Vitamin supplement consumption and breast cancer risk: a review
Alessandro M Misotti and Patrizia Gnagnarella
Division of Epidemiology and Biostatistics, European Institute of Oncology, Milan 20141, Italy
Correspondence to: Alessandro Maria Misotti. E-mail: alessandro.misotti@ieo.it
Abstract
Background: Breast cancer is the most frequently diagnosed cancer globally, and studies provide contradictory results about the possible effects of vitamin supplementation to reduce cancer risk. Our aim was to conduct a review to better investigate whether vitamin supplements given orally modify breast cancer risk.
Methods: We conducted a comprehensive, systematic bibliographic search of the medical literature to identify relevant studies. Case-control, cohort studies, and randomised controlled trials (RCTs) published up to August 2013 that reported cancer risk estimates for vitamin supplementation were included. For each study, we retrieved study characteristics, study population, exposure evaluation, and risk estimates.
Results: We identified 26 studies (14 cohort, 11 case-control, and one RCT) and overall, we found 104 estimates. We grouped all the estimates into six supplementation categories: vitamin A and beta-carotene, B-group vitamins and folic acid, vitamin C, vitamin D, vitamin E, and multivitamins. Only a few studies showed a statistically significant association between the consumption of supplemental vitamins and the occurrence of breast cancer, and most of the significant estimates were found in case-control studies. The results found in prospective studies seem to be in the opposite direction.
Conclusion: The role of vitamin supplements in preventing breast cancer still remains unclear, considering our review. Although biologic mechanisms exist to support the anticancer effects of vitamins, there is no clear evidence for an effect in cancer prevention for vitamin supplements. Further investigations are warranted to elucidate the mechanisms by which vitamin supplementation can modify breast cancer development.
Keywords: breast cancer, dietary supplements, vitamin supplementation, vitamins, antioxidants.
Introduction
Breast cancer is the most frequently diagnosed cancer globally and also the main cause of death among women [1]. The highest incidence rates are reported in Western and Northern Europe, Australia, and North America, which is mainly owing to changes in reproductive and hormonal factors, and the availability of screening programs [1]. Breast cancer is a complex disorder and can occur as a result of a process that includes oxidative stress and lipid peroxidation mechanisms [2] beyond gene--environment interactions and hormonal expression [3]. Biologic mechanisms exist to support the anticancer effects of dietary antioxidant vitamins and the overall impression is that intake of some vitamins may play a substantial role in the prevention of cancer [4–6], but the findings are not strong in this regard.
Observational studies and randomised controlled trials provide contradictory results about the possible preventive effects of vitamin supplementation to reduce cancer risk [7–10]. Bjelakovic et al [11], in a recent meta-analysis, found no evidence that antioxidant supplements can prevent gastrointestinal cancers; in contrast, they found a possible increasing effect on mortality for excessive consumption. The second World Cancer Research Fund/American Institute for Cancer Research expert report, published in 2007 [6], states that high-dose nutrient supplements can be protective or can cause cancer. The strongest evidence refers to beta-carotene supplementation and lung cancer; actually, high doses of beta-carotene can cause lung cancer in tobacco smokers. The evidence is limited for other vitamins, and there are no recommendations for breast cancer [6].
The use of dietary supplements, such as vitamins and multivitamins, is constantly increasing worldwide [12, 13]. Half of the US adult population (49%) regularly consumes one or more supplements. The use of supplements is different between genders: women tend to report a higher consumption than men (53% versus 44%) [14]. In Canada, the use of supplements is 48% in women and 32% in men [15], while in Australia, habitual consumers of supplements are 27% of women and 15% of men [16]. Women reporting a regular and high consumption of supplements are 41% and 64% in Sweden and in Denmark, respectively [17]. Consumption of dietary supplements of 28% (age: 35–50) and 34% are reported in Germany and Netherlands, respectively [18, 19]. These percentages tend to decrease in central European countries: 24% of the population uses supplements in the United Kingdom (mostly women), and 20% in France [20, 21]. Mediterranean countries present the lowest consumptions: 5% of the population in Italy [22], 0.5% in men and 6.7% in women in Greece [17]; 6.6% in men and 13.4% in women in Spain [17].
In view of evaluating the number of published reports on the relation between supplement use and breast cancer risk, we decided to conduct a review to investigate whether vitamin supplements given orally modify breast cancer incidence in humans.
Methods
We conducted a comprehensive, systematic bibliographic search of the medical literature to identify relevant studies on vitamin supplementation use and breast cancer risk. A literature search was performed up to August 2013 using the following databases: PubMed, ISIWeb of Science (Science Citation Index Expanded), and Embase. To identify all relevant published papers, our search consisted of a combination of the following MeSH terms: breast neoplasms, dietary supplements, vitamin E, vitamin D, vitamin A, ascorbic acid, vitamin B complex, vitamin B12, carotenoids, folic acid. We also searched for other non-indexed citations, using keywords such as ‘breast’ and ‘vitamin$ or ascorbic’ and ‘supplement$’. The related-records feature was used to amplify the search. In addition, reference lists of relevant articles and reviews were reviewed. The search was limited to human studies but no language or time restrictions were applied. We searched for publications with frequencies or estimates of the relative risk (RR), odd ratio (OR), incidence rate ratio (IRR), and hazard ratio (HR) for breast cancer. We decided to consider, for the review, only cohort studies and case-control studies that analysed the correlation between vitamin supplementation and breast cancer risk. Randomised controlled trials (RCTs) were included whenever they reported analysis on baseline vitamin consumptions, but they were excluded if they provided estimates on experimental vitamin supplementation. Reviews and meta-analyses were not considered eligible but were used to retrieve articles.
Studies reporting the biological mechanism of vitamins in cancer, analysis on dietary vitamins, and supplementation were excluded. We excluded two reports [23, 24] because we found more recent publications based on the same study population [25, 26].
We considered only data about vitamin intake coming exclusively from supplementation. Studies that analysed food plus supplementation intake of vitamins were not considered. We also rejected studies regarding other tumour sites than breast cancer.
For each study, we retrieved the following information:
• Study characteristics: first author, publication year, country and name of the cohort (if available), study design, starting year of the study, type of supplement
• Study population: number of cases and controls or cohort size, age range, menopausal status (if available)
• Exposure evaluation: the upper category of daily intake of supplement (Q max versus the lowest category Q1) or duration in years or users versus non-users
• Statistic: risk estimates, RR, OR, IRR, or HR and 95% confidence intervals (CIs).
Results
We identified 26 eligible studies to investigate the associations of vitamin supplementations with breast cancer risk. Selected studies were 14 cohort [4, 25–37], 11 case-control studies [2, 12, 38–46], and one RCT [47]. The main characteristics of the reviewed studies are presented in Table 1. Most of the studies (n. 19, 73%) were carried in the United States, two in Canada, three in Europe (three cohorts), and two in Asia. The most recent study was a case-control study carriedout in Pakistan from 2009 [46], and the oldest one is from the NHANES I Cohort (1971) [29]. The largest study is the Women’s Health Initiative Observational Study cohort [35] including more than 160 000 subjects, while the smallest is a nested case-control study reported by Wu et al (486 subjects) [42]. Freudenheim et al [39] and Lin et al [37] analysed women in the premenopausal status, the other eight studies [4, 26, 30–32, 35–37] included women in postmenopausal status, and 12 studies [12, 25, 28, 33, 34, 38, 40, 42–46] analysed both categories. In the other studies, no information about menopausal status was reported. We grouped all the estimates into six categories: vitamin A and beta-carotene, B-group vitamins and folic acid, vitamin C, vitamin D, vitamin E, and multivitamins (Table 2).
Vitamin A and beta-carotene
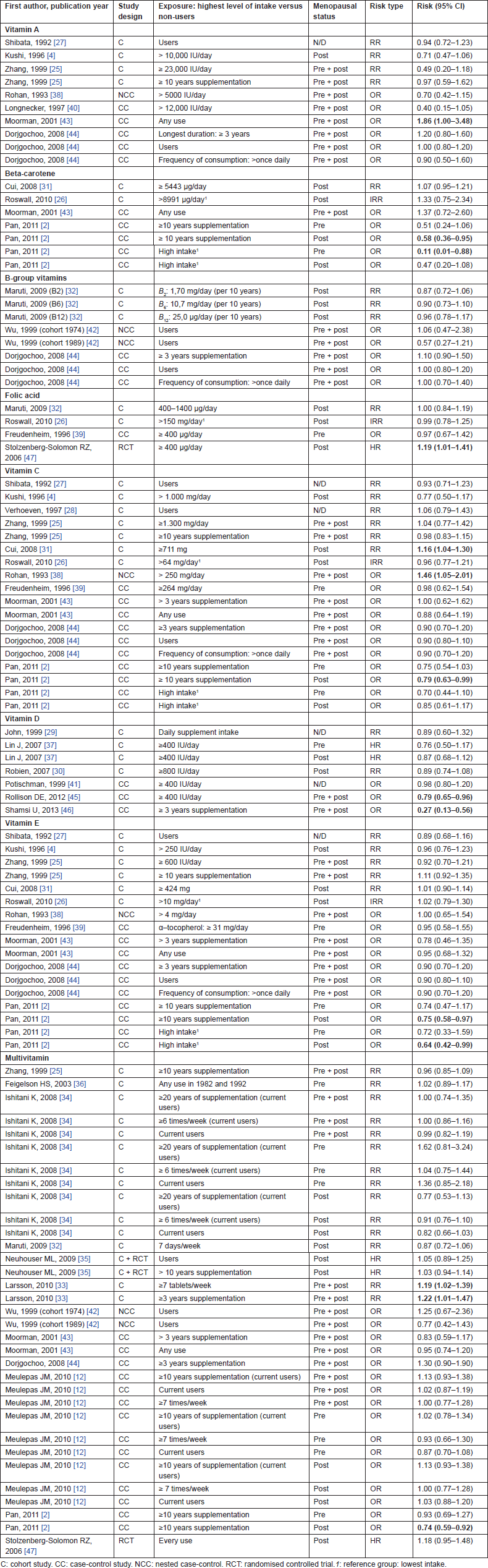
We identified seven studies to investigate the associations of vitamin A supplementation with breast cancer risk. Only one case-control study reported a statistically significant increased risk. Moorman et al [43] found that any use of supplemental vitamin A can increase the risk of breast cancer (OR = 1.86; 95% CI: 1.00–3.48). Examining the other studies, three cohort studies reported a decrease of cancer risk but this effect is not statistically significant [4, 25, 27]. Similar results were found in two case-control studies reported by Longnecker et al [40] and Rohan et al [38]. On the contrary, a case-control study by Dorjgochoo et al [44] found a not significant increased risk (OR = 1.20; 95% CI: 0.80–1.60) for the longest supplemental use.
For beta-carotene supplementation, we found four studies, two cohort [26, 31] and two case-control [2, 43]. The case-control by Pan et al [2] reported a significantly inverse association between high intake of beta-carotene and breast cancer risk (OR = 0.11; 95% CI: 0.01–0.88) in premenopausal women, but this effect was not found in postmenopausal women.
B-group vitamins
We identified six studies to investigate the associations of vitamin B supplementations with breast cancer risk. The authors reported estimates for single vitamin B, for vitamin B complex, and for folic acid (Table 2).
Maruti et al [32] found no association in the VITAL cohort [vitamin B2: RR = 0.87 (95% CI: 0.72–1.06), vitamin B6: RR = 0.90 (95% CI: 0.73–1.10), vitamin B12: RR = 0.96 (95% CI: 0.78–1.17)]. In the Shanghai Breast Cancer Study, Dorjgochoo et al [44] evaluated the consumption of vitamin B1, B2, and niacin supplementation, but the results did not support a relationship with breast cancer incidence for the use of supplements (user versus non-user, duration, and frequency of supplementation).
Wu et al [42] analysed two cohorts consuming a vitamin B-complex. The results were not statistically significant, and the estimates were contrasting (first cohort, 1974: users versus non-users OR=1.06; second cohort, 1989: OR=0.57).
Regarding folic acid supplementation, few data have been published. Data collected from two cohorts [26, 32] and one case-control [39] study did not indicate any effect. Stolzenberg-Solomon et al [47] found an increased risk for a folic acid supplementation greater than 400 µg/day (HR=1.19; 95% CI: 1.01–1.41) in their RCT analysis.
Table 1. Characteristics of the studies included in the review on the association between vitamin supplementations and breast cancer risk.
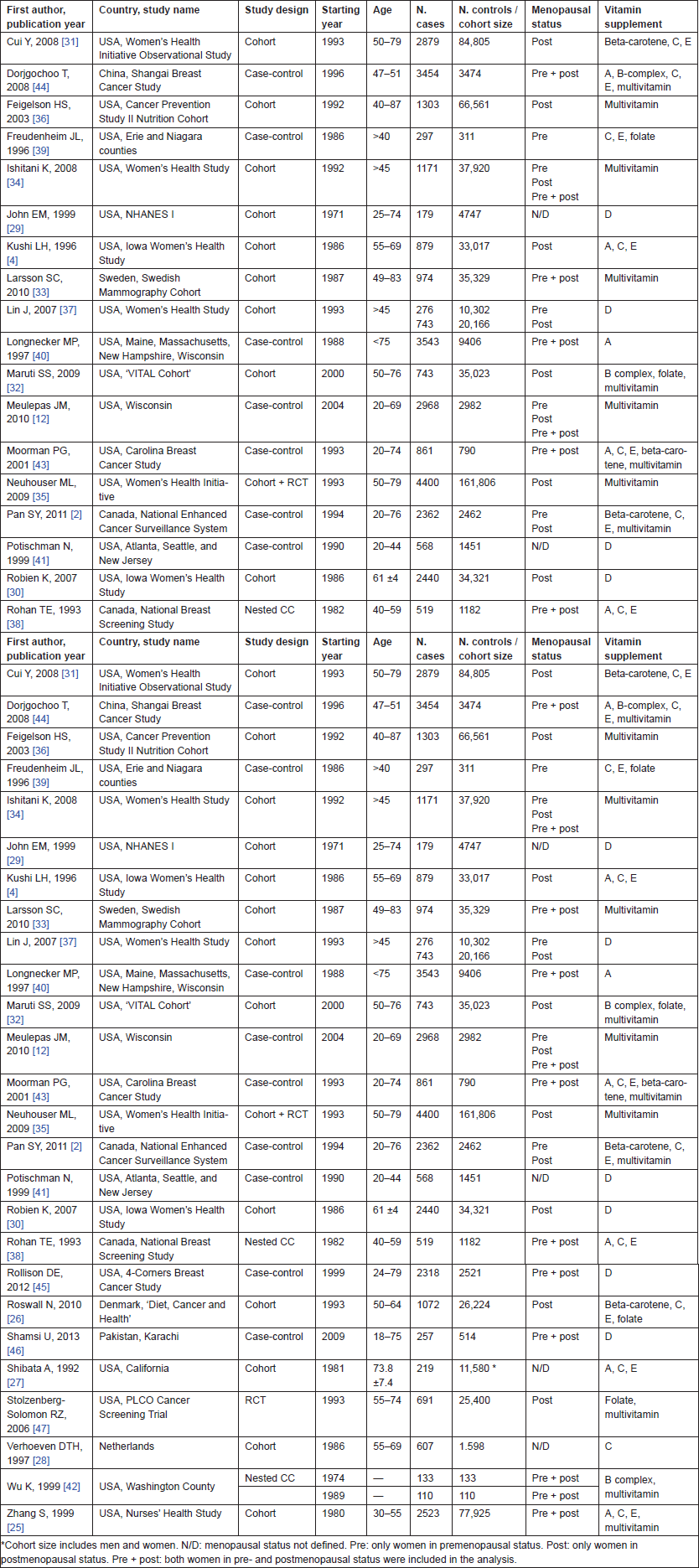
Table 2. Risk estimates for the association of breast cancer risk with vitamin supplementation by highest level of intake versus lowest or non-users
Vitamin C
We found 11 studies, six cohort [4, 25–28, 31], and five case-control studies (one nested case-control study) [2, 38, 39, 43, 44]. Two studies reported a significant increased risk of breast cancer. Cui et al [31] reported an increased risk (RR=1.16; 95% CI: 1.04–1.30) for high supplement intake (≥ 711mg) in a cohort of postmenopausal women. Rohan et al [38] found an increased risk (OR=1.46; 95% CI: 1.05–2.01) for high consumption (250 mg) in a case-control. The results were not statistically significant in the other studies also considering long-duration supplement use or frequency of consumption [25, 43, 44].
Vitamin D
The association between breast cancer risk and vitamin D supplementation has been analysed by three cohort studies [29, 30, 37]. The results suggest a decreased risk of breast cancer, although not significant. We found a statistically significant decreased risk in two case-control studies: Shamsi et al [46] found a reduced risk for vitamin D supplementation lasting more than three years (OR=0.27; 95% CI: 0.13–0.56) and Rollison et al [45] found a protective effect of more than 400 IU/day vitamin D supplementation (OR=0.79; 95% CI: 0.65–0.96).
Vitamin E
Five cohort [4, 25–27, 31] and four case-control [38, 39, 43, 44] studies analysed the relation between vitamin E supplementation and the risk of breast cancer. None of them reported an effect on breast cancer risk, except Pan et al [2] who reported a protective effect of high intake and long duration of vitamin E supplementation in postmenopausal women (OR=0.64; 95% CI: 0.42–0.99; OR=0.75; 95% CI: 0.58–0.97, respectively).
Multivitamins
The association between breast cancer risk and multivitamin supplementation has been analysed by six cohort [25, 32–36] and six case-control studies (one nested) [2, 12, 42–44]. The results are contradictory. Larsson et al [33] found an increased breast cancer risk associated both with high frequency (RR=1.19; 95% CI: 1.02–1.39) and high duration of multivitamin use (RR=1.22; 95% CI: 1.01–1.47) in the Swedish Mammography Cohort study. Pan et al [2] found contrasting results in the National Enhanced Cancer Surveillance System (Canada, case-control study). They found a protective effect for a supplementation lasting more than ten years in postmenopausal women (OR=0.74; 95% CI: 0.59–0.92) but not in premenopausal. In the remaining studies, no associations were found.
Discussion
In the present review, we examined the intake of vitamin supplements and its association with breast cancer risk. Out of the 26 studies, we identified 104 estimates evaluating the effect of vitamin A and beta-carotene, B-group vitamins and folic acid, vitamin C, vitamin D, vitamin E, and multivitamin. We found 14 significant estimates. This review addresses the supposition that the use of multivitamin or single vitamins does not have an effect on breast cancer risk.
A point to note is that most of the significant estimates were found in a large-population case-control study within the National Enhanced Cancer Surveillance System in Canada [2] (Table 2). Pan et al found that supplementations of ten years or longer of multiple vitamins, beta-carotene, vitamin C, and vitamin E were associated with statistically significant reductions for breast cancer risk for postmenopausal women. Moreover, they found a reduced cancer risk also for high intake of beta-carotene in premenopausal women and vitamin E in postmenopausal women. It is known that the oxidative stress and lipid peroxidation are linked to the aetiology of breast cancer, and antioxidants may exert their effect, blocking free radicals, inducing apoptosis in cancer cells, and inhibiting cell proliferation [48–49]. However, it seems that other factors might interfere with cancer development, such as menopausal status. This status is physiological but it produces significant modifications in women (body fat distribution, bone and breast density, ovarian and endometrial changes, cardiovascular modifications). It may influence breast cancer development and/or the interaction between antioxidants and the breast tissue, but it is difficult to fully understand the consequences and the potential clinical implications. Another factor that might interfere with cancer development is the receptor status [estrogen receptor (ER) or progesterone receptor (PR)]. These are the most widely studied markers in breast tissue, and their presence has an important clinical relevance. Cui et al found, in the Women’s Health Initiative Observational Study on postmenopausal women, an inverse association between breast cancer risk and dietary carotenoids in subject with ER and PR , but not with other breast cancer groups [31]. However, whether hormone receptor-defined breast cancers are etiologically different is not well understood, and it is difficult to identify an effect linked to the vitamin supplementation. We can underline that the results presented by Pan et al can be affected by recall bias inherent in case-control design. Cases might bias their responses to questions on diet after cancer diagnosis. In contrast, cohort and prospective studies have an important advantage because diet assessment is made before diagnosis, and it should be unbiased by cancer experience. The results seem to be in the opposite direction when found in prospective studies (Table 2), and they are also limited, making it difficult to draw any conclusions.
Regarding the use of multivitamins, Larsson et al [33] highlighted an increase in the risk of developing breast cancer both for high frequency of consumption (19%) and for long duration of multivitamin supplementation (22%) in the Swedish Mammography cohort study. It is not possible to know the composition of the multivitamins and to separate the effect of the single component for the observed associations. Multivitamins are usually a heterogeneous group of products with no standard composition [2, 33]. The composition may depend on the manufacturer, year of production, and batches [2, 12]. Manufacturers frequently change formulations, and detailed information is not reported [50].
For vitamin C supplementations, we found an increased breast cancer risk of 16% and 46%, respectively, in the Women’s Health Initiative Observational Study cohort [31] and in a nested case-control Study (National Breast Screening) [38]. Regarding vitamin A, only Moorman et al [43] in their case-control found an increased risk of breast cancer of 86%, considering any use of supplements. These effects may be due to an increment of oxidative damage [51, 52].
We found an increased risk of breast cancer associated with folic acid supplementation. Stolzenberg-Solomon et al [47] found that a supplementation of more than 400 µg/day of folic acid is associated with a 19% increase of the risk of developing breast cancer in the PLCO cancer screening trial. A high folate concentration could contribute to epigenetic changes in gene-regulatory mechanisms, which may result in enhanced cancer development and its promotion. However, results suggest that the role of folic acid in breast cancer development may be more complex, and these mechanisms must be considered with caution [47].
Biological as well as epidemiologic data suggest that vitamin D status could affect cancer risk and play a role in cancer prevention. All the studies included about vitamin D supplementation seem to show a decreased risk in breast cancer, though only two estimates were statistically significant. Rollison et al [45] and Shamsi et al [46] found a decreased risk of 21% and 73% in case-control studies. The link between vitamin D and breast cancer is based on the concept that the vitamin D receptor (VDR) and its ligand 1,25D (the biologically active form of vitamin D) promotes or maintains the differentiated phenotype in normal mammary cells [53]. Nevertheless, other differences in the vitamin D synthesis from sunlight exposure, circulating vitamin D levels, and ethnicity can affect the association [29] and this may explain the null association found in the other studies.
In addition, we found other differences between studies that made it difficult to compare results. Most of the studies collected information about vitamin supplement consumption throughout food frequency questionnaires, estimating average intakes over the preceding year at baseline, or open-ended questions [28, 33]. Some researchers reconstructed the consumption of supplements over long periods of time [2, 32, 43], estimating average intakes over the 5, 10, 20 years preceding (at baseline) or re-administering the questionnaire (cohort study). Their aim is to study the effect of latency time (time from exposure to cancer diagnosis). The specific time intervals after exposure or the relevant exposure time is unknown in diseases with long incubation periods such as cancer. Therefore, it is difficult to identify the relevant exposure period and antioxidants may exert their effect over a long period of time. The lack of evidence underlines the need to develop a more reliable and standardised method to assess supplement consumption.
Another interesting aspect is the dose of vitamin supplementation reported in selected studies (Table 2). The ‘upper level of intake’ and also the measurement unit are very heterogeneous. For instance, vitamin A doses vary from 5,000 to 23,000 IU/day, while vitamin C supplementation varies from 64 to 1,300 mg/day. Vitamin E varies from 4 to 424 mg/day. Vitamin consumption is reported in milligrams/micrograms or International Units. However, few studies provided evidences of a dose--response relationship between dose of supplementation and breast cancer risk [31, 45, 47]. Moreover, in some studies, authors reported only the frequency of consumption, the duration of use, or the user versus non-user data, not enabling us to make a direct comparison between estimates.
We decided to evaluate only vitamin consumption from supplementation, not including dietary intake. It is known that nutritional status and food intake may vary and may influence cancer risk [6], but we would separate these effects, trying to provide a picture of the role of vitamin supplementation in breast cancer risk. The food is constituted of a wide variety of intended and unintended chemicals which may act singly on human metabolism, but more likely act in groups in a synergistic way. Vitamins and bioactive components may interact between them, not acting in isolation [26, 54, 55].
We retrieved all relevant studies through our systematic search. The data extraction from studies have been performed and checked by individual reviewers, in order not to miss any data. Unfortunately, it should also be noted that some limitations exist. First, the number of studies was relatively small, which may limit our evaluation. We extracted fully adjusted estimates, for known confounding factors, however, in some studies the estimate can be modified by other aspects. It is possible that the observed relationships could be partly due to unmeasured or residual confounding such as other medical conditions or other factors (dietary intake, alcohol intake, sun exposure, lifestyle factors, physical activities, hormone receptor status) that could modify the estimates.
Conclusion
In conclusion, while dietary factors may be crucial in modifying cancer risk, the role of vitamin supplements in preventing breast cancer still remains unclear, considering our review. Although biologic mechanisms exist to support the anticancer effects of vitamins, this review did not address whether supplement use may have an effect on breast cancer risk. Considering the current recommendations and expert report published [6, 55], there is no clear evidence for an effect in cancer prevention for vitamin supplements due to the fact that few studies have generated relevant data and some results have been contradictory. Further investigations are warranted to elucidate the mechanisms by which vitamin supplementation may modify breast cancer development.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank the librarian William Russell-Edu of the European Institute of Oncology for retrieving the literature.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E and Forman D (2011) Global cancer statistics CA Cancer J Clin 61(2) 69–90 DOI: 10.3322/caac.20107 PMID: 21296855
2. Pan SY, Zhou J, Gibbons L, Morrison H and Wen SW (2011) Antioxidants and breast cancer risk—a population-based case-control study in Canada BMC Cancer 11 372 DOI: 10.1186/1471-2407-11-372 PMID: 21864361 PMCID: 3224257
3. Kang DH (2002) Oxidative stress, DNA damage, and breast cancer AACN Clin Issues 13 540–9 DOI: 10.1097/00044067-200211000-00007 PMID: 12473916
4. Kushi LH, Fee RM, Sellers TA, Zheng W and Folsom AR (1996) Intake of vitamins A, C, and E and postmenopausal breast cancer: The Iowa Women’s Health Study Am J Epidemiol 144 165–74 DOI: 10.1093/oxfordjournals.aje.a008904 PMID: 8678048
5. Michels KB, Holmberg L, Bergkvist L, Ljung H, Bruce A and Wolk A (2001) Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women Int J Cancer 91(4) 563–7 DOI: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1079>3.0.CO;2-9 PMID: 11251982
6. World Cancer Research Fund/American Institute for Cancer Research (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective (American Institute for Cancer Research, Washington, DC)
7. The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers N Engl J Med 330 1029–35 PMID: 8127329
8. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S and Hammar S (1996) Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease N Engl J Med 334 1150–5 DOI: 10.1056/NEJM199605023341802 PMID: 8602180
9. Lee BM and Park KK (2003) Beneficial and adverse effects of chemopreventive agents Mutat Res 523–24 265–78 PMID: 12628524
10. Paolini M, Abdel-Rahman SZ, Sapone A, Pedulli GF, Perocco P, Cantelli-Forti G and Legator MS (2003) Beta-carotene: A cancer chemopreventive agent or a co-carcinogen? Mutat Res 543(3) 195–200 DOI: 10.1016/S1383-5742(03)00002-4 PMID: 12787812
11. Bjelakovic G, Nikolova D, Simonetti RG and Gluud C (2004) Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis Lancet 364 1219–28 DOI: 10.1016/S0140-6736(04)17138-9 PMID: 15464182
12. Meulepas JM, Newcombe PA, Burnett-Hartman AN, Hampton JM and Trentham-Dietz A (2010) Multivitamin supplement use and risk of invasive breast cancer Public Health Nutr 13(10) 1540–5 DOI: 10.1017/S1368980009992187 PMCID: 2923261
13. Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M and Sempos C (2011) Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief 61 1–8 PMID: 21592424
14. Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT and Picciano MF (2011) Dietary supplement use in the United States, 2003–2006 J Nutr 141 261–6 DOI: 10.3945/jn.110.133025 PMCID: 3021445
15. Guo X, Willows N, Kuhle S, Jhangri G and Veugelers PJ (2009) Use of vitamin and mineral supplements among canadian adults Can J Public Health 100(5) 357–60 PMID: 19994737
16. McLennan W and Podger A (1997) National Nutrition Survey: Selected Highlights, Australia, 1995 (Canberra, Australia: Australian Bureau of Statistics)
17. Skeie G, Braaten T, Hjartåker A, Lentjes M, Amiano P, Jakszyn P, Pala V, Palanca A, Niekerk EM, Verhagen H, Avloniti K, Psaltopoulou T, Niravong M, Touvier M, Nimptsch K, Haubrock J, Walker L, Spencer EA, Roswall N, Olsen A, Wallström P, Nilsson S, Casagrande C, Deharveng G, Hellström V, Boutron-Ruault MC, Tjønneland A, Joensen AM, Clavel-Chapelon F, Trichopoulou A, Martinez C, Rodríguez L, Frasca G, Sacerdote C, Peeters PH, Linseisen J, Schienkiewitz A, Welch AA, Manjer J, Ferrari P, Riboli E, Bingham S, Engeset D, Lund E and Slimani N (2009) Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study Eur J Clin Nutr 63 S226–38 DOI: 10.1038/ejcn.2009.83 PMID: 19888276
18. Max Rubner Institut. Nationale Verzehrs-Studie II [www.was-esse-ich.de/uploads/media/NVS_II_Ergebnisbericht_Teil_1.pdf] Date accessed: 23/05/13
19. van Rossum CTM, Fransen HR, Verkaik-Kloosterman J, Buurma-Rethans EJM and Ocké MC (2011) Dutch National Food Consumption Survey 2007-2010. Diet of children and adults aged 7 to 69 years (Bilthoven, The Netherlands: National Institute for Public Health and the Environment)
20. Bates B, Lennox A and Swan G (2009) National Diet and Nutrition Survey (London: Food Standards Agency and the Department of Health)
21. Agence Française de Sécurité Sanitaire des Aliments (AFSSA) (2007) Summary of the Individual and National Study on Food Consumption 2 (INCA 2) 2006–2007
22. Piccinelli R, Arcella D, Buonocore P, Capriotti M, D’Addezio L, Le Donne C, Mistura L, Pettinelli A, Sette S, Turrini A and Leclercq C (2011) L’indagine nazionale sui consumi alimentari in Italia INRAN-SCAI 2005-06. Parte B2 - I consumi in termini di gruppi e sottogruppi alimentari (g/die) Osservatorio Consumi Alimentari, INRAN, Rome, Italy
23. Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE and Willett WC (1993) A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer N Engl J Med 329(4) 234–40 DOI: 10.1056/NEJM199307223290403 PMID: 8292129
24. Nissen SB, Tjønneland A, Stripp C, Olsen A, Christensen J, Overvad K, Dragsted LO and Thomsen B (2003) Intake of vitamins A, C, and E from diet and supplements and breast cancer in postmenopausal women Cancer Causes Control 14(8) 695–704 DOI: 10.1023/A:1026377521890 PMID: 14674733
25. Zhang S, Hunter DJ, Forman MR, Rosner BA, Speizer FE, Colditz GA, Manson JE, Hankinson SE and Willett WC (1999) Dietary carotenoids and vitamins A, C, and E and risk of breast cancer J Natl Cancer Inst 91(6) 547–56 DOI: 10.1093/jnci/91.6.547 PMID: 10088626
26. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K and Tjonneland A (2010) Micronutrient intake and breast cancer characteristics among postmenopausal women Eur J Cancer Prev 19 360–5 DOI: 10.1097/CEJ.0b013e32833ade68 PMID: 20698054
27. Shibata A, Paganini-Hill A, Ross RK and Henderson BE (1992) Intake of vegetables, fruits, -carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: A prospective study Br J Cancer 66 673–9 DOI: 10.1038/bjc.1992.336 PMID: 1419605 PMCID: 1977409
28. Verhoeven DTH, Assen N, Goldbohm RA, Dorant E, van’t Veer P, Sturmans F, Hermus RJJ and van den Brandt PA (1997) Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: A prospective cohort study Br J Cancer 75(1) 149–55 DOI: 10.1038/bjc.1997.25 PMID: 9000614 PMCID: 2222693
29. John EM, Schwartz GG and Dreon DM (1999) Vitamin D and breast cancer risk: The NHANES I Epidemiologic Follow-up Study, 1971-1975 to 1992 Cancer Epidemiol Biomarkers Prev 8 399–406 PMID: 10350434
30. Robien K, Cutler GJ and Lazovich D (2007) Vitamin D intake and breast cancer risk in postmenopausal women: The Iowa Women’s Health Study Cancer Causes Control 18 775–82 DOI: 10.1007/s10552-007-9020-x PMID: 17549593
31. Cui Y, Shikany JM, Liu S, Shagufta Y and Rohan TE (2008) Selected antioxidants and risk of hormone receptor–defined invasive breast cancers among postmenopausal women in the Women’s Health Initiative Observational Study Am J Clin Nutr 87 1009–18 PMID: 18400726
32. Maruti SS, Ulrich CM and White E (2009) Folate and one-carbon metabolism nutrients from supplements and diet in relation to breast cancer risk Am J Clin Nutr 89 624–33 DOI: 10.3945/ajcn.2008.26568 PMID: 19116331 PMCID: 2647765
33. Larsson SC, Akesson A, Bergkvist L and Wolk A (2010) Multivitamin use and breast cancer incidence in a prospective cohort of Swedish women Am J Clin Nutr 91(5) 1268–72 DOI: 10.3945/ajcn.2009.28837 PMID: 20335555
34. Ishitani K, Lin J, Manson JE, Buring JE, Zhang SM (2008) A prospective study of multivitamin supplement use and risk of breast cancer Am J Epidemiol 167(10) 1197–206
35. Neuhouser ML, Wassertheil-Smoller S, Thomson C, Aragaki A, Anderson GL, Manson JE, Patterson RE, Rohan TE, van Horn L, Shikany JM, Thomas A, LaCroix A, Prentice RL (2009) Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts Arch Intern Med 169(3) 294–304 DOI: 10.1001/archinternmed.2008.540 PMID: 19204221
36. Feigelson HS, Jonas CR, Robertson AS, McCullough ML, Thun MJ, Calle EE (2003) Alcohol, folate, methionine, and risk of incident breast cancer in the American Cancer Society Cancer Prevention Study II Nutrition Cohort Cancer Epidemiol Biomarkers Prev 12(2) 161–4 PMID: 12582027
37. Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM (2007) Intakes of calcium and vitamin D and breast cancer risk in women Arch Intern Med 167(10) 1050–9 PMID: 17533208
38. Rohan TE, Howe GR, Friedenreich CM, Jain M and Miller AB (1993) Dietary fiber, vitamins A, C, and E, and risk of breast cancer: A cohort study Cancer Causes and Control 4 29–37 DOI: 10.1007/BF00051711 PMID: 8381678
39. Freudenheim JL, Marshall JR, Vena JE, Laughlin R, Brasure JR, Swanson MK, Nemoto T and Graham S (1996) Premenopausal breast cancer risk and intake of vegetables, fruits, and related nutrients J Natl Cancer Inst 88(6) 340–8 DOI: 10.1093/jnci/88.6.340 PMID: 8609642
40. Longnecker MP, Newcombe PA, Mittendorf R and Willet WC (1997) Intake of carrots, spinach, and supplements containing vitamin A in relation to risk of breast cancer Cancer Epidemiol Biomarkers Prev 6 887–92 PMID: 9367061
41. Potischman N, Swanson CA, Coates RJ, Gammon MD, Brogan DR, Curtin J and Brinton LA (1999) Intake of food groups and associated micronutrients in relation to risk of early-stage breast cancer Int J Cancer 82 315–21 DOI: 10.1002/(SICI)1097-0215-(19990730)82:3<315::AID-IJC1>3.0.CO;2-N PMID: 10399945
42. Wu K, Helzlsouer KJ, Comstock GW, Hoffman SC, Nadeau MR and Selhub J (1999) A prospective study on folate, B12, and pyridoxal 5′-phosphate (B6) and breast cancer Cancer Epidemiol Biomarkers Prev 8(3) 209–17 PMID: 10090298
43. Moorman PG, Ricciuti MF, Millikan RC and Newman B (2001) Vitamin supplement use and breast cancer in a North Carolina population Public Health Nutr 4 821–7 DOI: 10.1079/PHN2001121 PMID: 11415490
44. Dorjgochoo T, Shrubsole MJ, Shu XO, Lu W, Ruan Z, Zheng Y, Cai H, Dai Q, Gu K, Gao YT and Zheng W (2008) Vitamin supplement use and risk for breast cancer: The Shanghai Breast Cancer Study Breast Cancer Res Treat 111(2) 269–78 DOI: 10.1007/s10549-007-9772-8
45. Rollison DE, Cole AL, Tung KH, Slattery ML, Baumgartner KB, Byers T, Wolff RK, Giuliano AR (2012) Vitamin D intake, vitamin D receptor polymorphisms, and breast cancer risk among women living in the southwestern U.S. Breast Cancer Res Treat 132(2) 683–91 DOI: 10.1007/s10549-011-1885-4 PMID: 22130867 PMCID: PMC3390020
46. Shamsi U, Khan S, Usman S, Soomro S, Azam I (2013) A multicenter matched case control study of breast cancer risk factors among women in Karachi, Pakistan Asian Pac J Cancer Prev 14(1) 183–8 PMID: 23534721
47. Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, Johnson KA, Johnson C, Buys SS, Hoover RN, Ziegler RG (2006) Folate intake, alcohol use, and postmenopausal breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial Am J Clin Nutr 83(4) 895–904 PMID: 16600944
48. Valko M, Rhodes CJ, Moncol J, Izakovic M and Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer Chem Biol Interact 160 1–40 DOI: 10.1016/j.cbi.2005.12.009 PMID: 16430879
49. Martínez ME, Jacobs ET, Baron JA, Marshall JR and Byers T (2012) Dietary supplements and cancer prevention: balancing potential benefits against proven harms J Natl Cancer Inst 104(10) 732–9 DOI: 10.1093/jnci/djs195 PMID: 22534785 PMCID: 3352833
50. Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE and Manson JE (2009) Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial J Nat Cancer Inst 101 14–23 DOI: 10.1093/jnci/djn438 PMID: 19116389 PMCID: 2615459
51. Lee KW, Lee HJ, Surh YJ and Lee CY (2003) Vitamin C and cancer chemoprevention: Reappraisal Am J Clin Nutr 78 1074–8 PMID: 14668266
52. Huang HY, Helzlsouer KJ and Appel LJ (2000) The effects of vitamin C and vitamin E on oxidative DNA damage: Results from a randomized controlled trial Cancer Epidemiol Biomarkers Prev 9 647–52 PMID: 10919732
53. Welsh J (2007) Vitamin D and prevention of breast cancer Acta Pharmacol Sin 28(9) 1373–82 PMID: 17723171
54. Goodman M, Bostick RM, Kucuk O and Jones DP (2011) Clinical trials of antioxidants as cancer prevention agents: Past, present, and future Free Radic Biol Med 51 1068–84 DOI: 10.1016/j.freeradbiomed.2011.05.018 PMID: 21683786
55. Kamangar F and Emadi A (2012) Vitamin and mineral supplements: Do we really need them? Int J Prev Med 3(3) 221–6 PMID: 22448315 PMCID: 3309636






