Survival analysis of HER2 receptor negative and HER2 receptor low breast cancer patients at a Philippine Tertiary Government Hospital
Carl Arenos, Steven Lim, Andreu Lominoque, Isabela Reveldez, Angeli Sison-Dimaano, Nehar Pagandaman, Vincent Tatoy, Timothy Uy and Michael San Juan
Division of Medical Oncology, Department of Medicine, University of the Philippines-Philippine General Hospital (UP-PGH), Manila 1008, Philippines
ahttps://orcid.org/0009-0001-6070-6924
Abstract
Background and objectives: Human epidermal receptor 2 (HER2)-low breast cancer, characterised by specific immunohistochemistry (IHC) scores (+1 or +2) or negative fluorescence in-situ hybridiszation (FISH), has unique biological traits, therapeutic outcomes and prognosis. Recent studies highlight the effectiveness of Trastuzumab-Deruxtecan (T-Dxd) for HER2-low patients. This study seeks to deepen understanding of HER2 negative and low patients for tailored treatment by determining the 3-year disease-free survival (DFS) rates and prognostic variables of different subsets of HER2 negative (HER2 0) and HER2-low breast cancer patients (HER2 +1, HER2 +2 and HER2 FISH negative).
Methodology: We analysed the records of 138 patients with non-metastatic breast cancer, exhibiting HER2 IHC 0, +1 or +2/FISH negative. Data on 3-year DFS in months, age, stage (early stage versus locally advanced) and menopausal status were collected. Kaplan–Meier survival curves were used to plot 3-year DFS, while Cox Proportional Analysis assessed the prognostic significance of age and menopausal status in the HER2-low population.
Results: Three-year DFS for HER2 0 was 84.4%, HER2 +1 was 81.8% and HER2 +2/FISH negative was 52.9%, displaying statistically significant differences (p = 0.00012). Subgroup analyses revealed consistently worse DFS for HER2 +2/FISH negative patients in both early and advanced stages (p = 0.0042, 0.0057). Cox proportional analysis showed a recurrence hazard ratio of 4.0–4.5 for HER2 +2/FISH negative patients. Among prognostic factors, post-menopausal status correlated with a decreased risk of recurrence (HR 0.4387), signifying a 56.13% lower recurrence risk compared to pre-menopausal patients (p = 0.00723). On the other hand, patient age was not correlated with a reduced risk of recurrence (HR 0.97; p = 0.0544).
Conclusion: This study reveals a worse 3-year DFS in HER2 +2/FISH negative patients across both early and advanced disease stages. The findings highlight the prognostic importance of HER2-low status and may guide future therapeutic strategies, including the use of targeted therapies like T-Dxd in non-metastatic patients.
Keywords: breast cancer, Her2-low, prognosis
Correspondence to: Carl Arenos
Email: ccarenos@up.edu.ph
Published: 06/02/2025
Received: 12/06/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Background and review of related literature
Cancer is one of the four epidemic non-communicable diseases in our country, affecting 189 of every 100,000 Filipinos with four patients dying every hour due to the condition [1]. According to the latest Global Cancer Observatory data, breast cancer is the most prevalent cancer in the world amounting to 8.1 million women worldwide diagnosed with the condition in the past 5 years [2]. In the Philippines, breast cancer comprises up to 17.5% of all newly diagnosed cases and is the leading cause of cancer-related death in the country [2]. A considerable factor in the high rates of breast cancer incidence is the lack of awareness/education about the disease [3].
One of the adjuncts in the treatment and prognostication of breast cancer patients is the determination of hormone status, such as estrogen (ER) and progesterone receptors (PR), and human epidermal receptor 2 (HER2) receptors. These are determined using immunohistochemistry (IHC) or in-situ hybridisation (ISH). IHC is used to characterise cellular characteristics using certain markers that detect intracellular proteins and cell surfaces. IHCs can be used to guide treatment and prognostic decisions by determining tumour subtypes, cell origin and distinguishing metastatic from primary tumour.
The most common IHC markers used in breast cancer are ER, PR, Ki-67 antigen and HER2/Neu expression. Breast cancer tissues are considered ER or PR positive if at least 1% of the examined cells are stained. Ki-67 antigen is expressed by cells in the G1, S, G2 and M phases of the cell cycle and is used as an index of proliferation of neoplastic cells. It is expressed as a percentage of positive cells in a tissue sample.
HER2 receptor is a tyrosine kinase that has effects on cell proliferation and survival. In breast cancer patients, HER2 overexpression is one of the main catalysts for cancer cell progression in a subset of breast cancer patients [4–8]. Patients who test positive for HER2 receptors can avail of treatment of HER2 receptor antagonists such as Trastuzumab, Pertuzumab and Lapatinib. HER2 expression ranges from HER2 positive (with a score of +3) to HER2 negative (score of 0).
The American Society of Clinical Oncology/College of American Pathologists defines the HER2 spectrum as:
HER2 score 3+ is defined as complete membrane staining, intense and >10% of tumour cells. HER2 score 2+ is defined as weak-moderate complete membrane staining in >10% of tumour cells or membrane staining is intense but in ≤ 10% of tumour cells. HER2 score 1+ qualifies as incomplete membrane staining that is faint or barely perceptible and >10% of tumour cells. HER2 ultra-low is outlined as incomplete membrane staining and faint/ barely perceptible in <10% of tumour cells. HER2 negative refers to samples that have no staining results upon analysis.
Currently, an evolving trend in cancer is the clinical implication of HER2-low breast cancer. It is defined as breast cancer subtypes with HER2 IHC score of 1+ or 2+ with a negative ISH result [4–8]. Approximately more than half of breast cancers may be classified under HER2 low subtype. HER2-low patients were noted to have distinct biological characteristics and show different outcomes in terms of therapy and prognosis. Current literature demonstrates conflicting results over the years, which shows that more studies are needed to shed more light on this matter.
Survival studies have been done comparing HER2 negative and HER2 low patients. It was seen that HER2 negative and HER2 low patients have similar prognosis regardless of hormone receptor status [9, 12] but it was noted that HR-positive tumours with HER2 low status have better disease-free survival (DFS) rates than HR-positive/ HER2 negative [9]. While further analysis of survival rates between HER2 negative and HER2 low patients show that patients with the latter have a longer DFS and overall survival (OS) compared to the former [10, 11]. Certain applications regarding HER2 low have been applied to patients. In a study involving HER2 low breast cancer patients, there was no prognostic value [13]
Recent studies show the effectiveness of certain medications for patients diagnosed with HER2-low cancers. In particular, the anti-HER2 antibody-drug conjugate Trastuzumab-Deruxtecan (T-Dxd) has been proven to provide survival benefits for this subset of patients [14, 15].
Implications and importance of the study
This study highlights the importance of HER2 negative and HER2 low patients in terms of prognostication and determines which subset of patients will benefit from emerging regimens targeted at HER2 low patients.
Research question
Is there a difference in the DFS between non-metastatic breast cancer patients diagnosed with HER2 negative status and those with HER2 low status?
Objectives
General objectives
Determine the 3-year DFS of different subsets of HER2 negative (HER2 0) and HER2 low breast cancer patients (HER2 +1, HER2 +2 and HER2 fluorescence in-situ hybridisation (FISH) negative).
Specific objectives
- Analyse the association between triple-negative breast cancer (TNBC) with HER2 negative and HER2 low patients with respect to DFS.
- Analyse the DFS of HER2 negative and HER2-low patients on early-stage breast cancer patients.
- Analyse the DFS of HER2 negative and HER2-low patients on locally advanced breast cancer patients.
- Determine the relation of other variables, such as age and menopausal status in the prognosis of HER2 low patients.
Methodology
Study design
This is a retrospective cohort study on HER2 negative and HER2 low patients diagnosed in 2020 or earlier.
Study setting
The Medical Oncology clinic of the Philippine General Hospital Cancer Institute was the study setting. Patient charts (physical and electronic) were retrieved with the assistance of the Cancer Institute Medical Records Division. The Medical Records Division of the Cancer Institute served as the provider of patient records in this study because they are responsible stewards of patient records within the institute.
Study population
A total of 138 patient records were included in the analysis [16]. Subjects included in the study are: 1) Patients aged more than 18 years old; 2) Breast cancer patients diagnosed from 2017 or earlier; 3) Resected non-metastatic breast cancer patients tumour, node and metastasis (TNM stage from I to IIIC) 4). IHC results bearing HER2 status 0, +1 or +2/equivocal with a FISH result of ‘HER2 not detected.’
Patients were excluded from the study if they present with any of the following: 1) non-breast cancer patients; 2) Patient aged below 18 years old; 3) HER2 IHC result of positive (3+) OR FISH result of ‘HER2 detected’; 4) Metastatic or non-resected breast cancer patients; 5) Patients with synchronous double primary malignancies (Figure 1).
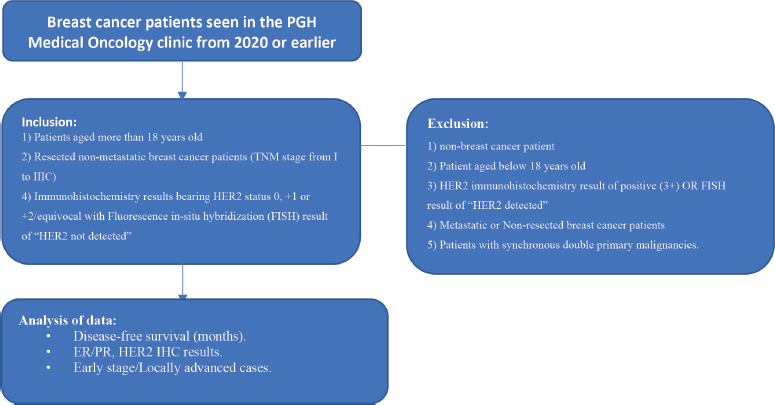
Figure 1. Study flow procedure.
Data collection
The study was conducted from 01 May to 18 August 2023. Patient charts of patients diagnosed with breast cancer from the year 2020 or earlier were included in the study. The earliest patient enrolled was diagnosed with breast cancer on October 2002. All investigators of the study collected the predetermined data using electronic medical records and/or physical medical records of the patients. The following data were obtained from the patient’s record: Name, age, cancer type, year of diagnosis, TNM stage, intrinsic subtype (Luminal status, HER2 and Basal-like), neo/adjuvant chemotherapy history, menopausal status and record of disease progression (radiographic or histopathologic) or mortality. The TNM staging will be based on the American Joint Committee on Cancer Staging Manual 7th Edition [17].
The HER2 status was based according to the American Society of Clinical Oncology/College of American Pathologists:
HER2 score +3 is defined as complete membrane staining, intense and >10% or tumour cells. HER2 score +2 is defined as weak-moderate complete membrane staining in >10% of tumour cells or membrane staining is intense but in ≤ 10% of tumour cells. HER2 score 1+ qualifies as incomplete membrane staining that is faint or barely perceptible and >10% of tumour cells. HER2 negative refers to samples that have no staining results upon analysis. Those samples that fall under Her2 +1/+2/FISH negative will be categorised under HER2-low status.
The intrinsic subtype definition was based on the Clinicopathologic Surrogate definition by the European Society of Medical Oncology Clinical Practice Guidelines for Early Breast Cancer [18].
Luminal A intrinsic subtype will correspond to patients with clinicopathologic surrogate definition fulfilling the following:
Luminal A-like: ER-positive, HER2-negative, Ki67 low, PR high (cut-off value of >20%) and low-risk molecular signature (if available).
Luminal B intrinsic subtype will correspond to patients with clinicopathologic surrogate definition fulfilling the following:
Luminal B-like (HER2-negative): ER-positive, HER2-negative, Ki67 high OR PR-low (cut-off value of <20%) and high-risk molecular signature (if available).
Luminal B-like (HER2-positive): ER-positive, HER2-positive, Any Ki67 or any PR status.
HER2 intrinsic subtype refers to patients with HER2-positive and ER/PR-negative results.
Basal-like intrinsic subtype will refer to patients with triple-negative profile (ER/ PR and HER2--negative results).
Menopausal status is defined in this study are individuals who fulfill any of the following criteria: 1) more than 60 years of age; 2) underwent bilateral oophorectomy/irradiation; or 3) patients who are amenorrheic for more than 1 year with a hormonal status within the menopausal range upon surveillance testing (follicle stimulating hormone, luteinising hormone and estradiol serum levels).
The data were extracted from physical charts and electronic medical records from the PGH Medical Records Division. All patient data will be kept in confidentiality with their corresponding alphanumeric patient codes. The electronic data will be recorded and stored using Microsoft Excel.
Data analysis
Descriptive statistics for summarising the categorical data, correlation analysis and Kaplan–Meier curve were used for statistical analysis. The confidence interval used is 95% (p-value cut-off of 0.05). Pearson’s chi-squared test was used to establish if the HER2-low and HER2-negative subgroups are comparable in terms of treatment allocation, confirming the population’s homogeneity for subsequent survival and prognostic analyses. DFS is defined as the length of time a patient or cohort survives with no evidence of primary or metastatic disease. The time from diagnosis to the recurrence of tumour was detected pathologically (via biopsy) or radiographically using imaging studies (CT scan, PET-CT and bone scan). OS pertains to the length of time from the date of diagnosis that patients diagnosed with cancer which are still alive [19, 20]. The data collected was analysed using the Rstudio package. Kaplan–Meier survival curves were used to plot the DFS data and Cox proportional analysis was used to account for the other factors related to the patients such as age and menopausal status.
Ethical considerations
Ethics approvals
The study is approved by the University of the Philippines Manila Research Ethics Board (UPMREB) with the corresponding UPMREB CODE: 2023-0189-01.
Anonymity and privacy/data protection plan
All the data that were retrieved from the medical records of the included patients are kept with utmost anonymity and privacy. The data gathered are stored in a computerised database accessible only to the investigators.
All information collected from the medical records, including their name, age and contact numbers, are kept confidential and accordingly assigned to unique alphanumerical case identifiers upon encoding into the dataset (de-identification and use of coded data).
Unique alphanumerical case identifiers are assigned for each patient and are used in data encoding and analysis. Only the investigators have access to the control numbers assigned for each patient.
All electronic data were stored and secured by the primary investigator in a password-protected and encrypted hard drive and will also be stored until the end of the manuscript writing. In case of a breach of privacy/confidentiality despite de-identification and use of coded information, the primary investigator will review to confirm if a breach of confidentiality was indeed done and accordingly seek legal consultation for the following steps. The PGH Data Privacy Officer will also be accordingly informed.
Possible benefits, risks and hazards
Since there is no direct interaction with the study population, there are no expected risks to the study population.
The results of this study could benefit breast cancer patients who present with HER2-low IHC results. It will guide future patients with additional possible therapeutic regimens that would fit their breast cancer subtype.
Preliminary results will be relayed to the primary physicians and concerned parties.
Recruitment process and consent to participate
Patient records using physical charts and electronic medical records were used for this study, no actual patient participation was required.
Compensation
(not applicable).
Research funding, conflicts of interest and disclosures
All research-related expenses were covered by the primary investigator. The principal investigator and co-investigators have no disclosures or conflicts of interest related to the study.
Data dissemination plan
After completion of the study, efforts will be made to have the study published in a local or an international journal. It will also be submitted for presentation in scientific meetings of both local and international societies, such as the Philippine Society of Medical Oncology, American Society of Clinical Oncology and the European Society for Medical Oncology Asia. The results of the study will not, in any form, cause stigma or any negative impact the well-being of the breast cancer patient community.
Good clinical practice certification
The investigators ensured that their good clinical practice certification is updated prior to and during the implementation of the study.
Ethics review board and approval
In compliance with the university and hospital regulations, this study protocol was submitted to the University of the Philippines Manila Research Ethics Board (UPMREB) for review and approval.
A waiver of informed consent was requested from the UPMREB panel since (1) the investigators only used hospital medical records (electronic and physical); (2) there are no risks associated with the study since there is no active involvement of human participants (National Ethical Guidelines for Health and Health-Related Research 2017 guidelines provision 16.2.1-4,17.1;4). The investigators ensured that the highest standards of confidentiality and anonymity of patient data will be strictly implemented in accordance with the National Ethical Guidelines for Health and Health-Related Research 2017.
Results
The results were tabulated and analysed using Microsoft Excel and RStudio statistical software (see Appendix). This study determined if there is a statistical difference in the DFS of HER2 0 versus HER2 low (+1 and +2/FISH negative) subset with further subgroup analysis between Her +1 and HER2 +2/FISH negative. Subgroup analysis on early stage and locally advanced breast cancer, luminal and TNBC were also performed. Cox proportional analysis was used to account for the patient-related factors such as age and menopausal status.
A comprehensive review of 138 patient records was conducted at the Medical Oncology clinic. The study population's mean age was determined to be 55 years, with a standard deviation of 10.4, encompassing an age range from 26 to 74 years of age. Within the cohort, 96 patients were identified with early-stage breast cancer, while the remaining 42 were classified as having locally advanced disease (Table 1). In the early stage group, 83% of patients underwent adjuvant cytotoxic chemotherapy while 96% of patients eligible for adjuvant hormonal therapy were given. In the locally advanced breast cancer cohort, 57% of patients underwent neoadjuvant cytotoxic chemotherapy while the rest (42.86%) underwent adjuvant cytotoxic chemotherapy. Almost all patients (except for 1 patient) in the luminal group received adjuvant hormonal therapy. None of the patients in the study were given adjuvant Cyclin-dependent kinase (CDK) 4/6 inhibitors because access to these drugs were limited (logistically and financially) in our institution.
Table 1. Descriptive statistics.
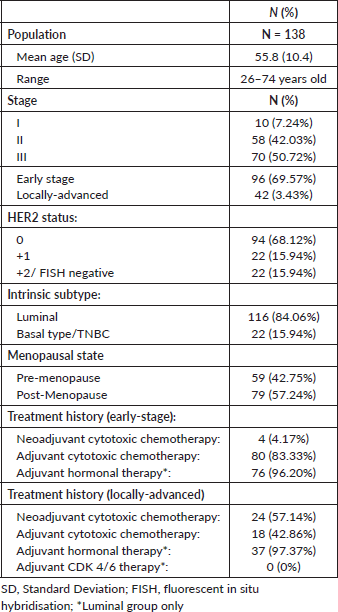
The distribution of HER2 status among the study population was as follows: 68% were HER2 0 by IHC, and 16% each were classified as HER2 +1 and HER2 +2/FISH negative. The Pearson’s chi squared test analysis was performed which indicated that the study population exhibited homogeneity in terms of HER2 status and treatment allocation. The test revealed no statistically significant association between HER2 status and the administration of neoadjuvant or adjuvant therapy, with p-values of 0.531 and 0.2194, respectively. This lack of association suggests that the HER2 expression levels did not influence the choice of therapy, and therefore, patients in this cohort were not selectively treated with either neoadjuvant or adjuvant therapy based on their HER2 status. These findings support the homogeneity of the study population concerning HER2 status and treatment strategy, allowing us to proceed with the analysis of DFS and prognostic factors.
A detailed breakdown of molecular subtypes revealed that 84% of the study group consisted of Luminal subtypes, whereas the remaining 16% were characterised as basal type or triple negative. It must be noted that the Luminal subtype population could not be further subdivided into Luminal A and Luminal B classifications, due to the absence of Ki-67 IHC data within the majority of the patient records. Menopausal status data was also recorded with the majority of the patients (n = 79) were post-menopause while the rest of the sample population was pre-menopausal. The results of statistical analysis generated by the RStudio software were based on a 95% confidence interval.
Figure 2 shows an overview of the 3-year DFS curves for patients with TNBC and conventional HER2-negative patients. The latter refers to the original group identified with a HER2 negative status, encompassing HER2 0, HER2 +1 and HER2 +2 as determined by IHC results. This shows an estimate of the general survival curve of patients in the cohort group. For the TNBC group, the 36-month DFS was 76.7%, with a standard error of 0.0916 and a confidence interval ranging from 60.7% to 96.9%. In contrast, the conventional HER2 negative group exhibited a 36-month DFS of 79.2%, with a standard error of 0.0377 and a confidence interval from 72.2% to 87.0%. When comparing the survival curves of these two groups, no statistically significant difference is observed, as evidenced by a p-value of 0.62.
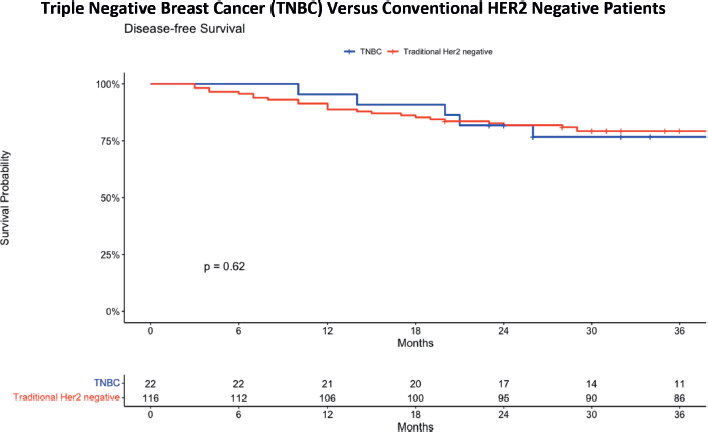
Figure 2. DFS comparing TNBC and traditional HER2 negative patients.
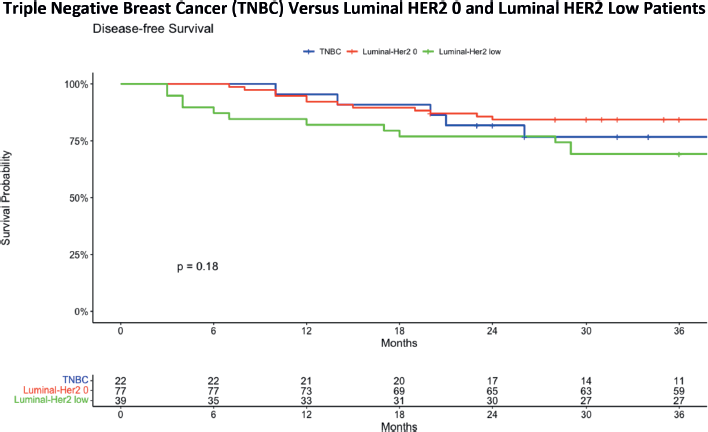
Figure 3. DFS comparing TNBC with luminal HER2 0 and luminal HER2-low patients.
Figure 3 presents the Kaplan–Meier survival curve in which the conventional Luminal HER2 negative group is further subdivided into Luminal-HER2 0 and Luminal HER2-low categories. In the TNBC group, the 36-month DFS was 76.7%, with a standard error of 0.0916 and a confidence interval ranging from 60.7% to 96.9%, showing a gradual decrease in survival over the 36-month period. The HER2 0 group exhibited a more stable survival rate, with a 36-month DFS of 84.4%, a standard error of 0.0414 and a confidence interval from 76.6% to 92.9%. In contrast, the HER2 low group experienced a more pronounced decrease in survival, with a 36-month DFS of 69.2%, a standard error of 0.0739 and a confidence interval from 56.2% to 85.3%. When analysing the 3-year DFS data for patients with either HER2 0 or HER2 low results, no statistically significant difference is found at a p-value of 0.18.
The study group has further broken down the Luminal HER2-low patients into Luminal HER2 +1 and Luminal HER2 +2/ FISH negative (Figure 4). The 36-month DFS analysis for three Luminal HER2 subgroups reveals distinct outcomes. The Luminal HER2 0 group exhibited a DFS of 84.4%, with a confidence interval ranging from 76.6% to 92.9%. The Luminal HER2 +1 group showed a slightly lower DFS at 81.8%, with a confidence interval from 67.2% to 99.6%. Most notably, the Luminal HER2 +2/FISH negative group demonstrated a significantly lower DFS at 52.9%, with a confidence interval from 33.8% to 82.9%. These findings underscore the varying survival outcomes among these subgroups, highlighting the lower survival rate in the HER2 +2/FISH negative group over a 36-month period. The statistical evaluation revealed a statistically significant difference in survival outcomes among the groups, with a p-value of 0.00012. This finding suggests a worse DFS in patients with a Luminal HER2 +2/FISH negative status.
The 3-year DFS analysis for early-stage (Figure 5) Luminal HER2 subgroups provides insights into the survival outcomes for these patients. For the Luminal HER2 0 group, the DFS was 87.0%, with a confidence interval ranging from 78.5% to 96.5%. In the Luminal HER2 +1 group, the DFS was slightly lower at 81.2%, with a confidence interval from 64.2% to 100%. The Luminal HER2 +2/FISH negative group demonstrated the lowest DFS at 54.5%, with a confidence interval from 31.8% to 93.6%. These results emphasise the differences in survival outcomes among the early stage Luminal HER2 subgroups, with the HER2 +2/FISH negative group showing a notably lower survival rate over the 36-month period. The data shows a statistically significant difference in terms of DFS in early stage breast cancer patients with Luminal Her 0, HER2 +1 and HER2 +2/FISH negative status. The Kaplan–Meier curve for Luminal HER2 +2 patients shows a significant downtrend compared to other groups.
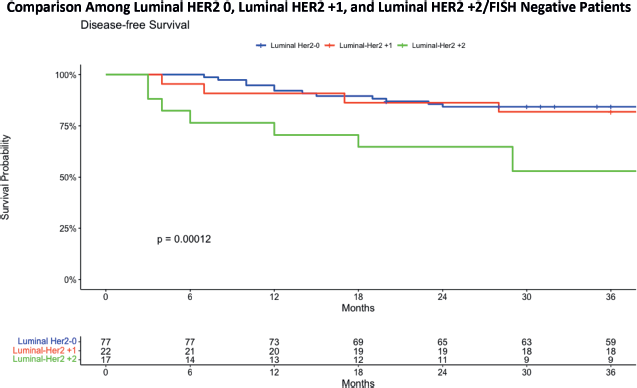
Figure 4. DFS comparing luminal HER2 0 with luminal HER2 +1 and luminal HER2 +2.
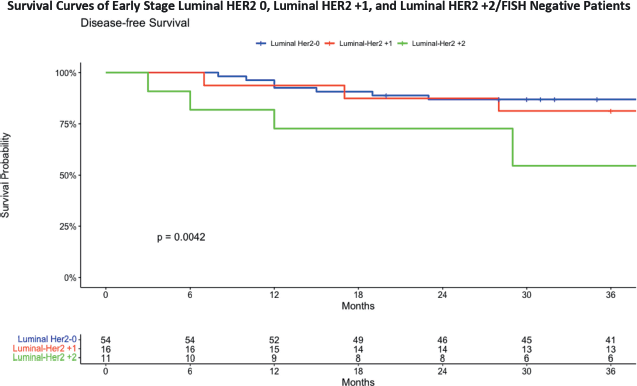
Figure 5. DFS in early stage breast cancer patients with HER2 0, HER2 +1 and HER2 +2/FISH negative status.
The 3-year DFS analysis for locally advanced (Figure 6) Luminal HER2 subgroups reveals distinct survival outcomes for these patients. In the Luminal HER2 0 group, the DFS was 78.3%, with a confidence interval ranging from 63.1% to 97.1%. For the Luminal HER2 +1 group, the DFS was 83.3%, with a confidence interval from 58.3% to 100%. The Luminal HER2 +2/FISH negative group exhibited the lowest DFS at 50.0%, with a confidence interval from 22.5% to 100%.
These results indicate that locally advanced Luminal HER2 0 and HER2 +1 patients have relatively similar survival outcomes, while the HER2 +2/FISH negative group demonstrates a significantly lower survival rate over the 36-month period. Figure 6 presents a notable statistically significant downward trend in the DFS of patients in patients with HER2 +2/FISH negative patients compared to HER2 negative and HER2 low patients (p = 0.0057).
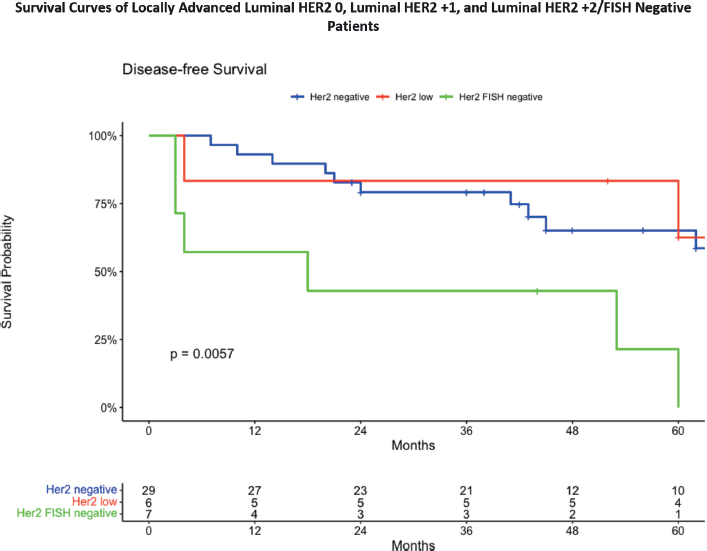
Figure 6. DFS in locally advanced breast cancer patients.
Table 2. Cox proportion analysis for age (with HER2 0 as reference).
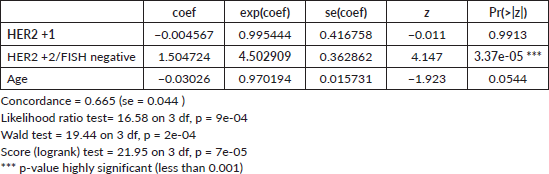
Table 3. Cox proportion analysis for menopause (with HER2 0 as reference).
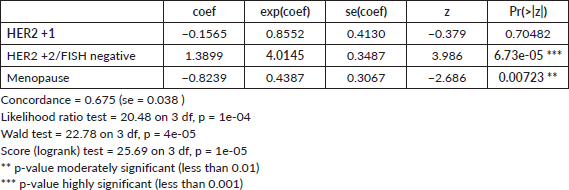
Cox proportion analysis
To understand the complex relation of factors that influence DFS outcomes, our study employed a Cox Proportional Hazards analysis in the HER2-low groups to investigate the role of age and menopausal status. These variables were selected based on their potential biological and clinical relevance, as well as their prominence in existing literature.
The Cox Proportional Hazards model while controlling for the effect of age (Table 3) revealed distinct effects of HER2 status and age on DFS. Having a HER2 +1 subtype was associated with a negligible decrease in hazard risk of the event compared to the HER2 negative reference group, but this was not statistically significant (p-value = 0.9913). Conversely, the HER2 +2/FISH negative showed a substantial increase in hazard, with a risk roughly 350.3% higher than the reference group, and this effect was highly significant (p-value = 3.37e-05). Age also appeared to influence the hazard, with a 2.98% lower risk for every 1-year increase, though the result was not statistically significant (p-value = 0.0544). The model's concordance of 0.665 indicates good discrimination, and the highly significant global tests (p-values ranging from 7e-05 to 9e-04) provide strong evidence that at least one predictor has a significant effect. Overall, the analysis underscores that having a HER2 +2/FISH negative status is a significant predictor of the hazard in this sample with a hazard ratio (HR) of 4.5, while the effects of HER2 +1 status and age were not conclusively demonstrated.
In the conducted Cox proportional hazards analysis (Table 3) while controlling for the effect of menopause, three variables were examined: HER2 +1 status (Group B), HER2 +2 status (Group C) and menopausal state (Menopause), with HER2 negative status serving as the reference category. The analysis revealed that HER2 +1 status was not statistically significant in influencing the hazard, with a hazard ratio (HR) of 0.8552 (p-value = 0.70482). In contrast, HER2 +2/FISH negative subtype was associated with a significant increase in hazard, with an HR of 4.0145 (p-value = 6.73e-05), with a 300% increase in the risk for recurrence. The menopausal state was also found to be a significant predictor, associated with a decreased hazard, with an HR of 0.4387 (p-value = 0.00723). The hazard ratio (HR) of the menopausal state indicates that being in menopause is associated with a decreased risk of recurrence. In other words, the risk is roughly 56.13% lower for post-menopausal individuals compared to pre-menopausal individuals.
The model's concordance of 0.675 and significant global tests (p-values ranging from 1e-05 to 1e-04) indicate a robust fit. Overall, the findings suggest that HER2 +2 status and menopausal state are significant predictors in the model, while HER2 +1 status does not appear to have a significant effect.
Discussion
The comprehensive analysis of 3-year DFS in various patient cohorts revealed mixed findings. While there was no statistically significant difference between TNBC and the conventional luminal HER2-negative patients. Further analysis subdividing the conventional HER2 negative group into HER2 0 and HER2 low patients also shows no significant difference in the 3-year survival analysis. A more detailed breakdown of the Luminal HER2-low group into Luminal HER2 +1 and Luminal HER2 +2/FISH negative revealed a significant difference in survival outcomes (p-value = 0.00012), with worse 3-year DFS in the Luminal HER2 +2/FISH negative group. The luminal cohort was divided into early stage and locally advanced breast cancer patients. A significant difference in DFS was observed among early-stage breast cancer patients with Luminal HER2 0, HER2 +1 and HER2 +2/FISH negative status. The Luminal HER2 +2/FISH negative group, in particular, showed a significant downtrend in DFS probability for both early stage and locally advanced cancer patients.
In summary, the analysis highlights the complexity of HER2 status in predicting DFS, with significant variations observed in specific subgroups. The findings underscore the importance of nuanced categorisation and understanding of HER2 status in breast cancer prognosis and treatment planning. The statistically significant differences in some categories emphasise the need for further investigation and potential consideration in clinical decision-making particularly in the HER2-low population.
In the conducted Cox proportional analyses, the HER2 +2/FISH negative group presented with a statistically significant increase the risk for recurrence (HR 4.0–4.5) amounting to an increased risk of 300%–350% when compared to HER2 0 and HER2 +1 group. Adjusting for the effect of age, it was seen that age appeared to influence the risk of recurrence, with a 2.98% lower risk for every 1-year increase in age, although this finding is not statistically significant (p-value 0.0544). Cox proportion analysis for menopause showed that there is a 56.13% lower risk for recurrence in post-menopausal patients compared to pre-menopausal patients.
In terms of prognosis, large-scale studies done overseas presented evidence that HER2 low breast cancer patients present with different tumour characteristics depending on the hormone receptor positive status. HER2-low breast cancer patients with the HR-positive subtype showed fewer T4 tumours, higher histologic profile and negative lymphatic invasion in contrast to HER2-low TNBC patients who have higher lymph node ratios and presentation of positive lymphatic invasion. What is notable in this study is that HER2-low breast cancer patients show significantly better breast cancer-specific survival rates compared to HER2 0 breast cancer patients irrespective of HR status [21]. Other studies show no significant difference in the survival rates between HER2 0 and HER2 low patients. A recent study done in China by Zhu et al [22] also showed mixed results. Out of 969 cases, 777 were hormone receptor positive and 192 were hormone receptor negative. In the hormone receptor positive group, HER2 low expressors showed no significant difference in the DFS and OS when compared to HER2 0 patients despite the former having a younger study population, higher pre-menopausal patients and more lymph node involvement.
In the hormone receptor-negative group, there was a better DFS in the HER2-low expressors when compared to the HER2 0 group but the OS benefit was not proven here [22]. Two meta-analyses comparing HER2 and HER2 low patients was done for early stage breast cancer patients. This study compiled 600 thousand cases and showed that having a HER2-low status is associated with an improved DFS and OS regardless of hormone receptor status [23–25]. In terms of the statistically significant worse DFS in HER2 +2/FISH negative patients, there are currently no studies that observed the same trend seen in this study as well as the absence of studies that divided the analysis of HER2 low patients into Hr2 +1 and HER2 +2/FISH negative. Perhaps this could be an avenue for further research in the future.
The cox proportional analysis for age showed that the risk for breast cancer recurrence is decreases by approximately 3% for each increase in the age of the patient. An analysis of the Surveillance, Epidemiology and End Results database from 2004 to 2008 showed that younger patients aged less than 40 years old showed worse OS and breast cancer-specific survival. What is noteworthy in this study is that further analysis showed an age of 60 and above was a significant independent predictor for poor prognosis [26]. A study with a 10-year follow-up during 1961–1991 investigated the link between age of diagnosis and breast-cancer-specific mortality. Analysis done in this study showed that patients aged less than 40 and older than 80 had a statistically significant 1-year mortality rate when compared to women aged 40–49 years old. Similar trends could not be replicated in our study because the oldest patient in the cohort is only 74 years of age [27]. A similar trend was seen in a nationwide cohort study done in Denmark which showed that the cumulative incidence of late recurrence was higher in younger patients compared to older patients. With adjusted hazards of recurrence for women who were diagnosed before 40 years of age [28].
In terms of menopausal status, the results seen in this study were consistent with a few studies. In a retrospective study done in 2022, post-menopausal patients were seen to have better DFS in the Kaplan-Meier curve in contrast to pre-menopausal patients with HER2 enriched, Luminal/HER2 type,
Luminal A and triple-negative subtype, although this trend was only statistically significant for the triple-negative subtype (p = 0.01) [29]. However, another study done by Lao et al [30] on women with stage I–III breast cancer patients aged 44–55 years old demonstrated that menopausal patients with ER+ and/or PR + subtypes have a higher risk for metastatic relapse at an HR of 1.38. Although this was not proven to be true as well for women with ER and PR-negative disease [30]. A commentary done on this study that pointed out certain limitations like the age range of patients within the study and the absence of their treatment details of the individual patients. The author of the commentary also pointed at other details that could pinpoint pre-menopausal (or younger patients in general) patients having increase risk for worse prognosis when compared to post-menopausal patients include: 1) younger women may be diagnosed late due to higher breast density; 2) younger women are more likely to have subtypes with worse prognosis such as HE negative, HER2 positive and TNBC; 3) older women are more likely to be diagnosed by screening due to lower breast density 4) older women are more likely to be diagnosed with HR-positive disease (which have more available therapeutic options that can decrease the risk of recurrence) [31].
There are certain limitations in this study, one problem is the sample size. Although there are analyses done in this paper that showed statistically significant results, the significance of the other subgroups could be confirmed by increasing the power of the study (increasing the population group). There was also paucity in patients with TNBC but with HER2 IHC samples of +1/+2/FISH negative, hence, meaningful statistical analysis cannot be done for this group. The access of ideal treatments and prognostic tests for breast cancer are also lacking in the study population. The majority of the patients in the Philippine General Hospital are indigent patients that have limited financial support, thereby limiting their access and compliance to certain expensive interventions such as gonadotropin receptor agonists, immunotherapy, Oncotype-DX testing and timely radiologic imaging.
A significant limitation of the current study stems from the substantial number of surveillance patients lost to follow-up. The recent COVID-19 pandemic has greatly impacted the ability to track these patients, making the update of their patient status a challenging task. This loss to follow-up has inevitably affected the comprehensiveness and potentially the accuracy of the study's findings.
In light of the findings seen in this study, the following recommendations can be made to bridge the knowledge gap in this field:
- Create a separate study on Filipino patients with HER2 low status, since the results of this study do not reflect what is seen in scientific literature.
- Create a larger study for locally advanced HER2 low patients.
- Create studies with larger samples that will tackle the difference in survival of patients with HER2 +1 and HER2 +2/FISH negative patients.
Conclusion
The 3-year DFS for the Luminal HER2 0 (negative) group is 84.4% while the Luminal HER2 +1 group showed a lower 3-year DFS at 81.8%. Most notably, the Luminal HER2 +2/FISH negative group demonstrated the lowest 3-year DFS at 52.9%. The study identified a significant lower 3-year DFS in HER2 +2/FISH negative patients in both early stage and locally advanced breast cancer. The findings underscore the importance of HER2 status in treatment planning and may inform future therapeutic strategies, including targeted therapies like T-Dxd.
Conflicts of interest
The authors do not declare any conflicts of interest in this study.
Funding
Personal funding.
References
1. Philippine Cancer Control Program: Department of Health (2022) Philippine cancer control program [https://doh.gov.ph/philippine-cancer-control-program] Date accessed: 9/9/2022
2. Ferlay J, Ervik M, and Lam F, et al (2024) Global Cancer Observatory: Cancer Today (Lyon: International Agency for Research on Cancer) [https://gco.iarc.who.int/today] Date accessed: 31/7/2024
3. Wu T and Lee J (2018) Promoting breast cancer awareness and screening practices for early detection in low-resource settings Eur J Breast Health 15(1) 18–25 https://doi.org/10.5152/ejbh.2018.4305
4. Krishnamurti U and Silverman J (2014) HER2 in breast cancer: a review and update Adv Anat Pathol 21(2) 100–107 https://doi.org/10.1097/PAP.0000000000000015 PMID: 24508693
5. Zaha DC (2014) Significance of immunohistochemistry in breast cancer World J Clin Oncol 5(3) 382–392 https://doi.org/10.5306/wjco.v5.i3.382 PMID: 25114853 PMCID: 4127609
6. Ronchi A, Pagliuca F, and Zito Marino F, et al (2021) Current and potential immunohistochemical biomarkers for prognosis and therapeutic stratification of breast carcinoma Semin Cancer Biol 72 114–122 https://doi.org/10.1016/j.semcancer.2020.03.002
7. Zhang H, Katerji H, and Turner BM, et al (2022) HER2-low breast cancers Am J Clin Pathol 157(3) 328–336 https://doi.org/10.1093/ajcp/aqab117
8. Venetis K, Crimini E, and Sajjadi E, et al (2022) HER2 low, ultra-low, and novel complementary biomarkers: expanding the spectrum of HER2 positivity in breast cancer Front Mol Biosci 9 834651 https://doi.org/10.3389/fmolb.2022.834651 PMID: 35372498 PMCID: 8965450
9. Xu H, Han Y, and Wu Y, et al (2022) Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: a single-institution experience Front Oncol 12 906011 https://doi.org/10.3389/fonc.2022.906011 PMCID: 9245921
10. Woo JW, Lee K, and Chung YR, et al (2020) The updated 2018 American Society of Clinical Oncology/College of American Pathologists guideline on human epidermal growth factor receptor 2 interpretation in breast cancer: comparison with previous guidelines and clinical significance of the proposed in situ hybridization groups Hum Pathol 98 10–21 https://doi.org/10.1016/j.humpath.2020.01.003 PMID: 32027910
11. Almstedt K, Heimes AS, and Kappenberg F, et al (2022) Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer Eur J Cancer 173 10–19 https://doi.org/10.1016/j.ejca.2022.06.012 PMID: 35839597
12. Tan R, Ong WS, and Lee KH, et al (2022) HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis BMC Med 20(1) 105 https://doi.org/10.1186/s12916-022-02284-6 PMID: 35296300 PMCID: 8928638
13. de Moura Leite L, Cesca MG, and Tavares MC, et al (2021) HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer Breast Cancer Res Treat 190(1) 155–163 https://doi.org/10.1007/s10549-021-06365-7 PMID: 34409551
14. Tarantino P, Curigliano G, and Tolaney SM (2022) Navigating the HER2-low paradigm in breast oncology: new standards, future horizons Cancer Discov 12(9) 2026–2030 https://doi.org/10.1158/2159-8290.CD-22-0703 PMID: 35856622
15. Adams E, Wildiers H, Neven P, and Punie K (2021) Sacituzumab govitecan and trastuzumab deruxtecan: two new antibody-drug conjugates in the breast cancer treatment landscape ESMO Open 6(4) 100204 https://doi.org/10.1016/j.esmoop.2021.100204 PMID: 34225076 PMCID: 8259232
16. Epitools (2022) Sample size for a cohort study [https://epitools.ausvet.com.au/cohorts] Date accessed: 23/10/22
17. Amin MB, Edge SB, and Greene FL, et al (2018) AJCC Cancer Staging Manual 8th edn (Chicago: Springer)
18. ESMO (2023) ESMO Interactive Guidelines (Lugano: ESMO) [http://interactiveguidelines.esmo.org/esmo-web-app/gl_toc/index.php?GL_id=73] Date accessed: 1/2/23
19. ScienceDirect Topics (2022) Disease free survival – an overview [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/disease-free-survival] Date accessed: 26/12/2022
20. National Cancer Institute (2022) NCI Dictionary of Cancer Terms (Bethesda: National Cancer Institute) [https://www.cancer.gov/publications/dictionaries/cancer-terms/def/overall-survival] Date accessed: 26/12/22
21. Won HS, Ahn J, and Kim Y, et al (2022) Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society Breast Cancer Res 24 22 https://doi.org/10.1186/s13058-022-01519-x PMID: 35307014 PMCID: 8935777
22. Zhu XJ, Zhang H, and Zhang S, et al (2023) Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression Beijing Da Xue Xue Bao Yi Xue Ban 55(2) 243–253 https://doi.org/10.19723/j.issn.1671-167X.2023.02.007 PMID: 37042134 PMCID: 10091245
23. Ergun Y, Ucar G, and Akagunduz B (2023) Comparison of HER2-zero and HER2-low in terms of clinicopathological factors and survival in early-stage breast cancer: a systematic review and meta-analysis Cancer Treat Rev 115 102538 https://doi.org/10.1016/j.ctrv.2023.102538
24. Li C, Yuan Q, and Deng T, et al (2023) Prognosis difference between HER2-low and HER2-zero breast cancer patients: a systematic review and meta-analysis Breast Cancer 30(6) 965–975 https://doi.org/10.1007/s12282-023-01487-w PMID: 37470943
25. Yang M, Sun J, and Liu L, et al (2023) Clinicopathological characteristics of HER2-low breast cancer: a retrospective study Sci Rep 13(1) 12382 https://doi.org/10.1038/s41598-023-39372-3 PMID: 37524746 PMCID: 10390573
26. Chen HL, Zhou MQ, and Tian W, et al (2016) Effect of age on breast cancer patient prognoses: a population-based study using the SEER 18 database PLoS one 11(10) e0165409 https://doi.org/10.1371/journal.pone.0165409 PMID: 27798652 PMCID: 5087840
27. Brandt J, Garne JP, and Tengrup I, et al (2015) Age at diagnosis in relation to survival following breast cancer: a cohort study World J Surg Onc 13 33 https://doi.org/10.1186/s12957-014-0429-x
28. Fillon M (2022) Breast cancer recurrence risk can remain for 10 to 32 years CA Cancer J Clin 72(3) 197–199 https://doi.org/10.3322/caac.21724 PMID: 35285943
29. Nishimura R, Osako T, and Okumura Y, et al (2022) Triple negative breast cancer: an analysis of the subtypes and the effects of menopausal status on invasive breast cancer J Clin Med 11(9) 2331 https://doi.org/10.3390/jcm11092331 PMID: 35566456 PMCID: 9103495
30. Lao C, Elwood M, and Kuper-Hommel M, et al (2021) Impact of menopausal status on risk of metastatic recurrence of breast cancer Menopause 28(10) 1085–1092 https://doi.org/10.1097/GME.0000000000001817 PMID: 34260475
31. Bell RJ (2021) Menopausal status at diagnosis of breast cancer and risk of metastatic recurrence Menopause 28(10) 1079–1080 https://doi.org/10.1097/GME.0000000000001830 PMID: 34260476
Appendix
Statistical results from RStudio
TNBC versus conventional HER2 negative
Group = TNBC
time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 22 0 1.000 0.0000 1.000 1.000
6 22 0 1.000 0.0000 1.000 1.000
12 21 1 0.955 0.0444 0.871 1.000
18 20 1 0.909 0.0613 0.797 1.000
24 17 2 0.818 0.0822 0.672 0.996
30 14 1 0.767 0.0916 0.607 0.969
36 11 0 0.767 0.0916 0.607 0.969
Group= HER2 negative time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 116 0 1.000 0.0000 1.000 1.000
6 112 5 0.957 0.0189 0.921 0.995
12 106 8 0.888 0.0293 0.832 0.947
18 100 4 0.853 0.0328 0.791 0.920
24 95 4 0.819 0.0358 0.752 0.892
30 90 3 0.792 0.0377 0.722 0.870
36 86 0 0.792 0.0377 0.722 0.870
TNBC versus HER2 0 versus HER2 low
Group=TNBC time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 22 0 1.000 0.0000 1.000 1.000
6 22 0 1.000 0.0000 1.000 1.000
12 21 1 0.955 0.0444 0.871 1.000
18 20 1 0.909 0.0613 0.797 1.000
24 17 2 0.818 0.0822 0.672 0.996
30 14 1 0.767 0.0916 0.607 0.969
36 11 0 0.767 0.0916 0.607 0.969
Group=HER2 0 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 77 0 1.000 0.0000 1.000 1.000
6 77 0 1.000 0.0000 1.000 1.000
12 73 6 0.922 0.0305 0.864 0.984
18 69 2 0.896 0.0348 0.830 0.967
24 65 4 0.844 0.0414 0.766 0.929
30 63 0 0.844 0.0414 0.766 0.929
36 59 0 0.844 0.0414 0.766 0.929
Group=HER2 low time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 39 0 1.000 0.0000 1.000 1.000
6 35 5 0.872 0.0535 0.773 0.983
12 33 2 0.821 0.0615 0.708 0.950
18 31 2 0.769 0.0675 0.648 0.914
24 30 0 0.769 0.0675 0.648 0.914
30 27 3 0.692 0.0739 0.562 0.853
36 27 0 0.692 0.0739 0.562 0.853
Luminal HER2 0, HER2 +1, HER2 +2/FISH negative
Group= Luminal HER2 0 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 77 0 1.000 0.0000 1.000 1.000
6 77 0 1.000 0.0000 1.000 1.000
12 73 6 0.922 0.0305 0.864 0.984
18 69 2 0.896 0.0348 0.830 0.967
24 65 4 0.844 0.0414 0.766 0.929
30 63 0 0.844 0.0414 0.766 0.929
36 59 0 0.844 0.0414 0.766 0.929
Group= Luminal HER2 +1 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 22 0 1.000 0.0000 1.000 1.000
6 21 1 0.955 0.0444 0.871 1.000
12 20 1 0.909 0.0613 0.797 1.000
18 19 1 0.864 0.0732 0.732 1.000
24 19 0 0.864 0.0732 0.732 1.000
30 18 1 0.818 0.0822 0.672 0.996
36 18 0 0.818 0.0822 0.672 0.996
Group= Luminal HER2 +2/FISH negative time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 17 0 1.000 0.000 1.000 1.000
6 14 4 0.765 0.103 0.587 0.995
12 13 1 0.706 0.111 0.519 0.959
18 12 1 0.647 0.116 0.455 0.919
24 11 0 0.647 0.116 0.455 0.919
30 9 2 0.529 0.121 0.338 0.829
36 9 0 0.529 0.121 0.338 0.829
Early stage luminal HER2 0, HER2 +1 and HER2 +2/FISH negative
Group = Luminal HER2 0 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 54 0 1.000 0.0000 1.000 1.000
6 54 0 1.000 0.0000 1.000 1.000
12 52 4 0.926 0.0356 0.859 0.998
18 49 1 0.907 0.0394 0.833 0.988
24 46 2 0.870 0.0458 0.785 0.965
30 45 0 0.870 0.0458 0.785 0.965
36 41 0 0.870 0.0458 0.785 0.965
Group= Luminal HER2 +1 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 16 0 1.000 0.0000 1.000 1
6 16 0 1.000 0.0000 1.000 1
12 15 1 0.938 0.0605 0.826 1
18 14 1 0.875 0.0827 0.727 1
24 14 0 0.875 0.0827 0.727 1
30 13 1 0.812 0.0976 0.642 1
36 13 0 0.812 0.0976 0.642 1
Group= Luminal HER2 +2/FISH negative time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 11 0 1.000 0.000 1.000 1.000
6 10 2 0.818 0.116 0.619 1.000
12 9 1 0.727 0.134 0.506 1.000
18 8 0 0.727 0.134 0.506 1.000
24 8 0 0.727 0.134 0.506 1.000
30 6 2 0.545 0.150 0.318 0.936
36 6 0 0.545 0.150 0.318 0.936
Locally advanced luminal HER2 0, HER2 +1 and HER2 +2/FISH negative
Group= Luminal HER2 0 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 23 0 1.000 0.0000 1.000 1.000
6 23 0 1.000 0.0000 1.000 1.000
12 21 2 0.913 0.0588 0.805 1.000
18 20 1 0.870 0.0702 0.742 1.000
24 19 2 0.783 0.0860 0.631 0.971
30 18 0 0.783 0.0860 0.631 0.971
36 18 0 0.783 0.0860 0.631 0.971
Group= Luminal HER2 +1 time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 6 0 1.000 0.000 1.000 1
6 5 1 0.833 0.152 0.583 1
12 5 0 0.833 0.152 0.583 1
18 5 0 0.833 0.152 0.583 1
24 5 0 0.833 0.152 0.583 1
30 5 0 0.833 0.152 0.583 1
36 5 0 0.833 0.152 0.583 1
Group= Luminal HER2 +2/FISH negative time n.risk n.event survival std.err lower 95% CI upper 95% CI
0 6 0 1.000 0.000 1.000 1
6 4 2 0.667 0.192 0.379 1
12 4 0 0.667 0.192 0.379 1
18 4 1 0.500 0.204 0.225 1
24 3 0 0.500 0.204 0.225 1
30 3 0 0.500 0.204 0.225 1
36 3 0 0.500 0.204 0.225 1






