Information perception, wishes, and satisfaction in ambulatory cancer patients under active treatment: patient-reported outcomes with QLQ-INFO25
Ana Catarina Pinto1,2, Fernando Ferreira-Santos3,4, Lissandra Dal Lago1, Evandro de Azambuja1,2, Francisco Luís Pimentel5, Martine Piccart-Gebhart1 and Darius Razavi6,7
1Medicine Department, Medical Oncology Unit, Institut Jules Bordet, Université Libre de Bruxelles, Boulevard de Waterloo, 121 (7th Floor), 1000 Brussels, Belgium
2Br.E.A.S.T. Data Centre, Institut Jules Bordet, Brussels 1000, Belgium
3Laboratory of Neuropsychophysiology, Faculty of Psychology and Education Sciences, University of Porto, Porto 4200-135, Portugal
4Developmental Cognitive Neuroscience Unit, UCL Institute of Child Health, London WC1N 1EH, UK
5Health Sciences, University of Aveiro, Aveiro 3810-193, Portugal
6Psychosomatic and Psycho-Oncology Research Unit, Université Libre de Bruxelles, Brussels 1050, Belgium
7Psycho-Oncology Clinic, Institut Jules Bordet, Université Libre de Bruxelles, Brussels 1000, Belgium
Correspondence to: Ana Catarina Pinto. Email: ana-catarina.pinto@bordet.be
Abstract
Background: Information is vital to cancer patients. Physician–patient communication in oncology presents specific challenges. The aim of this study was to evaluate self-reported information of cancer patients in ambulatory care at a comprehensive cancer centre and examine its possible association with patients’ demographic and clinical characteristics.
Patients and methods: This study included adult patients with solid tumours undergoing chemotherapy at the Institute Jules Bordet’s Day Hospital over a ten-day period. EORTC QLQ-C30 and QLQ-INFO25 questionnaires were administered. Demographic and clinical data were collected. Descriptive and inferential statistics were used.
Results: 101 (99%) fully completed the questionnaires. They were mostly Belgian (74.3%), female (78.2%), with a mean age of 56.9 ± 12.8 years. The most frequent tumour was breast cancer (58.4%). Patients were well-informed about the disease and treatments, but presented unmet information domains. The Jules Bordet patients desired more information on treatment side effects, long-term outcome, nutrition, and recurrence symptoms. Patients on clinical trials reported having received less information about their disease and less written information than patients outside clinical trials. Higher information levels were associated with higher quality of life (QoL) scores and higher patient satisfaction.
Conclusion: Patients were satisfied with the information they received and this correlated with higher QoL, but they still expressed unmet information wishes. Additional studies are required to investigate the quality of the information received by patients enrolled in clinical trials.
Keywords: ambulatory care facility, cancer, information, physician–patient relations, quality of life, questionnaires
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Patients reporting good communication with their doctors are more likely to be satisfied with their care, share pertinent information for accurate diagnosis, follow advice, and adhere to treatments [1–3]. Surveys consistently show that patients want better communication with their doctors [2]. Moreover, they show that communication problems correlate positively with patients’ distress and anxiety, causing anticipatory nausea and vomiting during chemotherapy, criticism about information received during hospital visits, and even low rates of recruitment into clinical trials [2, 3]. These circumstances are also professionally and personally unrewarding for health-care staff [1, 2].
Earlier studies evaluating the delivery of information according to patients’ needs recognised that giving patient-tailored information contributed to lowering their anxiety levels [4]. Such studies have also shown that doctors’ decision-making on giving information is based on subjective criteria with a strong cultural influence; it correlates poorly with paired patients’ assessments and causes the physician to attain a conservative attitude [4–6]. Reasons may include both personality and attitudinal characteristics of patients and their health carers. Issues at the level of the cancer care delivery systems also constitute an impediment to good communication [5].
Doctors rate poorly in studies identifying patient misunderstanding, typically underestimating the proportion of patients misreporting information [7]. These results are in agreement with the body of research demonstrating that most clinicians cannot accurately detect patient emotional states [7, 8]. Evidence shows that few doctors recognise the different preferences that their patients have for both type and amount of information. The desire for more information is sometimes confused with a presumed wish to participate in clinical decision-making. For example, in women with breast cancer, research has shown that the majority prefers a relatively passive role in decision-making but require, nevertheless, a large amount of information regarding treatment options [9].
To better understand how cancer patients perceive the information they receive from health-care professionals and making use of a tool devised with this specific purpose, with the aim of delivering tailored standardised information to patients in our centre at a later time point, we carried out a cross-sectional observational study assessing the self-reported information regarding disease, treatment, prognosis, and supportive care in patients attending the ambulatory care facility of our comprehensive cancer centre.
Patients and methods
Patients
Eligible patients were adults (≥18 years) with histologically proven solid tumours, receiving any line of intravenous chemotherapy at the Institute Jules Bordet’s (IJB) Day Hospital in Brussels, Belgium. They also had to be literate and fluent in either French or English. Patients with neurological impairment preventing the comprehension of the questionnaires were excluded. Ethics committee approval was obtained, as was informed consent for all patients before study enrollment.
Questionnaires
Patients completed the EORTC QLQ-C30 questionnaire (version 3.0) and its information module EORTC QLQ-INFO25. The EORTC QLQ-C30 (v. 3.0) is a validated, widely used questionnaire, suitable for observational, experimental, and randomised clinical trials, containing five functional scales (physical, role, emotional, social, and cognitive function), three symptom scales (fatigue, pain, and nausea/vomiting), one global health status/quality of life (QoL) scale, and six single items (symptoms and financial impact) [10]. It contains 30 questions, and the response format is a four-point Likert scale (1 = not at all, 2 = a little, 3 = quite a bit, and 4 = very much) for questions 1–28. The response format for questions 29 and 30 is a seven-point scale (ranging from 1—very poor to 7—excellent). The questions are answered by circling the option the patient considers applicable to him/her [10].
Validated by Arraras et al [11] as a reliable measure of patients’ perceptions of received information, QLQ-INFO25 (hereupon referred to as INFO25) is a module of the EORTC QLQ-C30 that can be used in clinical practice and research to evaluate information-based interventions and programmes [11]. It contains four multi-item scales (information about the disease, medical tests, treatments, and other services) and eight single items (e.g., places of care, self-help to get well, information channels, and information satisfaction and usefulness). INFO25 also includes two open questions allowing patients to write about topics of their choice. Overall, INFO25 comprises 25 questions in a four-point Likert scale response format (1 = not at all, 2 = a little, 3 = quite a bit, and 4 = very much), except for the dichotomous (yes/no) questions 51 and 52 and 54 and 55 (for questionnaire specimens please visit http://groups.eortc.be/qol/eortc-qlq-c30 and http://groups.eortc.be/qol/why-do-we-need-modules).
Demographic data (age, gender, marital status, level of education, profession, and place of birth) were collected. Clinical data (primary tumour site, extent of disease [limited/disseminated], World Health Organisation performance status [ECOG], and treatment line received) were also collected, through medical chart review and direct questioning of patients in case of missing chart information.
Procedures
Every one out of three eligible patients was identified from IJB’s Day Hospital treatment daily schedules and was invited to participate while in the Day Hospital setting. Participation only took place after informed consent signature.
The same person (A.C.P.) applied the questionnaires for all the patients included in this study. Questionnaires were administered on a paper-printed format, self-reported on a paper-and-pencil basis. Administration time was estimated to be 10–15 min for each of the EORTC questionnaires. The investigator could read out the questions for the patients if required, and write the answers on the form, without exerting any kind of influence towards answer choice. Patients answered the questionnaire in the presence of the investigator and in the absence of proxy (to allow for privacy and avoid interference).
Statistical considerations
A power analysis using G*Power3 software (version 3.1.2.) was conducted to estimate the appropriate sample size (12). Following Arraras et al’s specifications [11], it was estimated that a sample of 94 patients would have 90% power to detect a ten-point difference in responses in a two-tailed Mann–Whitney U test at 0.05 significance.
To address the primary objective (participants’ self-reported information), the data collected were summarised by obtaining descriptive statistics of central tendency and variability (significance level of 0.05). Cronbach’s alpha values were computed for the INFO25 scales defined by Arraras et al to assess their internal consistency [11]. Descriptive statistics of scale responses for subgroups of interest were also computed, namely those defined by age, gender, birthplace, marital status, education level, profession, primary tumour site, performance status (ECOG), disease extent, and treatment line received. To address association between self-reported information and socio-demographic and clinical characteristics, eight a priori hypotheses (H) based on the literature [6, 13–17] were tested, using Mann–Whitney U tests (one or two-tailed, as appropriate):
H1: Women’s information level > men’s information level.
H2: Women’s information satisfaction < men’s information satisfaction.
H3: Younger patients’ information level (≤65 years) > older patients’ information level (66–84; ≥85 years).
H4: Information level of patients with higher education > information level of patients with lower education.
H5: Written information of less educated patients < written information of more educated patients.
H6: Wishes of patients with lower education < wishes of patients with higher education.
H7: Information level under curative treatment ≠ information level under palliative treatment.
H8: Information level on ≥ second chemotherapy line ≠ information level on first chemotherapy line.
The remaining clinical and demographic characteristics of the patients were subjected to an exploratory analysis, and comparison of item and scale responses between clinical and demographic subgroups were performed by means of Mann–Whitney U tests (for comparisons between two groups) or Kruskal–Wallis one-way analysis of variance (for comparisons between more than two groups). Unplanned exploratory hypotheses tests may lead to the inflation of the Type I error rate due to multiple comparisons. However, because of the potential clinical relevance of the findings, the significance level (α) for the exploratory analyses was kept at 0.05, and inferences were performed on uncorrected p-values. Bonferroni corrected results were further calculated and statistically significant comparisons that survive the correction were signaled where appropriate.
All statistical analyses were conducted using Microsoft Excel and SPSS v17.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Demographic and clinical variables—descriptive statistics
Between 30 May and 8 June 2011, 102 patients were offered the chance to participate in the study, and all agreed to participate. 101 patients (99%) fully completed both questionnaires. One patient was too tired to complete the questionnaire and was therefore excluded from the analysis.
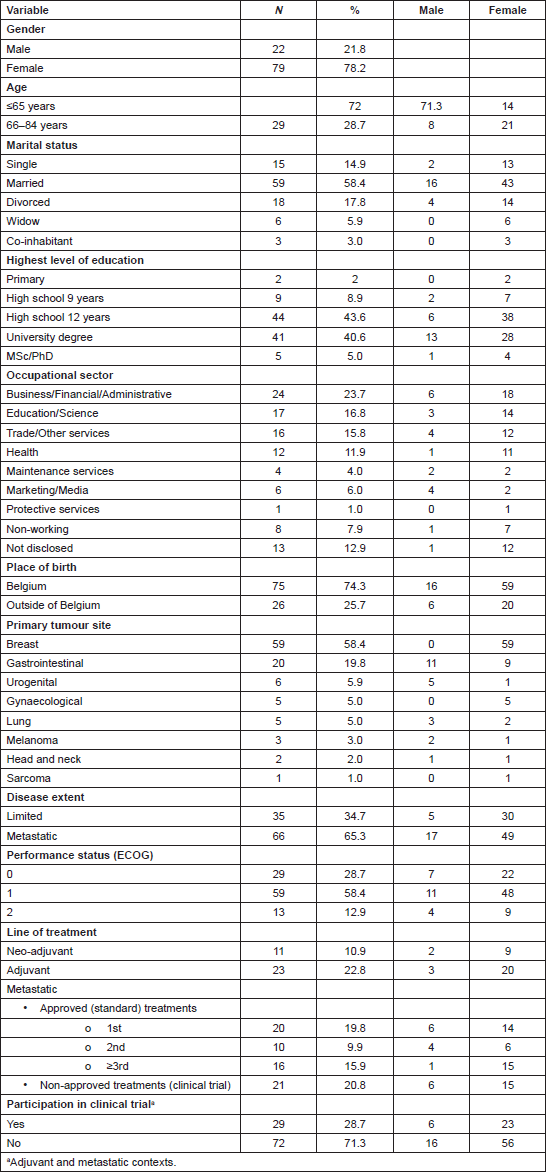
The patients’ demographic and clinical characteristics are presented in Table 1. Twenty-nine patients (28.7%) were participating in a clinical trial. The majority had breast cancer diagnosis (59 patients; 58.4%).
INFO25 questionnaire—descriptive statistics
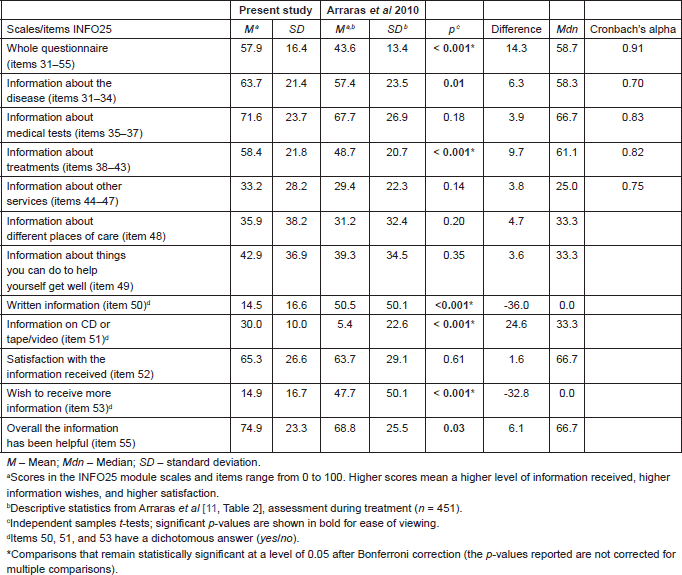
The INFO25 scales showed good internal consistency, with Cronbach’s alpha values in line with the findings of Arraras et al [11]. The descriptive statistics of INFO25 scales/items, internal consistency and comparison with the module’s validation study are shown in Table 2.
INFO25 open questions (wishes about more or less information)
Patients were asked to respond to the two open questions contained in INFO25, that is, to list topics they would either like to receive more information about (question 53b, following affirmative response to 53a), or less information about (question 54b, following affirmative response to 54a). Regarding question 53b, we separated the answers dichotomously into: (1) topics already mentioned in INFO25 and (2) new topics suggested by the patients. As for question 54a, only one patient responded affirmatively, writing in 54b that she desired to receive less information ‘when her health condition deteriorated’.
Fifty-six patients answered item 53a affirmatively (three did not answer question 53b: designated as missing items). The most frequent INFO25 topics about which patients expressed their wish to receive more information were possible treatment side effects (item 40; 11 patients) and results of the medical tests already performed (item 37; nine patients) [data not shown]. The predominant new topics on which patients wanted more information were long-term outcome (eight patients), nutrition (four patients), and recurrence symptoms (four patients) [data not shown].
Inferential statistics
Of the eight hypotheses on association between self-reported information and the socio-demographic and clinical characteristics of patients, three associations were significant while five were not. The statistically significant associations were related to age (H3), treatment type (H7), treatment lines (H8), the non-significant ones were linked to gender (H1 and H2), and education (H4, H5, and H6).
Table 1. Demographic and clinical characteristics of the patients.
Regarding age (H3), self-reported information was statistically significantly higher in older patients (≥66 years old) for written information (M = 62.1, SD = 49.4 versus M = 36.1, SD = 48.4; p = 0.02) and information on CD/tape/video (M = 100.0, SD = 0 versus M = 86.1, SD = 34.8; p = 0.03). In younger patients (≤65 years old) the results were significant regarding satisfaction with the information received (M = 67.6, SD = 28.0 versus M = 59.8, SD = 22.5; p = 0.049) and helpfulness of the overall information received (M = 78.2, SD = 22.5 versus M = 66.7, SD = 23.6; p = 0.01).
With reference to treatment type (H7), self-reported information was statistically significantly higher in patients undergoing treatment with curative intent (M = 60.0, SD = 35.1) than in patients receiving palliative treatment (M = 33.8, SD = 34.8) with respect to self-help to get well (p = 0.001) and also to overall helpfulness of information (M = 82.9, SD = 20.4 versus M = 70.7, SD = 23.8; p = 0.01). The reverse association was true with regard to the information on CD/tape/video, with higher mean scores for patients undergoing palliative treatment than those being treated with curative intent (M = 98.5, SD = 12.3 versus M = 74.3, SD = 44.3; p < 0.001, respectively).
Finally, regarding treatment lines (H8), patients who had undergone two or more chemotherapy lines presented a higher mean score for the information obtained from CD/tape/video than patients having received only one line (M = 100.0, SD = 0 versus M = 81.5, SD = 39.2; p = 0.002).
Table 2. Descriptive statistics of INFO25 scales/items, reliability, and comparison with the module’s validation study.
Exploratory analyses
Regarding place of birth and marital status, the only statistically significant association was a higher score for satisfaction with information received by patients born outside of Belgium (M = 74.4, SD = 23.7 versus M = 62.2, SD = 27.0; p = 0.05).
Figure 1 shows the comparisons made with the Global QoL scale of the QLQ-C30 questionnaire. Self-reported information was statistically significantly higher in patients with higher QoL scores for the whole questionnaire, information about medical tests, and satisfaction with information.
Regarding information about the disease and written materials, Figure 2 shows that self-reported information was statistically significantly higher for patients not participating in clinical trials than for those who were.
Figure 3 portrays statistically significant associations between satisfaction with information and all the scales/items of INFO25 depicted, except for items 50 and 51. This means that patients were satisfied with the received information. Similar results were obtained for the association between information wishes and all the scales/items of INFO25 depicted, with the exception of item 51 (Figure 4).
Discussion
Our study showed that patients attending the Day Hospital at IJB presented higher global information than the validation study sample [11], that their wishes to receive more information were lower and the helpfulness of the overall information received were higher [11].
Our study found similar data to the INFO25 validation study conducted by Arraras et al [11] regarding age distribution and gender. The education level of our sample was high, reflecting the educational attainment level of Belgium itself [18].
Open questions in health-related QoL evaluation questionnaires have been shown to be valued by patients, giving them freedom to list what they consider most important [19]; nevertheless, the analyses performed on these data are mainly qualitative. Patients wanting the results of medical tests stressed the desire to receive them on a hard-paper copy (e.g., blood tests or imaging), and also of having radiological findings explained to them during consultations, which has also been found by others [16]. Our working hypotheses H1, H2, and H4 to H6 were not supported. This is contrary to most published studies [1, 5, 6, 13, 15, 20, 21] and might be partially explained by the patients’ homogeneous and high levels of education.
Younger patients were more satisfied and found the information given more helpful than older patients (H3), which might derive from less attention so far given to the older cancer age groups regarding informational needs [22–24]. Nevertheless, older patients received more written and CD/tape/video information than younger patients. We can only speculate, but it might be an attempt of oncologists to overcome possible geriatric syndromes (e.g., hearing loss, cognitive dysfunction) in this age group by delivering information preferably on those formats [22].
Patients treated with palliative intent seem less well-informed about self-help and find the information given them less helpful. Others have shown that these patients have specific information needs, namely on physical symptoms, euthanasia [25], and complementary and alternative medicine (CAM) than patients treated with curative intent [13, 26] and that physicians are viewed as offering little useful input in this respect [20]. Furthermore, patients on palliative treatment or who received more than two lines of chemotherapy reported obtaining more written and CD/tape/video information than patients on earlier cancer stages, probably due to their longer disease duration, rendering them more likely to have received the results of computed tomography and positron emission tomography scans in such formats, which is becoming increasingly common.
Our exploratory analyses indicate that patients with higher information levels also report a higher QoL. More satisfied patients may require less additional information about specific topics, but they do continue to be open to novel information in general. However, our exploratory analyses indicated–unexpectedly–that patients outside clinical trials had more information about their disease, including more written information. Although we conducted additional statistical tests to look for confounding variables, none were found. These data mirror other recent evidence indicating that patients exhibit an unacceptably low understanding about the trials they join. Studies have shown that a fundamental factor in poor trial recruitment comes down to the communication skills of doctors [27, 28]. These are worrisome findings since they may be preventing many patients from the benefice of innovative therapies. On the other hand, interventions to improve the skills of doctors explaining clinical trials (Phase I, II, and III trials) do not suffice to provide key clinical information to patients [7, 27] and for them to understand it correctly. This underlines the urgent need to improve the content of patient informed sheet/consent forms, among other information provided to patients in connection with clinical trials.
We acknowledge that our study has some limitations, namely the fact that it was a single-centre study restricted to patients in an ambulatory setting. The sample contained both early and advanced cancer patients, preventing a clear separation among people in different disease stages and such, together with the fact that a longitudinal approach was not carried out, hinders the possibility of an assessment of the information given to cancer patients throughout the disease process from diagnosis to cure or end of life. It was also a single-institution study, and may therefore not represent the reality of Day Hospital patients’ of other institutions and also other countries.
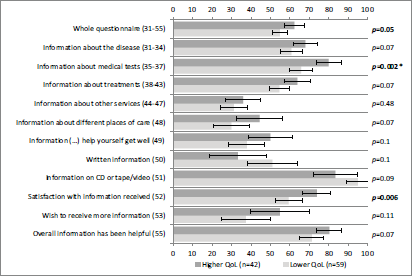
Figure 1: Self-reported information according to the QoL scale of QLQ-C30. A lower QoL (lowest through mean) and higher QoL (mean through highest) correspond to scores on the QoL scale of QLQ-C30. Vertical axis: INFO25 questions; horizontal axis: scores on the INFO25 questions (items range from 0 to 100). Higher scores mean a higher level of information received, higher information wishes, and higher satisfaction. Error bars represent 95% confidence intervals. P-values (Mann–Whitney U tests) appear on the right, with significant p-values shown in bold and comparisons that remain statistically significant after Bonferroni correction signalled with an asterisk.
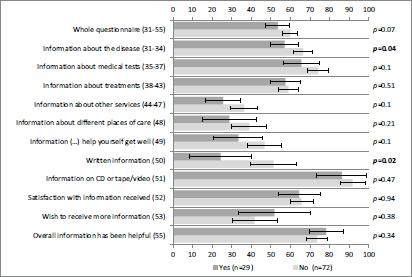
Figure 2: Self-reported information according to clinical variables: clinical trial participation. Vertical axis: INFO25 questions; horizontal axis: scores on the INFO25 questions (items range from 0 to 100). Higher scores mean a higher level of information received, higher information wishes, and higher satisfaction. Error bars represent 95% confidence intervals. P-values appear on the right, with significant p-values (Mann–Whitney U tests) shown in bold and comparisons that remain statistically significant after Bonferroni correction signalled with an asterisk.
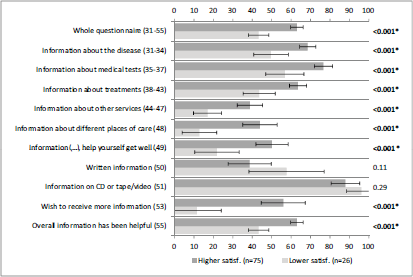
Figure 3: Self-reported information according to satisfaction with information. A lower satisfaction (lowest through mean) and higher satisfaction (mean through highest) correspond to answers to item 52 (‘satisfaction with the amount of information received‘) of INFO25. Vertical axis: INFO25 questions; horizontal axis: scores on the INFO25 questions (items range from 0 to 100). Higher scores mean a higher level of information received, higher information wishes, and higher satisfaction. Error bars represent 95% confidence intervals. P-values (Mann–Whitney U tests) appear on the right, with significant p-values shown in bold and comparisons that remain statistically significant after Bonferroni correction signalled with an asterisk.
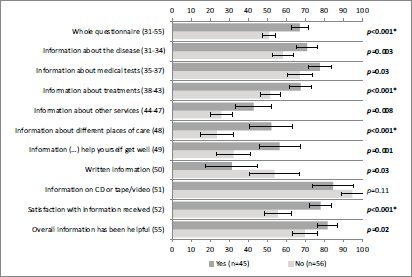
Figure 4: Self-reported information according to wishes for more information. More information wishes (yes) and less information wishes (no) correspond to answers to item 53a (‘Do you wish to receive more information?’) of INFO25. Vertical axis: INFO25 questions; horizontal axis: scores on the INFO25 questions (items range from 0 to 100). Higher scores mean a higher level of information received, higher information wishes, and higher satisfaction. Error bars represent 95% confidence intervals. P-values (Mann–Whitney U tests) appear on the right, with significant p-values shown in bold and comparisons that remain statistically significant after Bonferroni correction signalled with an asterisk.
Additional shortcomings concern INFO25 itself, particularly the items about the format in which information was given (written information and information on CD/tape/video). Unfortunately, those items do not discriminate which information is displayed in which support (it can refer to, e.g., test results, radiological images, and rehabilitation services announcements), possibly misleading patients in their responses, and thus rendering the interpretation of results challenging. Moreover, the item on CD/tape/video may even be considered outmoded, since information access presently emphasises the Internet and other technology-based health resources that are likely to rank high in terms of patients’ preferences, particularly the youngest [24, 28].
Conclusions
Patients attending a day hospital in a busy comprehensive cancer centre in Europe are generally well-informed but would appreciate additional information on nutrition, long-term outcome, and recurrence symptoms, along with more details about medical tests and treatment side effects. The information perceived by patients in clinical trials is deficient, however, and interventional studies using a standardised format of delivering information are needed. INFO25 is a useful instrument for baseline assessment and intervention measures, although it would need to be updated to accommodate the role of new information sources available to patients, especially the Internet and other technology-based health tools that are now widespread.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank Dr Dominique de Valeriola and Dr Yassine Lalami of IJB’s Day Hospital and all the staff working there for their helpfulness and cooperation. We also thank the patients who participated in the study for their extraordinary kindness, patience, and cooperation. Finally, we thank Carolyn Straehle and Dr Hatem A Azim Jr for kindly revising the text.
References
1. Duffy FD et al (2004) Assessing competence in communication and interpersonal skills: the Kalamazoo II report Acad Med 79(6) 495–507 DOI: 10.1097/00001888-200406000-00002 PMID: 15165967
2. Brédart A, Bouleuc C and Dolbeault S (2005) Doctor-patient communication and satisfaction with care in oncology Curr Opin Oncol 17(4) 351–4 DOI: 10.1097/01.cco.0000167734.26454.30 PMID: 15933466
3. Baile WF et al (2000) SPIKES-A six-step protocol for delivering bad news: application to the patient with cancer Oncologist 5(4) 302–11 DOI: 10.1634/theoncologist.5-4-302 PMID: 10964998
4. De Valck C, and van de Woestijne KP (1996) Communication problems on an oncology ward Patient Educ Couns 29(2) 131–6 DOI: 10.1016/0738-3991(96)00908-1 PMID: 9006230
5. Fallowfield L and Jenkins V (1999) Effective communication skills are the key to good cancer care Eur J Cancer 35(11) 1592–7 DOI: 10.1016/S0959-8049(99)00212-9
6. Fallowfield L, Ford S and Lewis S (1994) Information preferences of patients with cancer Lancet 344(8936) 1576 DOI: 10.1016/S0140-6736(94)90386-7 PMID: 7983974
7. Gattellari M et al (1999) Misunderstanding in cancer patients: why shoot the messenger? Ann Oncol 10(1) 39–46 DOI: 10.1023/A:1008336415362 PMID: 10076720
8. Hack TF et al (2005) The communication goals and needs of cancer patients: a review Psychooncology 14(10) 831–45 discussion 846–7 DOI: 10.1002/pon.949 PMID: 16200519
9. Beaver K et al (1996) Treatment decision making in women newly diagnosed with breast cancer Cancer Nurs 19(1) 8–19 DOI: 10.1097/00002820-199602000-00002 PMID: 8904382
10. Aaronson NK et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology J Natl Cancer Inst 85(5) 365–76 DOI: 10.1093/jnci/85.5.365 PMID: 8433390
11. Arraras JI et al (2010) An international validation study of the EORTC QLQ-INFO25 questionnaire: an instrument to assess the information given to cancer patients Eur J Cancer 46(15) 2726–38 DOI: 10.1016/j.ejca.2010.06.118 PMID: 20674333
12. Faul F et al (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences Behav Res Methods 39(2) 175–91 DOI: 10.3758/BF03193146 PMID: 17695343
13. Hardy R and Hardy G (1979) Patterns of communication to cancer patients: a descriptive analysis J Tenn Med Assoc 72(9) 656–8 PMID: 537355
14. Wilkinson S (1991) Factors which influence how nurses communicate with cancer patients J Adv Nurs 16(6) 677–88 DOI: 10.1111/j.1365-2648.1991.tb01726.x PMID: 1869716
15. Fallowfield L (1993) Giving sad and bad news Lancet 341(8843) 476–8 DOI: 10.1016/0140-6736(93)90219-7 PMID: 8094499
16. Fujimori M and Uchitomi Y (2009) Preferences of cancer patients regarding communication of bad news: a systematic literature review Jpn J Clin Oncol 39(4) 201–16 DOI: 10.1093/jjco/hyn159 PMID: 19190099
17. Rutten LJF et al (2005) Information needs and sources of information among cancer patients: a systematic review of research (1980-2003) Patient Educ Couns 57(3) 250–61 DOI: 10.1016/j.pec.2004.06.006 PMID: 15893206
18. EUROSTAT (2013) Tertiary educational attainment by sex, age group 25-64 and NUTS 2 regions [http://epp.eurostat.ec.europa. eu/tgm/table.do?tab=table&plugin=1&language=en&pcode=tgs00109] Date accessed: 14/06/13
19. Hearn J and Higginson IJ (1999) Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group Qual Health Care 8(4) 219–27 DOI: 10.1136/qshc.8.4.219
20. Ha JF and Longnecker N (2010) Doctor-patient communication: a review Ochsner J 10(1) 38–43
21. Yardley IE, Yardley SJ and Wu AW (2010) How to discuss errors and adverse events with cancer patients Curr Oncol Rep 12(4) 253–60 DOI: 10.1007/s11912-010-0109-0 PMID: 20473648
22. Van Weert JCM et al (2013) Older cancer patients’ information and communication needs: what they want is what they get? Patient Educ Couns 92(3) 388–97 DOI: 10.1016/j.pec.2013.03.011 PMID: 23602863
23. Puts MTE et al (2012) A systematic review of unmet needs of newly diagnosed older cancer patients undergoing active cancer treatment Support Care Cancer 20(7) 1377–94 DOI: 10.1007/s00520-012-1450-7 PMID: 22476399
24. Xie B (2009) Older adults’ health information wants in the internet age: implications for patient-provider relationships J Health Commun 14(6) 510–24 DOI: 10.1080/10810730903089614 PMID: 19731124
25. Englert M (2005) [Depenalized practice of euthanasia in Belgium] Rev Med Liege 60(4) 227–30 PMID: 15943099
26. Whippen DA and Canellos GP (1991) Burnout syndrome in the practice of oncology: results of a random survey of 1,000 oncologists J Clin Oncol 9(10) 1916–20 PMID: 1919641
27. Jenkins V et al (2011) What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions J Clin Oncol 29(1) 61–8 DOI: 10.1200/JCO.2010.30.0814
28. Maddock C et al (2012) Online information as a decision making aid for cancer patients: recommendations from the Eurocancercoms project Eur J Cancer 48(7) 1055–9 DOI: 10.1016/j.ejca.2011.08.018






