Effect on cardiovascular outcome of sodium-glucose co-transporter-2 (SGLT2) inhibitors among cancer patients treated with anthracycline: a systematic review and meta-analysis
Chalothorn Wannaphut1, Phuuwadith Wattanachayakul2,3, Sakditad Saowapa4, Ben Ponvilawan5, Manasawee Tanariyakul1, Jakrin Kewcharoen6, Pitchaporn Yingchoncharoen4, Thanathip Suenghataiphorn7, Noppawit Aiumtrakul1 and Jared Acoba8,9
1Department of Medicine, John A. Burns School of Medicine, University of Hawai’i, Honolulu, HI 96813, USA
2Department of Medicine, Albert Einstein Healthcare Network, Philadelphia, PA 19141, USA
3Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA 19107, USA
4Department of Internal Medicine, Texas Tech Health Science Center, Lubbock, TX 79430, USA
5Department of Internal Medicine, University of Missouri–Kansas City School of Medicine, Kansas City, MO 64108, USA
6Division of Cardiology, Department of Medicine, Loma Linda University Health, Loma Linda, CA 92354, USA
7Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand
8University of Hawai’i Cancer Center, Honolulu, HI 96813, USA
9Queen’s Medical Center, Honolulu, HI 96813, USA
Abstract
Background/objectives: Sodium-glucose-co-transporter-2 (SGLT2) inhibitors have shown benefit in reducing cardiovascular disease outcomes in diabetes patients. Anthracycline therapy is associated with a risk of cardiomyopathy. However, the impact of SGLT2 inhibitors in the prevention of cardiomyopathy and heart failure in cancer patients undergoing anthracycline treatment remains unclear. Thus, we conducted a systematic review and meta-analysis to explore the effect of the prevention of cardiovascular outcomes in patients with cancer and diabetes who had received anthracycline therapy.
Methods: We systematically reviewed Medline and EMBASE databases from inception to January 2024 for studies focusing on cancer patients with a history of anthracycline therapy. Eligible studies had to report relative risk (RR) with 95% confidence intervals (CIs) for the clinical endpoints of mortality outcomes and the risk of heart failure exacerbation, comparing cohorts with and without SGLT2 inhibitor use.
Results: Our study included four retrospective cohort studies in the meta-analysis (n = 6,708, 24% received SGLT2). There was significantly lower all-cause mortality in the SGLT2 inhibitors group (pooled RR of 0.52, 95% CI 0.35–0.77, I2 64%). However, there were no differences in the risk of heart failure exacerbation (pooled RR of 0.67, 95% CI 0.39–1.14, I2 17%).
Conclusion: Our study found that anthracycline-treated cancer patients using SGLT2 inhibitors experienced lower all-cause mortality compared to the control group. A randomised clinical trial is necessary to further elucidate these findings.
Keywords: SGLT2 inhibitors, cardiovascular outcomes, anthracycline, cancer, meta-analysis
Correspondence to: Chalothorn Wannaphut
Email: chalothorn.wan@gmail.com
Published: 12/02/2025
Received: 22/07/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Anthracycline is a cornerstone in the treatment of multiple solid organ and hematologic malignancies. Unfortunately, it is also associated with cardiotoxicities including heart failure, cardiomyopathy and arrhythmia. These adverse effects can markedly diminish patients' overall prognosis [1–3]. Sodium-glucose co-transporter-2 (SGLT2) inhibitors have emerged as a promising therapeutic class in the management of heart failure, offering benefits such as reduced mortality, fewer heart failure hospitalisations and improved cardiac function [4–8]. The observed benefits lead to growing interest in exploring their use beyond typical diabetes or heart failure therapy, notably in the treatment of cardiotoxicity in oncology patients.
However, the application of SGLT2 inhibitors in the context of anthracycline-induced cardiotoxicity remains limited. While preliminary evidence suggests potential protective effects, comprehensive clinical data are scarce. This gap underscores the need for a systematic evaluation of existing studies to formulate clearer insights into this specific scenario.
To address this knowledge gap, we aim to conduct a systematic review and meta-analysis focusing on the use of SGLT2 inhibitors in patients undergoing anthracycline therapy. By collating and analyzing data from various clinical trials and observational studies, we intend to assess the impact of SGLT2 inhibitors on mortality outcomes and HF exacerbation in this population. In this research, our goal is to enhance therapeutic approaches and improve long-term outcomes for patients with malignancies undergoing anthracycline therapy.
Methods
Literature search strategy
Two investigators (C.W. and P.W.) independently searched for published articles indexed in MEDLINE and EMBASE databases from inception to 14 January 2024, using the search strategy that included the terms for SGLT2 inhibitor, anthracycline and cardiac outcomes. The search strategy is available as Supplementary data 1. References of the included studies were also manually reviewed for additional eligible studies. This study was undertaken under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, which is available as Figure 1.
Selection criteria
To be eligible for the meta-analysis, the study must be a cohort study that investigated survival and clinical outcomes in cancer patients treated with anthracycline between SGLT2 inhibitor use and without SGLT2 inhibitor use. Eligible cohort study must consist of patients with SGLT2 inhibitors and comparators without SGLT2 inhibitors. The eligible study must also provide the magnitude of association, which could be odd ratio (OR), relative risk (RR), hazard ratio (HR), incidence rate ratio (IRR) or standardised incidence ratio along with its corresponding confidence interval (CI).
All retrieved articles were reviewed independently by the two investigators (C.W. and P.W.) for their eligibility. The last investigator (CW) reviewed all the included studies again to ensure that the inclusion criteria were met and also served as the deciding vote when different determinations of study eligibility were made by the first two investigators. Newcastle–Ottawa quality assessment scale was used to assess the quality of the included cohort studies [9]. This scale evaluates the quality of the included studies in three areas including recruitment of participants, comparability between the groups and ascertainment of the outcome of interest for cohort study.
Data extraction
A standardised data collection form was used to extract the following information: last name of the first author, study design, year(s) of study, country of origin, year of publication, sample size, baseline characteristics of participants, methods used to identify and verify the acute heart failure, hospitalisation confounders that were adjusted and adjusted effect estimates with 95% CI. This data extraction was independently performed by the same two investigators (C.W and P.W.) to minimise error. Any discrepancies found in the case record forms were resolved by referring back to the original articles.
Statistical analysis
Review Manager 5.3 software from the Cochrane Collaboration was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined using the generic inverse variance method as described by DerSimonian and Laird [10]. A random-effect model, rather than a fixed-effect model, was used because the included studies were of different methodologies and background populations. OR or HR of cohort study was used as an estimate for RR to calculate the pooled RR along with RR of cohort studies. Statistical heterogeneity was assessed using Cochran's Q test. This statistic is complemented with the I2 statistic which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. A value of I2 of 0%–25% represents insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity and >75% high heterogeneity [11]. The presence of publication bias would be assessed by visualisation of the funnel plot.
Results
The systematic search identified 234 relevant articles (163 articles from EMBASE and 71 articles from MEDLINE). After the exclusion of 32 duplicated articles, 202 articles underwent title and abstract review. A total of articles were excluded at this stage as they did not fulfill the eligibility criteria based on the type of article, study design, participants and outcome of interest. A total of 11 articles were retrieved for full-length article review, and 7 articles were excluded at this stage as they did not report the association of interest. Finally, 4 cohort studies [12–15] with 167,907 participants (1,616 patients were on SGLT2 inhibitors) were eligible for the meta-analysis. The literature retrieval, review and selection process are shown in Figure 1. The characteristics of the included studies and their quality assessment are described in Table 1.
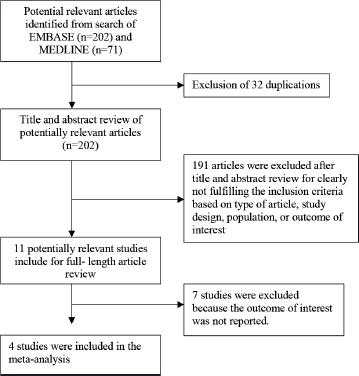
Figure 1. Flow chart of literature review process.
Table 1. Baseline characteristics of studies included in the meta-analysis.
Briefly, among the four included cohort studies [12–15] with 167,907 participants, four studies were retrospective cohort 2 studies from the United States [13, 14], 1 studies from Canada [12] and Korea [15]. Three studies reported effect estimates as HR [12, 14, 15], and one study reported as OR [13]. Therefore, HR of the three studies, and OR of one study was used as an estimate to calculate pooled RR. All studies reported a significant mortality rate in non SGLT 2 inhibitor use. All of the eligible studies adjusted their effect estimate for any potential confounders such as demographic data and commodities.
All cause of mortality of SGL2 inhibitor use
A total of four cohort studies [12–15] reported lower all causes of mortality among patients using SGLT2 inhibitors than those without SGLT 2 inhibitors with a pooled RR of 0.52 (95% CI 0.35–0.77). The between-study heterogeneity was high with an I2 of 64%. Figure 2 demonstrates the forest plot of this meta-analysis.
Risk of acute heart failure exacerbation
A total of three cohort studies [13–15] reported the risk of heart failure exacerbation was lower in those who received SGLT2 inhibitors, though the results were not statistically significant, with a pooled RR of 0.67 (95% CI 0.39–1.14, I2 17%). The between-study heterogeneity was high with an I2 of 12%. Figure 3 demonstrates the forest plot of this meta-analysis.
Evaluation for publication bias
Evaluation for publication bias using visualisation of funnel plots could not be performed due to the limited number of included studies.
Discussion
Several mechanisms of AC-induced cardiotoxicity have been proposed including oxidative stress, DNA damage, impaired iron metabolism, mitochondrial dysfunction and autophagy dysregulation [16]. Additionally, diabetes mellitus is associated with an increased risk of anthracycline-induced cardiotoxicity. Cardiac exposure to high blood glucose levels increases fatty acid and cytokines, resulting in the accumulation of fat droplets in myocardial cells and ultimately mediating cardiotoxicity [17]. Significantly, SGLT2 inhibitors exhibit noteworthy advantages in mitigating heart failure and cardiovascular mortality among individuals, irrespective of their diabetic status. To the best of our knowledge, this is the first systematic review and meta-analysis to evaluate the effect of SGLT2 inhibitors in the prevention of heart failure and cardiac outcomes in patients with cancer treated with anthracycline. Our findings indicate that patients with SGLT2 inhibitors have lower all-cause mortality compared to the non-SGLT2 inhibitors cohort with RR 0.52 with several possible explanations.
First, SGLT-2 inhibitors have demonstrated a cardiomyocyte-protective effect against anthracycline-induced cardiotoxicity through multiple mechanisms. At the molecular level, these mechanisms include the reduction of karyorrhexis and karyolysis, modulating the AMPK-mTOR signaling pathway to regulate autophagy and enhancing mitochondrial function by mitigating mitochondrial shrinkage [18]. Additionally, SGLT-2 inhibitors exhibit anti-inflammatory effects, decreasing the production of IL-1, IL-6, IL-8 and TNF-α and inhibiting reactive oxygen species formation in doxorubicin-treated cells. Regarding cardiovascular structure, function and outcomes, the utilisation of SGLT-2 inhibitors such as empagliflozin and dapagliflozin shows promise in reversing adverse effects on left ventricular ejection fraction. Empagliflozin, in particular, exhibits a reduction in markers of myocardial fibrosis, such as collagen [19]. SGLT-2 inhibitors also help rebalance intracellular sodium and calcium levels in cardiomyocytes, supporting improved left ventricular systolic function post-myocardial infarction and mitigating calcium overload caused by doxorubicin. Moreover, their diuretic effect may reduce heart failure risk associated with steroid use or fluid retention during anthracycline treatment [12]. In addition to these effects, SGLT2 inhibitors exhibit anticancer effects by modulating cancer cell metabolism, including inhibiting β-Catenin, activating AMPK and reducing ATP production, which suppresses DNA/RNA synthesis and proangiogenic factors [20].
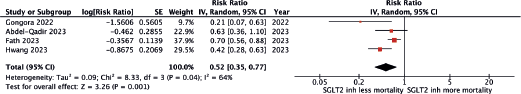
Figure 2. Forest plot of the meta-analysis.
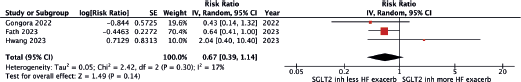
Figure 3. Forest plot of the meta-analysis.
Furthermore, SGLT2 inhibitors contribute to weight reduction through glycosuria, thereby improving insulin resistance. The reduction in body weight has been associated with a lowered risk of certain cancers, including obesity-associated breast and colon cancers [15]. However, our study shows no differences in the risk of heart failure exacerbation (pooled RR of 0.67, 95% CI 0.39–1.14, I2 17%). The mechanism by which SGLT2i can reduce HF in patients receiving anthracycline therapy is unclear. The possible mechanisms have been explained above.
This meta-analysis carries some limitations that should be acknowledged. First, the statistical heterogeneity of the meta-analysis of all-cause mortality was moderate. Different participant characteristics were probably one of the main reasons for the variation. Second, the majority of the included studies relied on diagnosis codes from administrative databases to identify diagnoses and treatments. Therefore, the completeness of case identification, accuracy and stage of the cancer treatment, type of SGLT2 and outcome occurrences outside the database are limited. Finally, the small number of included studies in the meta-analysis could jeopardise the validity and interpretation of the funnel plot.
Conclusion
The current study found that the SGLT2 inhibitors are associated with a lower all-cause of mortality in cancer patients treated with anthracycline but no difference in acute heart failure exacerbation. This supports conducting randomised trials testing the effect of SGLT2 inhibitors on cardiac outcomes in patients treated with an anthracycline.
Conflicts of interest
All authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices or materials described in this report.
Funding
None.
References
1. Cardinale D, Iacopo F, and Cipolla CM (2020) Cardiotoxicity of anthracyclines Front Cardiovasc Med 7 26 https://doi.org/10.3389/fcvm.2020.00026 PMID: 32258060 PMCID: 7093379
2. Armenian S and Bhatia S (2018) Predicting and preventing anthracycline-related cardiotoxicity Am Soc Clin Oncol Educ Book 38 3–12 https://doi.org/10.1200/EDBK_100015 PMID: 30231396
3. Volkova M and Russell R, 3rd (2011) Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment Curr Cardiol Rev 7(4) 214–220 https://doi.org/10.2174/157340311799960645
4. Bauersachs J and Soltani S (2024) Heart failure: update of the ESC 2023 guidelines Herz 49(1) 19–21 https://doi.org/10.1007/s00059-023-05221-2
5. Frak W, Hajdys J, and Radzioch E, et al (2023) Cardiovascular diseases: therapeutic potential of SGLT-2 inhibitors Biomedicines 11(7) 2085 https://doi.org/10.3390/biomedicines11072085 PMID: 37509724 PMCID: 10377079
6. Silverii GA, Monami M, and Mannucci E (2021) Sodium-glucose co-transporter-2 inhibitors and all-cause mortality: a meta-analysis of randomized controlled trials Diabetes Obes Metab 23(4) 1052–1056 https://doi.org/10.1111/dom.14286
7. Bhattarai M, Salih M, and Regmi M, et al (2022) Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in patients with type 2 diabetes and other risk factors for cardiovascular disease: a meta-analysis JAMA Netw Open 5(1) e2142078 https://doi.org/10.1001/jamanetworkopen.2021.42078 PMID: 34985519 PMCID: 8733833
8. Monzo L, Ferrari I, and Cicogna F, et al (2023) Sodium-glucose co-transporter 2 inhibitors in heart failure: an updated evidence-based practical guidance for clinicians Eur Heart J Suppl 25(Suppl C) C309–C315 https://doi.org/10.1093/eurheartjsupp/suad055 PMID: 37125324 PMCID: 10132577
9. Wells GA, Shea B, and O'Connell D, et al (2012) The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses [http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp]
10. DerSimonian R and Laird N (1986) Meta-analysis in clinical trials Control Clin Trials 7(3) 177–188 https://doi.org/10.1016/0197-2456(86)90046-2 PMID: 3802833
11. Higgins JP, Thompson SG, and Deeks JJ, et al (2003) Measuring inconsistency in meta-analyses BMJ 327(7414) 557–560 https://doi.org/10.1136/bmj.327.7414.557 PMID: 12958120 PMCID: 192859
12. Abdel-Qadir H, Carrasco R, and Austin PC, et al (2023) The association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in anthracycline-treated patients with cancer JACC CardioOncol 5(3) 318–328 https://doi.org/10.1016/j.jaccao.2023.03.011 PMID: 37397088 PMCID: 10308059
13. Fath AR, Aglan M, and Aglan A, et al (2023) Sodium glucose co-transporter-2 inhibitors and clinical outcomes in cancer patients treated with anthracyclines Circulation 148 https://doi.org/10.1161/circ.148.suppl_1.15808
14. Gongora CA, Drobni ZD, and Quinaglia Araujo Costa Silva T, et al (2022) Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines JACC Heart Failure 10(8) 559–567 https://doi.org/10.1016/j.jchf.2022.03.006 PMID: 35902159 PMCID: 9638993
15. Hwang HJ, Kim M, and Jun JE, et al (2023) Sodium-glucose cotransporter-2 inhibitors improve clinical outcomes in patients with type 2 diabetes mellitus undergoing anthracycline-containing chemotherapy: an emulated target trial using nationwide cohort data in South Korea Sci Rep 13(1) 21756 https://doi.org/10.1038/s41598-023-48678-1 PMID: 38066029 PMCID: 10709414
16. Russo M, Della Sala A, and Tocchetti CG, et al (2021) Metabolic aspects of anthracycline cardiotoxicity Curr Treat Options Oncol 22(2) 18 https://doi.org/10.1007/s11864-020-00812-1 PMID: 33547494 PMCID: 7864817
17. Qiu S, Zhou T, and Qiu B, et al (2021) Risk factors for anthracycline-induced cardiotoxicity Front Cardiovasc Med 8 736854 https://doi.org/10.3389/fcvm.2021.736854 PMID: 34660739 PMCID: 8511483
18. Vafa RG, Sabahizadeh A, and Mofarrah R (2024) Guarding the heart: how SGLT-2 inhibitors protect against chemotherapy-induced cardiotoxicity: SGLT-2 inhibitors and chemotherapy-induced cardiotoxicity Curr Probl Cardiol 49(3) 102350 https://doi.org/10.1016/j.cpcardiol.2023.102350
19. Basham HA, Keswani S, and Kumar A, et al (2024) Role of sodium-glucose co-transporter-2 inhibitor during anthracycline use: an updated review Cardiol Rev https://doi.org/10.1097/CRD.0000000000000638 PMID: 38189378
20. Dutka M, Bobinski R, and Francuz T, et al (2022) SGLT-2 inhibitors in cancer treatment-mechanisms of action and emerging new perspectives Cancers (Basel) 14(23) 5811 https://doi.org/10.3390/cancers14235811 PMID: 36497303 PMCID: 9738342
Supplementary data 1
Search strategy
Medline
1. Exp SGLT2 inhibitors/ or exp SGLT2 inhibitor.mp.
2. Anthracycline.mp. or exp anthracyclines
3. Exp doxorubicin/ or doxorubicin.mp.
4. Adriamycin.mp. or exp doxorubicin
5. Daunorubicin.mp. or exp daunorubicin
6. Epirubicin.mp. or exp epirubicin
7. Idarubicin.mp. or exp idarubicin
8. Cancer therapy.mp. or exp antineoplastic agents
9. Exp cardiovascular diseases/ or cardiovascular outcome.mp.
10. Exp cardiovascular system system/ or exp cardiovascular abnormalities/ or cardiovascular.mp. or exp cardiovascular diseases
11. 2 or 3 or 4 or 5 or 6 or 7 or 8
12. Exp myocardial infarction/ or cardiac outcome.mp. or exp heart failure
13. Exp hospital mortality/ or exp mortality/ or mortality.mp. or exp mortality, premature
14. 9 or 10 or 12 or 13
15. Exp SGLT2 inhibitors/ or empagliflozin.mp.
16. Canagliflozin.mp. or exp canagliflozin/
17. Exp SGLT2 inhibitors/ or dapagliflozin.mp.
18. Ertugliflozin.mp.
19. 1 or 15 or 16 or 17
20. 18 or 19
21. 11 and 14 and 20
Embase
1. SGLT2 inhibitor'/exp odd ratio (OR) 'SGLT2 inhibitor
2. Dapagliflozin
3. Empagliflozin
4. Canagliflozin
5. Ertugliflozin
6. Bexaglifloxin
7. Anthracycline
8. Doxorubicin
9. Daunorubicin
10. Epirubicin
11. Idarubicin
12. Antineoplastic agent
13. Cancer therapy
14. Cardiovascular outcome
15. Cardiovascular disease
16. Heart failure
17. Heart infarction
18. Mortality
19. #1 OR #2 OR #3 OR #4 OR #5 OR #6
20. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
21. #14 OR #15 OR #16 OR #17 OR #18
22. #19 AND #20 AND # 21