated with specific treatment modalities was reported in 17 studies in Japan, South Korea, Singapore, China, Sweden, Portugal, the UK and the US. The cost of endoscopic submucosal dissection (ESD) for early GC in Japan and South Korea was evaluated in three studies [31, 44, 94]. Hospitalisation costs were estimated at USD 4,681, USD 5,353 and USD 1,864 for open gastrectomy, laparoscopy-assisted gastrectomy and ESD, respectively, with no differences in the 1-year follow-up costs between ESD and conventional surgeries [94]. In Japan, between 2009 and 2011, the cost of ESD per patient decreased from USD 6,768 to USD 6,428 due to a significant decrease in the length of hospital stay from 10.5 to 9.5 days [31]. The cost of ESD was higher in elderly (≥80 years, USD 7,346) compared with non-elderly patients (<80 years, USD 6,296) due to the longer length of stay in elderly patients (12.2 days versus 9.3 days) [44].
The economic burden of adjuvant and neoadjuvant chemotherapy was reported in four studies [20, 24, 35, 38]. In China, the implementation of adjuvant therapy increased the overall cost compared with gastrectomy alone over the first 3 (USD 17,824 versus USD 9,051) and 5 (USD 23,364 versus USD 20,007) years but decreased the overall cost over 10 (USD 39,889 versus USD 48,284) and 30 (USD 71,537 versus USD 87,004) years. Furthermore, the total direct cost per patient per cycle was lower with adjuvant therapy with S1 (USD 1938 ± 236) than with XELOX (IV oxaliplatin and oral capecitabine, USD 2,317 ± 315) [20]. Similarly, another study showed higher total direct costs per cycle per patient of XELOX (USD 2,317 ± 315) compared with S1 (USD 1,938 ± 236), which was driven by the time cost [38]. A study conducted in Sweden assessed the cost of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from GC compared with systemic chemotherapy alone. The mean costs per patient were higher in the neoadjuvant group (USD 145,728) than in the systemic chemotherapy group (USD 59,314) [24]. A study conducted in Singapore showed that the implementation of a multidisciplinary gastrectomy pathway significantly reduced the mean total (11.29 versus 14.04 days; P = 0.023) and post-surgery (8.88 versus 11.00 days; P = 0.022) length of hospital stay and average hospitalisation costs per patient (before its implementation: USD 17,371; post-implementation: USD 13,338; P = 0.047) [35].
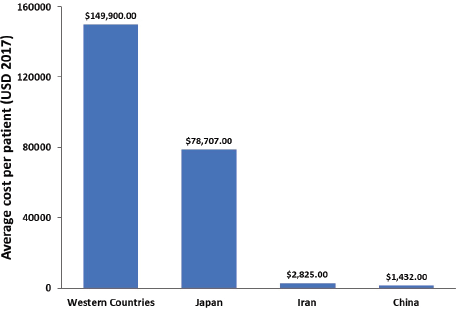
Figure 3. Average overall healthcare cost per GC and GEJC patient.
Table 3. Annual cost of GC and GEJC in different geographic regions (2017 value).
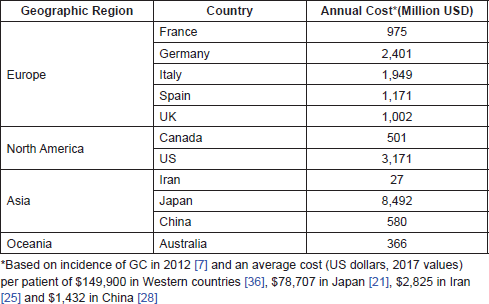
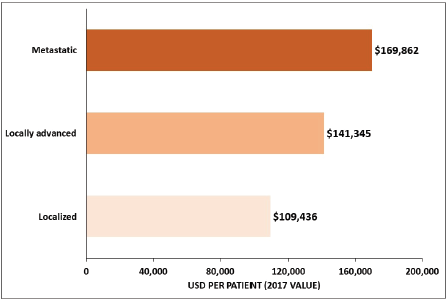
Figure 4. Average overall healthcare cost of GC by tumour stage for ≥65-year-old patients in the US. Source: Yabroff 2008 [36].
The cost of chemotherapy was assessed in ten studies [18, 19, 22, 26, 29, 33, 41–43, 68]. Two studies compared the costs associated with oral capecitabine versus IV 5-FU in combination with cisplatin as first-line treatment in patients with advanced GC and found that for 5.5 cycles of 21 days each, administration of capecitabine in place of 5-FU resulted in savings of £4210.29 [42] and €5,869 [29]. Two studies conducted in the UK [68] and Japan [33] investigated the economic burden associated with trastuzumab as first-line therapy for HER2-positive advanced GC and reported costs of approximately £26,100 and between €27,000–€30,000, respectively. One study in the UK evaluated the economic burden of ramucirumab either alone or with paclitaxel for advanced GC or GEJC adenocarcinoma previously treated with chemotherapy; total costs were £52,996 for ramucirumab/paclitaxel versus £13,400 for BSC, £18,779 for docetaxel in combination therapy and £36,678 for ramucirumab monotherapy versus £14,137 for BSC monotherapy [41]. A study assessing the cost effectiveness of second-line therapies in the US found that the total lifetime cost per patient ranged between USD 39,264 for irinotecan and USD 143,978 for ramucirumab plus paclitaxel [26]. The annual costs of third- or subsequent-line therapy in GC patients in China amounted to USD 9,915.48 for apatinib and USD 1,801.62 for BSC [18]. Another Chinese study of chemotherapy regimens in advanced GC reported that FAMTX (5-FU, calcium leucovorin, adriamycin and methotrexate) and DCF (docetaxel, cisplatin and 5-FU) were the least (RMB 1,756.95) and most expensive (RMB 9,979), respectively [19]. Total healthcare costs for patients with advanced/unresectable or metastatic GC receiving standard first-line chemotherapy in the US were evaluated in two studies. Between 2000 and 2009, the average costs for first-line, second-line and BSC as first-line were USD 36,810, USD 22,332 and USD 40,628, respectively [43]. Similar results were reported in a retrospective database analysis of chemotherapy treatment patterns and outcomes of patients with GC. Between 2004 and 2012, the costs for first- and second-line treatment were USD 40,810 and USD 26,587, respectively [22].
Based on the data reported in this review, we estimated that the annual financial burden of GC calculated for Europe (including France, Germany, Italy, Spain and the UK), Asia (including Iran, Japan and China), North America (Canada and the US) and Australia amounted to 20.6 billion USD in 2017.
Discussion
GC is an aggressive cancer that, in 2015, was the third leading cause of cancer-related mortality worldwide and the fifth in incidence [1]. Over the last two decades, a stable decline in the worldwide incidence of non-cardia GC has been observed in both developed and developing countries with marked differences across geographical regions [1, 6, 50, 51]. Possible reasons for this decline in Western countries include the increased availability of fresh fruits and vegetables, decreasing consumption of preserved foods and a decrease in smoking [12]. H. pylori infection has been strongly associated with non-cardia GC, suggesting that the widespread use of antibiotics may have contributed to the decrease in incidence [12]. In contrast, the incidence of cardia GC has increased in the US and several European countries possibly due to the growing incidence of gastroesophageal reflux disease associated with increased obesity [6, 12, 13]. Despite the overall decline in the global incidence of GC, and even if this trend continues, the prevalence of GC is expected to increase in the next decade due to the growing worldwide population. Most countries, particularly those with a low incidence of GC, lack specific screening programmes for GC, and therefore diagnosis often occurs at advanced stages, resulting in a poor prognosis [9–11]. In the past decades, survival rates have significantly improved in various Asian countries where GC screening programmes have been implemented [97], suggesting that efforts to reduce GC rates should focus on early diagnosis. It is likely that control of H. pylori infection and risk factors including reflux disease, obesity, tobacco use and diet would also contribute to decreasing the mortality associated with GC. This is supported by the evidence that in the last 25 years, the rate of oesophageal squamous-cell carcinoma has been declining in several countries, and since this type of oesophageal cancer is associated with alcohol and tobacco use, this decline could be due to a reduction in tobacco and alcohol use [98].
Table 4. Mean length of hospitalisation for GC patients.
Overall, the treatment guidelines for GC are similar across regions, with surgery and adjuvant chemoradiotherapy reserved for early stages and palliative chemotherapy for later stages, depending on the patient’s health status. Several chemotherapy regimens are available, yet no gold standard exists. In the US, 80% of patients with metastatic GC received first-line chemotherapy in 2016 [62].
The literature search conducted for this review did not identify any large longitudinal epidemiological studies that assessed the humanistic burden of GC. Based on the available data, GC patients have worse general well-being, functional difficulties and symptoms than patients with colon or rectal cancer [75], and disease progression is associated with lower HRQoL [77]. Total or subtotal gastrectomy was found to have a negative impact on physical, cognitive and role functioning, all of which gradually recover between 3 months [64, 87] and 5 years after surgery [78]. Chemotherapy was associated with improvement in the global HRQoL, and physical, emotional and social functioning scores compared with baseline in 30%─60% of patients [72].
Few high-quality studies assessing the economic burden of GC and GEJC were identified. Since most economic evaluations were conducted in Asia, where GC incidence is high and early diagnosis and treatment are more common, it is difficult to extrapolate the conclusions of these studies to other regions. Compared with other cancers, GC is associated with one of the highest economic burdens, with marked differences across different geographic regions. This burden increases as disease progresses, and the highest costs are associated with metastatic tumours. No studies were identified that reported the total cost per patient or the overall financial burden associated with GC in different regions. In Japan, the direct cost associated with GC has increased from 1996 to 2008 and is expected to continue growing until 2020 [21]. In the US, the 5-year cost of care for the elderly Medicare cancer patients diagnosed in 2004 was estimated at $624 million [36], and the healthcare costs incurred by GC patients in the US have been estimated to be at least 10 times those for non-cancer US subjects [23]. We have attempted to estimate the global costs of GC/GEJC based on published cost data and estimated prevalence in different countries. However, since most of the available data are not up-to-date (2015 or earlier), our estimate may not reflect the current global economic burden. This may also explain the significantly lower cost reported for China [28] compared with other countries despite the high GC/GEJC prevalence observed in this country. It is also important to consider that, even though the economic costs associated with GC/GEJC are largely affected by disease stage, this information was not always reported in the reviewed articles. Our calculated cost for the US was lower than the one estimated for Japan. Our estimate for the US was based on an average patient cost calculated across all disease stages and may, therefore, represent a conservative estimate. It is likely that this cost would increase if calculated based on the cost associated with advanced stages of the tumour.
Conclusion
GC represents a substantial burden to patients, associated with severe symptoms and limited availability of effective treatments, and with economic costs that are expected to continue growing worldwide in the next decade. There is still significant room for improvement with regard to early detection and intervention and the introduction of new life-extending therapies. Moreover, the use of predictive biomarkers to optimise patient selection for specific therapies may improve treatment efficiency and patient outcome.
List of Abbreviations
5-FU 5-Fluorouracil
ASIR Age standardised-incidence rate
BSC Best supportive care
DCF Docetaxel, cisplatin and 5-FU
EBV Epstein-Barr virus
ECF Epirubicin, cisplatin and fluorouracil
EORTC-QLQ European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
EOX Epirubicin, oxaliplatin and capecitabine
EPIC Early post-operative intraperitoneal chemotherapy
ESD Endoscopic submucosal dissection
FAMTX 5-FU, calcium leucovorin, adriamycin and methotrexate
FOLFOX 5-FU, calcium leucovorin and oxaliplatin
GC Gastric cancer
GEJ Gastroesophageal junction
GEJC Gastroesophageal junction cancer
HIPEC Hyperthermic intraperitoneal chemotherapy
HRQoL Health-related quality of life
HTA Health Technology Assessment
LV5FU2 Leucovorin plus 5-FU
OG25 Oesophago-gastric
PD-1 Programmed death receptor 1
SD Standard deviation
STO22 Gastric cancer module
USD United States dollar
XELOX IV oxaliplatin and oral capecitabine
Conflicts of Interest
M Casamayor reports personal fees from Astellas during the conduct of the study. R Morlock and H Maeda are employed by Astellas. J Ajani has no relevant conflict of interest to declare.
Acknowledgment
This work was sponsored by Astellas Pharma, Inc. (Northbrook, IL, USA). Editorial support was provided by Rosalba Satta and Mike Zbreski (SuccinctChoice Medical Communications, Chicago, IL) and was funded by Astellas Pharma, Inc.
References
1. Fitzmaurice C, Allen C, and Barber RM, et al (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study JAMA Oncol 3 524–548 https://doi.org/10.1001/jamaoncol.2016.5688
2. Dikken JL, van de Velde CJ, and Gonen M, et al (2012) The New American Joint Committee on Cancer/International Union Against Cancer staging system for adenocarcinoma of the stomach: increased complexity without clear improvement in predictive accuracy Ann Surg Oncol 19 2443–2451 https://doi.org/10.1245/s10434-012-2403-6 PMID: 22618718 PMCID: 3404274
3. Smyth EC, Verheij M, and Allum W, et al (2016) Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 27 v38–v49 https://doi.org/10.1093/annonc/mdw350 PMID: 27664260
4. Hu B, El Hajj N, and Sittler S, et al (2012) Gastric cancer: classification, histology and application of molecular pathology J Gastrointest Oncol 3 251–261 PMID: 22943016 PMCID: 3418539
5. Buas MF and Vaughan TL (2013) Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease Semin Radiat Oncol 23 3–9 https://doi.org/10.1016/j.semradonc.2012.09.008 PMCID: 3535292
6. Ang TL and Fock KM (2014) Clinical epidemiology of gastric cancer Singapore Med J 55 621–628 https://doi.org/10.11622/smedj.2014174
7. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136 E359–E386 https://doi.org/10.1002/ijc.29210
8. Torres J, Correa P, and Ferreccio C, et al (2013) Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America Cancer Causes Control 24 249–256 https://doi.org/10.1007/s10552-012-0114-8 PMCID: 3697934
9. Dicken BJ, Bigam DL, and Cass C, et al (2005) Gastric adenocarcinoma: review and considerations for future directions Ann Surg 241 27–39 PMCID: PMC1356843 PMID: 15621988
10. Ajani JA, D’Amico TA, and Almhanna K, et al (2016) Gastric cancer, Version 3.2016, NCCN clinical practice guidelines in oncology J Natl Compr Canc Netw 14 1286–1312 https://doi.org/10.6004/jnccn.2016.0137 PMID: 27697982
11. Sitarz R, Skierucha M, and Mielko J, et al (2018) Gastric cancer: epidemiology, prevention, classification, and treatment Cancer Manag Res 10 239–248 https://doi.org/10.2147/CMAR.S149619 PMID: 29445300 PMCID: 5808709
12. Fock KM (2014) Review article: the epidemiology and prevention of gastric cancer Aliment Pharmacol Ther 40 250–260 https://doi.org/10.1111/apt.12814 PMID: 24912650
13. Karimi P, Islami F, and Anandasabapathy S, et al (2014) Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention Cancer Epidemiol Biomarkers Prev 23 700–713. https://doi.org/10.1158/1055-9965.EPI-13-1057 PMID: 24618998 PMCID: 4019373
14. Yakoob J, Fatima SS, and Abbas Z, et al (2017) Distribution of gastric carcinoma in an area with a high prevalence of Helicobacter pylori Turk J Gastroenterol 28 98–103 https://doi.org/10.5152/tjg.2017.17607 PMID: 28119270
15. Baniak N, Senger JL, and Ahmed S, et al (2016) Gastric biomarkers: a global review World J Surg Oncol 14 212 https://doi.org/10.1186/s12957-016-0969-3 PMID: 27514667 PMCID: 4982433
16. Lyons TG and Ku GY (2017) Systemic therapy for esophagogastric cancer: targeted therapies Chin Clin Oncol 6 48 https://doi.org/10.21037/cco.2017.07.02 PMID: 29129088
17. Xu YH, Li ZL, and Qiu SF (2018) IFN-gamma induces gastric cancer cell proliferation and metastasis through upregulation of integrin beta3-mediated NF-kappaB signaling Transl Oncol 11 182–192 https://doi.org/10.1016/j.tranon.2017.11.008 PMID: 29306706 PMCID: 5755748
18. Chen HD, Zhou J, and Wen F, et al (2017) Cost-effectiveness analysis of apatinib treatment for chemotherapy-refractory advanced gastric cancer J Cancer Res Clin Oncol 143 361–368 https://doi.org/10.1007/s00432-016-2296-z
19. Chen XZ, Jiang K, and Hu JK, et al (2008) Cost-effectiveness analysis of chemotherapy for advanced gastric cancer in China World J Gastroenterol 14 2715–2722 https://doi.org/10.3748/wjg.14.2715 PMID: 18461656 PMCID: 2709040
20. Chongqing T, Liubao P, and Xiaohui Z, et al (2014) Cost-utility analysis of the newly recommended adjuvant chemotherapy for resectable gastric cancer patients in the 2011 Chinese National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: gastric cancer Pharmacoeconomics 32 235–243 https://doi.org/10.1007/s40273-013-0065-2
21. Haga K, Matsumoto K, and Kitazawa T, et al (2013) Cost of illness of the stomach cancer in Japan—a time trend and future projections BMC Health Serv Res 13 283 https://doi.org/10.1186/1472-6963-13-283
22. Hess LM, Michael D, and Mytelka DS, et al (2016) Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data Gastric Cancer 19 607–615 https://doi.org/10.1007/s10120-015-0486-z PMCID: 4824832
23. Hirst C, Ryan J, and Tunceli O, et al (2014) Cost profile of patients with gastric cancer using United States administrative claims data Value Health 17 A78 https://doi.org/10.1016/j.jval.2014.03.457
24. Hultman B, Lundkvist J, and Glimelius B, et al (2012) Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer Acta Oncol 51 112–121 https://doi.org/10.3109/0284186X.2011.594809
25. Izadi A, Sirizi MJ, and Esmaeelpour S, et al (2016) Evaluating direct costs of gastric cancer treatment in Iran—case study in Kerman City in 2015 Asian Pac J Cancer Prev 17 3007–3013 PMID: 27356726
26. Lam SW, Wai M, and Lau JE, et al (2017) Cost-effectiveness analysis of second-line chemotherapy agents for advanced gastric cancer Pharmacotherapy 37 94–103 https://doi.org/10.1002/phar.1870 PMID: 27870079
27. Li TY, Hsieh JS, and Lee KT, et al (2014) Cost trend analysis of initial cancer treatment in Taiwan PLoS One 9 e108432 https://doi.org/10.1371/journal.pone.0108432 PMID: 25279947 PMCID: 4184791
28. Li X, Cai H, and Wang C, et al (2016) Economic burden of gastrointestinal cancer under the protection of the New Rural Cooperative Medical Scheme in a region of rural China with high incidence of oesophageal cancer: cross-sectional survey Trop Med Int Health 21 907–916 https://doi.org/10.1111/tmi.12715 PMID: 27125226
29. Macedo A, Pereira C, and Goncalves J, et al (2009) [Economic evaluation of capecitabine use as first line treatment in patients with advanced gastric carcinoma in Portugal] Acta Med Port 22 827–832 PMID: 20350467
30. Mahar AL, El-Sedfy A, and Brar SS, et al (2015) Are we lacking economic evaluations in gastric cancer treatment? Pharmacoeconomics 33 83–87 https://doi.org/10.1007/s40273-014-0215-1
31. Murata A, Okamoto K, and Muramatsu K, et al (2014) Time trend of medical economic outcomes of endoscopic submucosal dissection for gastric cancer in Japan: a national database analysis Gastric Cancer 17 294–301 https://doi.org/10.1007/s10120-013-0282-6
32. Pinho I, Santos JV, and Dinis-Ribeiro M, et al (2015) Burden of digestive diseases in Portugal: trends in hospitalizations between 2000 and 2010 Eur J Gastroenterol Hepatol 27 279–289 https://doi.org/10.1097/MEG.0000000000000266 PMID: 25629572
33. Shiroiwa T, Fukuda T, and Shimozuma K (2011) Cost-effectiveness analysis of trastuzumab to treat HER2-positive advanced gastric cancer based on the randomised ToGA trial Br J Cancer 105 1273–1278 https://doi.org/10.1038/bjc.2011.390 PMID: 21959871 PMCID: 3241558
34. Smyth E, Schoder H, and Strong VE, et al (2012) A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer Cancer 118 5481–5488 https://doi.org/10.1002/cncr.27550 PMID: 22549558
35. So JB, Lim ZL, and Lin HA, et al (2008) Reduction of hospital stay and cost after the implementation of a clinical pathway for radical gastrectomy for gastric cancer Gastric Cancer 11 81–85 https://doi.org/10.1007/s10120-008-0458-7 PMID: 18595014
36. Yabroff KR, Lamont EB, and Mariotto A, et al (2008) Cost of care for elderly cancer patients in the United States J Natl Cancer Inst 100 630–641 https://doi.org/10.1093/jnci/djn103 PMID: 18445825
37. Yeh JM, Ho W, and Hur C (2010) Cost-effectiveness of endoscopic surveillance of gastric ulcers to improve survival Gastrointest Endosc 72 33–43 https://doi.org/10.1016/j.gie.2010.01.047 PMID: 20430384 PMCID: 2902548
38. He J, Wen F, and Yin X, et al (2013) Cost analysis of S1 and XELOX as adjuvant therapy for gastric cancer Anticancer Drugs 24 754–758 https://doi.org/10.1097/CAD.0b013e328361bef2 PMID: 23629479
39. Kim HR, Kim JH, and Rhee Y, et al (2016) Assessment of adrenal function and health-related quality of life in advanced gastric cancer patients who received first-line chemotherapy Oncology 90 248–254 https://doi.org/10.1159/000445010 PMID: 27070835
40. Shermock KM, Asche CV, and Seal B, et al Cost of care for gastric cancer in patients with and without metastases Value in Health 18 A201 https://doi.org/10.1016/j.jval.2015.03.1165
41. National Institute for Health and Care Excellence. Ramucirumab for treating advanced gastric cancer or gastro–oesophageal junction adenocarcinoma previously treated with chemotherapy [https://www.nice.org.uk/guidance/ta378/resources] Date accessed: 24/04/17
42. National Institute for Health and Care Excellence. Capecitabine for the treatment of advanced gastric cancer [https://www.nice.org.uk/guidance/ta191/resources] Date accessed: 24/04/17
43. Karve S, Lorenzo M, and Liepa AM, et al (2015) Treatment patterns, costs, and survival among medicare-enrolled elderly patients diagnosed with advanced stage gastric cancer: analysis of a linked population-based cancer registry and administrative claims database J Gastric Cancer 15 87–104 https://doi.org/10.5230/jgc.2015.15.2.87 PMID: 26161282 PMCID: 4496446
44. Murata A, Muramatsu K, and Ichimiya Y, et al (2014) Endoscopic submucosal dissection for gastric cancer in elderly Japanese patients: an observational study of financial costs of treatment based on a national administrative database J Dig Dis 15 62–70 https://doi.org/10.1111/1751-2980.12106
45. Spackman E, Rice S, and Norman G, et al (2013) Trastuzumab for the treatment of HER2-positive metastatic gastric cancer : a NICE single technology appraisal Pharmacoeconomics 31 185–194 https://doi.org/10.1007/s40273-013-0023-z PMID: 23371465
46. Centre for Reviews and Dissemination (2009) Systematic Reviews: CRD’s guidance for undertaking reviews in health care (York: University of York) 6–81
47. National Institute for Health and Care Excellence. Stomach cancer [https://www.nice.org.uk/guidance/conditions-and-diseases/cancer/stomach-cancer] Date accessed: 24/04/17
48. GLOBOCAN 2012 [http://globocan.iarc.fr/Pages/cancer.aspx] Date accessed: 19/04/18
49. Roberts SE, Morrison-Rees S, and Samuel DG, et al (2016) Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe Aliment Pharmacol Ther 43 334–345 https://doi.org/10.1111/apt.13474
50. Ferro A, Peleteiro B, and Malvezzi M, et al (2014) Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype Eur J Cancer 50 1330–1344 <a data-cke-saved-href="https://doi.org/10.1016/j.ejca.2014.01.029 PMID: 24650579
51. Sierra MS, Cueva P, and Bravo LE, et al (2016) Stomach cancer burden in Central and South America Cancer Epidemiol 44(Suppl 1) S62–S73 https://doi.org/10.1016/j.canep.2016.03.008 PMID: 27678324
52. Colquhoun A, Arnold M, and Ferlay J, et al (2015) Global patterns of cardia and non-cardia gastric cancer incidence in 2012 Gut 64 1881–1888 https://doi.org/10.1136/gutjnl-2014-308915 PMID: 25748648
53. National Cancer Institute. SEER [https://seer.cancer.gov/statfacts/html/stomach.html] Date accessed: 14/03/18
54. American Cancer Society [https://www.cancer.org/cancer/stomach-cancer/detection-diagnosis-staging/survival-rates.html] Date accessed: 14/03/18
55. Cancer Research UK [http://about-cancer.cancerresearchuk.org/about-cancer/stomach-cancer/survival] Date accessed: 14/03/18
56. Martin-Richard M, Custodio A, and Garcia-Giron C, et al (2015) Seom guidelines for the treatment of gastric cancer 2015 Clin Transl Oncol 17 996–1004 https://doi.org/10.1007/s12094-015-1456-y PMID: 26691658 PMCID: 4689778
57. Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer 20 1–19 https://doi.org/10.1007/s10120-016-0622-4 PMCID: 5215069
58. AIOM 2016. Linee Guida. Neoplasie dello stomaco [http://media.aiom.it/userfiles/files/doc/LG/2016_LG_AIOM_Stomaco.pdf] Date accessed: 02/04/17
59. Al-Batran SE, Hofheinz RD, and Pauligk C, et al (2016) Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial Lancet Oncol 17 1697–1708 https://doi.org/10.1016/S1470-2045(16)30531-9 PMID: 27776843
60. National Comprehensive Cancer Network (NCCN) Guidelines. Esophageal and Esophagogastric Junction Cancer Treatment Regimens (2016)
61. Curea FG, Hebbar M, and Ilie SM, et al (2017) Current targeted therapies in HER2-positive gastric adenocarcinoma Cancer Biother Radiopharm 32 351–363 https://doi.org/10.1089/cbr.2017.2249 PMID: 29265917
62. Kantar Health—CancerMPact. Treatment Architecture: United States Gastric Cancer. (2016) http://www.kantarhealth.com/docs/datasheets/cancermpact-treatment-architecture.pdf?sfvrsn=6 Date accessed: 24/04/17
63. Astin MP, Martins T, and Welton N, et al (2015) Diagnostic value of symptoms of oesophagogastric cancers in primary care: a systematic review and meta-analysis Br J Gen Pract 65 e677–e691 https://doi.org/10.3399/bjgp15X686941 PMID: 26412845 PMCID: 4582881
64. Dorcaratto D, Grande L, and Ramon JM, et al (2011) [Quality of life of patients with cancer of the oesophagus and stomach] Cir Esp 89 635–644 https://doi.org/10.1016/j.ciresp.2011.07.006 PMID: 21907976
65. Mahar AL, Coburn NG, and Karanicolas PJ, et al (2012) Effective palliation and quality of life outcomes in studies of surgery for advanced, non-curative gastric cancer: a systematic review Gastric Cancer 15(Suppl 1) S138–S145 https://doi.org/10.1007/s10120-011-0070-0
66. Kaptein AA, Morita S, and Sakamoto J (2005) Quality of life in gastric cancer World J Gastroenterol 11 3189–3196 https://doi.org/10.3748/wjg.v11.i21.3189 PMID: 15929166 PMCID: 4316047
67. Cuyun Carter G, King DT, and Hess LM, et al (2015) Health state utility values associated with advanced gastric, oesophageal, or gastro-oesophageal junction adenocarcinoma: a systematic review J Med Econ 18 954–966 https://doi.org/10.3111/13696998.2015.1066380
68. National Institute for Health and Care Excellence. Trastuzumab for the treatment of HER2-positive metastatic gastric cancer [https://www.nice.org.uk/guidance/ta208/resources] Date accessed: 24/04/17
69. Ajani JA, Moiseyenko VM, and Tjulandin S, et al (2007) Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group J Clin Oncol 25 3210–3216 https://doi.org/10.1200/JCO.2006.08.3956 PMID: 17664468
70. Bonnetain F, Bouche O, and Conroy T, et al (2005) Longitudinal quality of life study in patients with metastatic gastric cancer. Analysis modalities and clinical applicability of QoL in randomized phase II trial in a digestive oncology Gastroenterol Clin Biol 29 1113–1124 https://doi.org/10.1016/S0399-8320(05)82175-X
71. Curran D, Pozzo C, and Zaluski J, et al (2009) Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: results of a randomised phase III trial Qual Life Res 18 853–861 https://doi.org/10.1007/s11136-009-9493-z PMID: 19568958 PMCID: 2724642
72. Gubanski M, Glimelius B, and Lind PA (2014) Quality of life in patients with advanced gastric cancer sequentially treated with docetaxel and irinotecan with 5-fluorouracil and folinic acid (leucovin) Med Oncol 31 906 https://doi.org/10.1007/s12032-014-0906-7 PMID: 24627237
73. Bodoky G, Scheulen ME, and Rivera F, et al (2015) Clinical benefit and health-related quality of life assessment in patients treated with Cisplatin/S-1 versus Cisplatin/5-FU: secondary end point results from the first-line advanced gastric cancer study (FLAGS) J Gastrointest Cancer 46 109–117 https://doi.org/10.1007/s12029-014-9680-1 PMID: 25707610
74. Chung J, Ju G, and Yang J, et al (2018) Prevalence of and factors associated with anxiety and depression in Korean patients with newly diagnosed advanced gastrointestinal cancer Korean J Intern Med 33 585–594
75. Bektas DK and Demir S (2016) Anxiety, depression levels and quality of life in patients with gastrointestinal cancer in Turkey Asian Pac J Cancer Prev 17 723–731 https://doi.org/10.7314/APJCP.2016.17.2.723 PMID: 26925670
76. Bilgin S and Gozum S (2018) Effect of nursing care given at home on the quality of life of patients with stomach cancer and their family caregivers’ nursing care Eur J Cancer Care (Engl) 27(2) e12567 https://doi.org/10.1111/ecc.12567
77. McKernan M, McMillan DC, and Anderson JR, et al (2008) The relationship between quality of life (EORTC QLQ-C30) and survival in patients with gastro-oesophageal cancer Br J Cancer 98 888–893 https://doi.org/10.1038/sj.bjc.6604248 PMID: 18268490 PMCID: 2266859
78. Yu W, Park KB, and Chung HY, et al (2016) Chronological changes of quality of life in long-term survivors after gastrectomy for gastric cancer Cancer Res Treat 48 1030–1036 https://doi.org/10.4143/crt.2015.398 PMID: 27004956 PMCID: 4946352
79. Rhee YS, Yun YH, and Park S, et al (2008) Depression in family caregivers of cancer patients: the feeling of burden as a predictor of depression J Clin Oncol 26 5890–5895 https://doi.org/10.1200/JCO.2007.15.3957 PMID: 19029423
80. Lee JH, Lee HJ, and Choi YS, et al (2016) Postoperative quality of life after total gastrectomy compared with partial gastrectomy: longitudinal evaluation by European Organization for Research and Treatment of Cancer-OG25 and STO22 J Gastric Cancer 16 230–239 https://doi.org/10.5230/jgc.2016.16.4.230
81. Areia M, Alves S, and Brito D, et al (2014) Health-related quality of life and utilities in gastric premalignant conditions and malignant lesions: a multicentre study in a high prevalence country J Gastrointestin Liver Dis 23 371–378 PMID: 25531994
82. Cho MH, Dodd MJ, and Lee KA, et al (2006) Self-reported sleep quality in family caregivers of gastric cancer patients who are receiving chemotherapy in Korea J Cancer Educ 21 S37–S41 https://doi.org/10.1207/s15430154jce2101s_8 PMID: 17020500
83. Hofheinz R, Clouth J, and Borchardt-Wagner J, et al (2016) Patient preferences for palliative treatment of locally advanced or metastatic gastric cancer and adenocarcinoma of the gastroesophageal junction: a choice-based conjoint analysis study from Germany BMC Cancer 16 937 https://doi.org/10.1186/s12885-016-2975-9 PMID: 27923357 PMCID: 5139120
84. Kim S, Bae JM, and Kim YW, et al (2008) Self-reported experience and outcomes of care among stomach cancer patients at a median follow-up time of 27 months from diagnosis Support Care Cancer 16 831–839 https://doi.org/10.1007/s00520-007-0340-x
85. Nakada K, Takahashi M, and Ikeda M, et al (2016) Factors affecting the quality of life of patients after gastrectomy as assessed using the newly developed PGSAS-45 scale: a nationwide multi-institutional study World J Gastroenterol 22 8978–8990 https://doi.org/10.3748/wjg.v22.i40.8978 PMID: 27833389 PMCID: 5083803
86. Hong J, Wei Z, and Wang W (2015) Preoperative psychological distress, coping and quality of life in Chinese patients with newly diagnosed gastric cancer J Clin Nurs 24 2439–2447 https://doi.org/10.1111/jocn.12816 PMID: 25930090
87. Sun V, Kim JY, and Ruel N, et al (2017) Quality of life and self-management strategies after gastroesophageal cancer surgery J Clin Oncol 35 159. https://doi.org/10.1200/JCO.2017.35.4_suppl.159
88. Cuyun Carter G, Kaltenboeck A, and Ivanova J, et al (2017) Real-world treatment patterns among patients with advanced gastric cancer in South Korea Cancer Res Treat 49 578–587 https://doi.org/10.4143/crt.2016.001
89. Cuyun Carter G, Kaltenboeck A, and Ivanova J, et al (2017) Treatment patterns in patients with advanced gastric cancer in Taiwan Asia Pac J Clin Oncol 13 185–194 https://doi.org/10.1111/ajco.12497
90. Din NU, Ukoumunne OC, and Rubin G, et al (2015) Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK clinical practice research datalink PLoS One 10 e0127717
91. Hayran M, Yüce D, and Hüseyin B, et al (2015) Quality of life domains associated with readmission Value in Health 18 A211–A212. https://doi.org/10.1016/j.jval.2015.03.1225
92. Hung MC, Lai WW, and Chen HH, et al (2015) Comparison of expected health impacts for major cancers: integration of incidence rate and loss of quality-adjusted life expectancy Cancer Epidemiol 39 126–132
93. Rajan N, Cuyun Carter G, and Kaltenboeck A, et al (2014) Health care resource use among advanced gastric cancer patients in Taiwan and South Korea Value Health 17 A734 https://doi.org/10.1016/j.jval.2014.08.100 PMID: 27202630
94. Kim Y, Kim YW, and Choi IJ, et al (2015) Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea Gut Liver 9 174–180 https://doi.org/10.5009/gnl13299 PMCID: 4351023
95. Honda M, Wong SL, and Healy MA, et al (2017) Long-term trends in primary sites of gastric adenocarcinoma in Japan and the United States J Cancer 8 1935–1942 https://doi.org/10.7150/jca.19174 PMID: 28819392 PMCID: 5559953
96. Mahar AL, Coburn NG, and Kagedan DJ, et al (2016) Regional variation in the management of metastatic gastric cancer in Ontario Curr Oncol 23 250–257 https://doi.org/10.3747/co.23.3123 PMID: 27536175 PMCID: 4974032
97. Hua Z, Zheng X, and Xue H, et al (2017) Long-term trends and survival analysis of esophageal and gastric cancer in Yangzhong, 1991–2013 PLoS One 12 e0173896 https://doi.org/10.1371/journal.pone.0173896
98. Castro C, Bosetti C, and Malvezzi M, et al (2014) Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015 Ann Oncol 25 283–290 https://doi.org/10.1093/annonc/mdt486
Supplement
Table S1. Recommended first-line regimens for advanced GC in Europe, US and Japan.
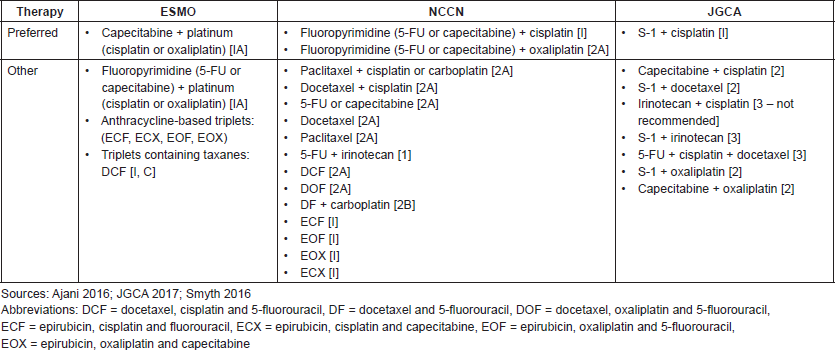
Table S2. Key recommendations for first- and second-line treatment of advanced and metastatic GC and GEJC.
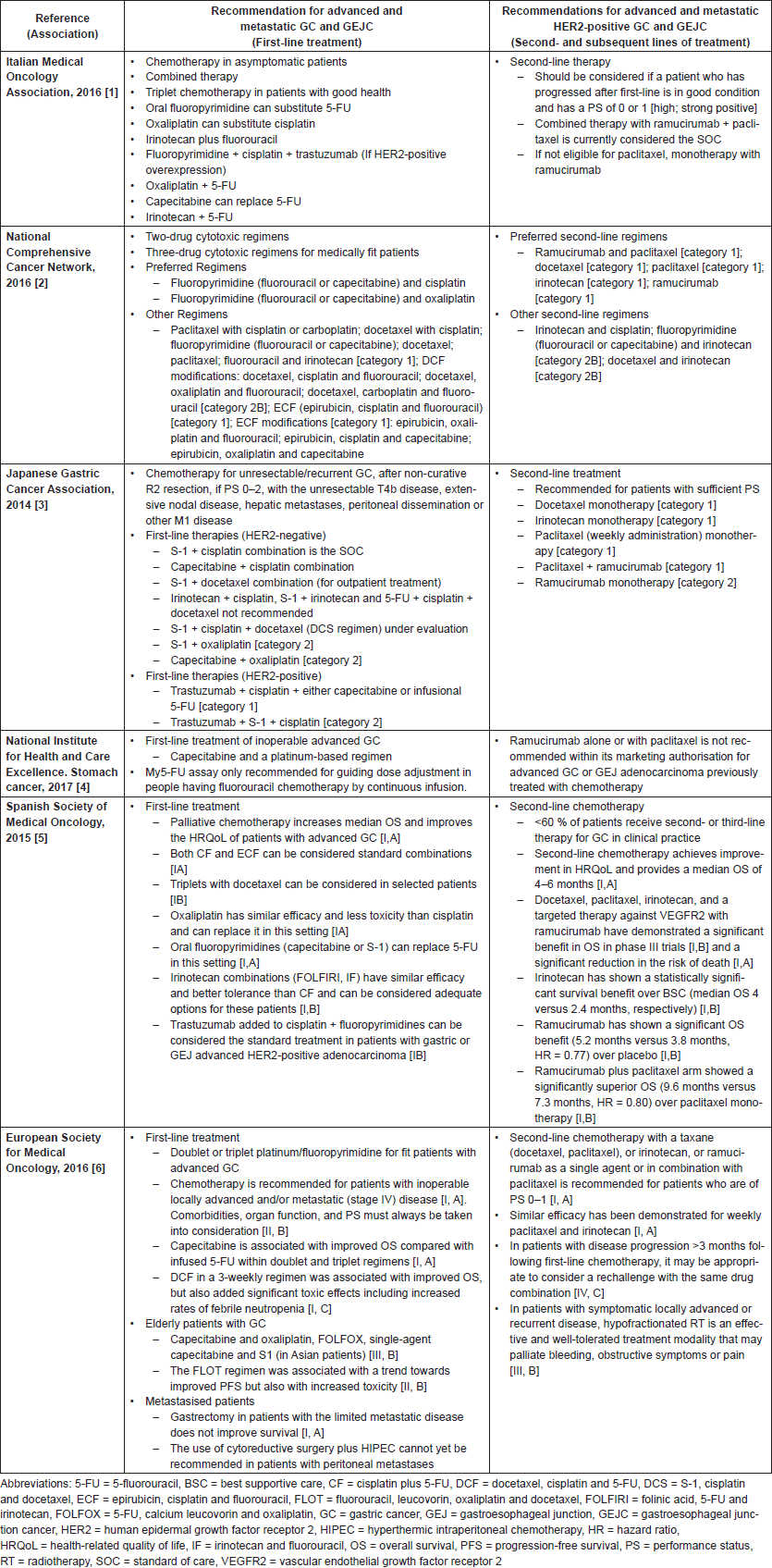
Table S3. Most prescribed chemotherapy regimens in Western Europe, the US and Japan.
References
1. AIOM 2016. Linee Guida. Neoplasie dello stomaco [http://www.aiom.it/professionisti/documenti-scientifici/linee-guida/stomaco/1,712,1] Date accessed: 02/04/17
2. National Comprehensive Cancer Network (NCCN) Guidelines. Esophageal and Esophagogastric Junction Cancer Treatment Regimens (2016)
3. Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer 20 1–19
4. National Institute for Health and Care Excellence. Stomach cancer [https://www.nice.org.uk/guidance/conditions-and-diseases/cancer/stomach-cancer] Date accessed: 24/04/17
5. Martin-Richard M, Custodio A, and Garcia-Giron C, et al (2015) Seom guidelines for the treatment of gastric cancer 2015 Clin Transl Oncol 17 996–1004
6. Smyth EC, Verheij M, and Allum W, et al (2016) Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 27 v38–v49






