Current perspectives on skeletal health and cancer progression across the disease continuum in breast cancer-the role of bisphosphonates
R Aft1
1 Department of Surgery, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110, USA
Correspondence to: Rebecca Aft. Email: aftr@wustl.edu
Abstract
Pre-clinical and clinical evidence suggest that bisphosphonates inhibit both bone resorption and cancer progression. New and updated analyses from several large, controlled studies in pre- and post-menopausal women with early stage breast cancer (BC) suggest that addition of bisphosphonates improves cancer-related outcomes, particularly in patients with a ‘low-estrogen environment’. Further, preliminary clinical data suggest that bisphosphonate therapy may reduce circulating tumour cell numbers (a negative prognostic indicator of disease-free and overall survival) in patients with advanced/metastatic disease. These new findings warrant reconsideration of the therapeutic role of bisphosphonates in BC.
Keywords: bisphosphonates, breast cancer, treatment, zoledronic acid
Introduction
Bisphosphonates are the most common pharmaceutical intervention for prevention of skeletal-related events (SREs) in patients with malignant skeletal involvement. Data from several studies suggest that in addition to inhibiting bone resorption, bisphosphonates may limit cancer progression through their effects within the bone (e.g. cancer cell–bone interactions) or their effects on extraskeletal processes such as host antitumour immunity, angiogenesis, and circulating tumour cells (CTCs). The potential of simultaneously limiting bone resorption and tumourigenesis by bisphosphonates is therapeutically relevant, and accordingly, several large, clinical programs have evaluated the anticancer benefits of adding bisphosphonates to standard-of-care in the adjuvant setting in earlier stage breast cancer (BC) and in patients with advanced/metastatic BC. These studies shed light on the anticancer benefits of bisphosphonate therapy and the subsets of patients who may benefit from such therapy. Recently, several large, controlled studies in patients with earlier stage disease reported data suggestive of potential anticancer benefit of zoledronic acid (ZOL) in particular patient subsets. In addition, smaller studies suggest that ZOL may have similar anticancer benefits in patients with advanced/metastatic disease. These data warrant reconsideration of clinical practice in early BC and further clinical exploration of the therapeutic role of bisphosphonates in advanced BC.
Early disease
Several trials have demonstrated the anticancer benefit of adding bisphosphonates to standard adjuvant treatment in patients with early stage disease (Table 1) [1–5]. For example, in the ABCSG-12 study in pre-menopausal patients with early BC undergoing complete estrogen blockade (a patient population highly susceptible to bone loss), 3 years’ ZOL treatment (4 mg every 6 months) significantly improved disease-free survival (DFS) at the 48-month follow-up (hazard ratio [HR] = 0.74; log-rank
Table 1: Controlled studies of antiresorptive agents in breast cancer.
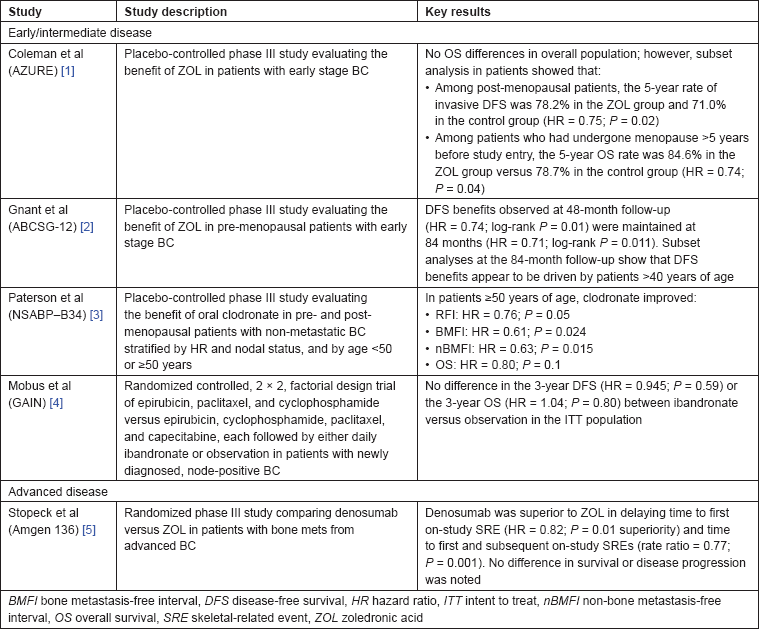
In the NSABP-B34 study evaluating the benefit of adding clodronate to adjuvant therapy in patients with earlier stage BC, clodronate treatment was associated with significant improvement in non-bone metastasis-free survival (HR = 0.743;
Consistent with these data, Coleman
More recently, the novel receptor activator of nuclear factor kappaB ligand (RANKL)-directed antibody denosumab was shown to significantly increase bone mineral density (BMD) over 24 months at trabecular and cortical bone in women with non-metastatic BC and low-bone mass receiving adjuvant aromatase inhibitor therapy [8]. However, it should be noted that the effects of denosumab on BMD are relatively transient, as was observed in a study that evaluated the effects of discontinuing and restarting denosumab treatment in post-menopausal women with low-bone mass [9]. In this study, discontinuation of denosumab was associated with a BMD decrease of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months after stopping treatment. This was paralleled by an increase in bone turnover marker levels as early as six months after discontinuation of denosumab treatment at dose levels comparable with those used in the early BC setting. These rates of bone loss were higher than those observed in placebo-treated patients at any point during the study. Although BMD benefits were restored by re-treatment, this rebound effect needs further consideration as this may be reflective of a bone microenvironment more conducive to tumour recurrence.
Thus, several large studies independently corroborate the benefit of bisphosphonates in the early BC setting in older or post-menopausal patients (low-estrogen environment) and support inclusion of ZOL as standard treatment for these patients. Further, these studies show that treatment with adjuvant bisphosphonates is safe and may provide sustained anticancer benefit—desirable treatment characteristics for this patient population with good prognosis and prone to recurrent disease.
Advanced disease
The results of a randomized, controlled phase III trial comparing denosumab with ZOL in patients with advanced BC and at least one bone lesion demonstrated that denosumab significantly delayed the time to first on-study SRE (non-inferiority
The effect of antiresorptives on cancer-related outcome may be influenced by both drug- and disease-related factors. First, nitrogen-containing bisphosphonates target a broad range of intracellular signal transduction intermediates, whereas denosumab acts exclusively by binding RANKL. Second, bisphosphonates have little or no systemic availability, unlike denosumab, so their effects are largely confined to bone. Conceivably, cancer-related outcomes may be influenced by effects on bone resorption alone, effects on tumour cells, or both. In addition, tumour type and disease burden may both be important determinants of treatment outcome. For example, in patients with multiple myeloma, wherein the majority of the cancer resides within the bone marrow,
The minimal inference from these data is that a reduction in bone turnover markers in patients with skeletal involvement is associated with improved outcomes, and that with the availability of new agents, clinicians may now consider sequencing of bone-conserving therapies. Recently, it was shown that denosumab may lower bone turnover markers in patients with elevated levels after previous bisphosphonate treatment [15]. However, it is unclear whether this denosumab-mediated normalization of bone markers correlates with improvements in disease outcomes. The role of markers in comparing treatment options therefore requires further study. Further trials are also needed to optimize sequencing strategies and dosing regimens as well as to identify baseline prognostic factors that may inform treatment decisions.
In addition to the effects in bone, ZOL may improve cancer-related outcome through extraskeletal effects. For example, recent exploratory data from the Z-ACT1 study show that ZOL induced rapid and sustained decrease in the proportion of metastatic BC patients (
Conclusions
With the availability of several antiresorptive agents, it is conceivable that skeletal- and cancer-related benefits may be considered collectively during treatment decisions throughout the disease continuum. Furthermore, the overall benefit may be optimized using patient-selection strategies based on menopausal status, age, CTCs, and/or bone metabolism markers. In addition to long-term risk–benefit considerations relating to bone health, the potential of antiresorptives to affect cancer-related outcomes may become an important consideration for treatment choice. Recent clinical evidence showing the potential anticancer activity of bisphosphonates, especially ZOL, suggests that re-evaluation of the benefits and roles of antiresorptive therapies is warranted.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals, East Hanover, NJ. The author thanks Jerome F. Sah, PhD, ProEd Communications, Inc.®, for his medical editorial assistance with this article.
References
1. Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Ritchie D, Pugh J, Gaunt C, Rea U, Peterson J, Davies C, Hiley V, Gregory W and Bell R (2011) Breast-cancer adjuvant therapy with zoledronic acid N Engl J Med 365 1396–405 DOI: 10.1056/NEJMoa1105195 PMID: 21995387
2. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Stoeger H, Dubsky P, Jakesz R, Singer C, Eidtmann H, Fesl C, Eiermann W, Marth C and Greil R (2011) Long-term follow-up in ABCSG-12: significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with endocrine-receptor-positive early breast cancer Presented at 2011 CTRC-AACR San Antonio Breast Cancer Symposium (Dec. 6–10, 2011), San Antonio, TX (Abstract S1-2)
4. Möbus V, Diel IJ, Elling D, Harbeck N, Jackisch C, Thomssen C, Untch M, Conrad B, Schneeweiss A, Kreienberg R, Huober J, Müller V, Lück HJ, Bauerfeind I, Loibl S, Nekljudova V and von Minckwitz G (2011) GAIN study: a phase III trial to compare ETC versus EC-TX and ibandronate versus observation in patients with node-positive primary breast cancer—1st interim efficacy analysis Presented at 2011 CTRC-AACR San Antonio Breast Cancer Symposium (Dec. 6–10, 2011), San Antonio, TX (Abstract S2-4)
5. Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S and Braun A (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study J Clin Oncol 28 5132–9 DOI: 10.1200/JCO.2010.29.7101 PMID: 21060033
6. Coleman RE, Thorpe HC, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Davies RD and Bell R (2010) Adjuvant treatment with zoledronic acid in stage II/III breast cancer. The AZURE trial (BIG 01/04) Presented at 33rd Annual San Antonio Breast Cancer Symposium (Dec. 8–12, 2010), San Antonio, TX (Abstract S4-5)
7. de Boer R, Bundred N, Eidtmann H, Neven P, von Minckwitz G, Martin N, Modi A and Coleman R (2011) Long-term survival outcomes among postmenopausal women with hormone receptor-positive early breast cancer receiving adjuvant letrozole and zoledronic acid: 5-year follow-up of ZO-FAST Presented at 2011 CTRC-AACR San Antonio Breast Cancer Symposium (Dec. 6–10, 2011), San Antonio, TX (Abstract S1-3)
8. Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, Fan M and Jun S (2008) Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer J Clin Oncol 26 4875–82 DOI: 10.1200/JCO.2008.16.3832 PMID: 18725648
9. Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y and San Martin J (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial Bone 43 222–9 DOI: 10.1016/j.bone.2008.04.007 PMID: 18539106
10. Henry D, von Moos R, Vadhan-Raj S, Hungria V, Spencer A, Hirsh V, Wang J, Jun S, Yeh H and Dansey R (2009) A double-blind, randomized study of denosumab versus zoledronic acid for the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma Presented at ECCO 15-34th ESMO Multidisciplinary Congress (Sept. 20–24, 2009), Berlin, Germany (Abstract 20LBA)
11. Xgeva [prescribing information] (2010) Accessed July 3, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125320s007lbl.pdf (Thousand Oaks, CA: Amgen Inc.)
12. Hirsh V, Major PP, Lipton A, Cook RJ, Langer CJ, Smith MR, Brown JE and Coleman RE (2008) Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity J Thorac Oncol 3 228–36 DOI: 10.1097/JTO.0b013e3181651c0e PMID: 18317064
13. Body J, Cook R, Costa L, Brown J, Terpos E, Saad F, Lipton A and Coleman R (2009) Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features Presented at IX International Meeting on Cancer Induced Bone Disease (Oct. 28–31, 2009), Arlington, VA (Abstract P71)
14. Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, Brown JE and Coleman RE (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumours and elevated bone resorption receiving zoledronic acid Cancer 113 193–201 DOI: 10.1002/cncr.23529 PMID: 18459173
15. Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W and Jun S (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates J Clin Oncol 27 1564–71 DOI: 10.1200/JCO.2008.19.2146 PMID: 19237632
16. Brufsky A, Beck J, Dakhil S, Hallmeyer S, Tezcan H, Yardley D, Tran D, Warsi G and Culver K (2011) Z-ACT1: Zometa combined with standard therapy in patients with metastatic breast cancer further decreases the proportion of patients with CTC counts of 5 or above Presented at 2011 CTRC-AACR San Antonio Breast Cancer Symposium (Dec. 6–10, 2011), San Antonio, TX (Abstract P1-18-01)
17. Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN and Terstappen LW (2005) Circulating tumour cells: a novel prognostic factor for newly diagnosed metastatic breast cancer J Clin Oncol 23 1420–30 DOI: 10.1200/JCO.2005.08.140 PMID: 15735118
18. Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV and Terstappen LW (2006) Circulating tumour cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival Clin Cancer Res 12 4218–24 DOI: 10.1158/1078-0432.CCR-05-2821 PMID: 16857794
19. Foroni C, Andreis D, Maldotti M, Cappelletti MR and Generali DG (2011) Proof of the anti-tumour effect of zoledronic acid (ZA) in naive bone-only metastatic and locally advanced breast cancer: results from the biological window therapy Presented at 2011 CTRC-AACR San Antonio Breast Cancer Symposium (Dec. 6–10, 2011), San Antonio, TX (Abstract P4-16-03)
20. Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, Zhai J, Kuo S, Shannon W, Diemer K, Herrmann V, Dietz J, Ali A, Ellis M, Weiss P, Eberlein T, Ma C, Fracasso PM, Zoberi I, Taylor M, Gillanders W, Pluard T, Mortimer J and Weilbaecher K (2010) Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial Lancet Oncol 11 421–8 DOI: 10.1016/S1470-2045(10)70054-1 PMID: 20362507