Outcomes of locally advanced gastric and gastroesophageal adenocarcinoma cancers treated with neoadjuvant FLOT in a tertiary care hospital in Pakistan
Tasneem Dawood1, Yasmin Abdul Rashid1, Saqib Raza Khan1, Adnan Abdul Jabbar2, Muhammad Nauman Zahir2 and Munira Shabbir Moosajee1
1Department of Medical Oncology, Aga Khan University Hospital, Karachi 74800, Pakistan
2Department of Medical Oncology, Dr. Ziauddin Hospital, Karachi 74700, Pakistan
Abstract
Background and aim: Docetaxel, oxaliplatin, leucovorin and 5-fluorouracil (FLOT) may improve overall survival (OS) in patients with locally advanced gastric and gastroesophageal cancer. Our study aims to determine the pathological response in these patients with the FLOT chemotherapy in the Neoadjuvant setting. This is the first study conducted in our country.
Methods: We conducted a retrospective cross-sectional study from March 2018 to December 2020. After ethical review committee approval, all patients who fulfilled the inclusion criteria and received treatment at our tertiary care center were included in the study. SPSS version 22 was used for data analysis. Frequencies and percentages were calculated for categorical. Values were presented as mean ± standard deviation (SD) for continuous variables. The chi-square test was used to determine the difference between categorical variables. A p-value of ≤0.05 was considered the level of significance. Kaplan-Meier curves were used to calculate survival analysis.
Results: Out of 41, 35 patients with locally advanced resectable gastric or gastroesophageal adenocarcinoma were included in our study analysis. The entire cohort had a male predominance, with a mean age of 59. All patients received neoadjuvant FLOT. Pathological treatment response achieved was 77%, of which 66% had partial and 11% had complete response. There is a significant association of pathological response with age, gender, stage, grade, co-morbid and number of chemotherapy cycles received (p-value =<0.05). The OS was 80% with the mean OS was 2.6 years (31 months).
Conclusion: Our study shows comparable response rates to other studies conducted internationally. Our findings confirm that FLOT is an effective and well-tolerated perioperative regimen with reasonable response rates in the Pakistani population. A more extensive longitudinal study would ensure these preliminary results in the local patient population.
Keywords: gastric cancer, gastroesophageal cancer, pathological response, neoadjuvant, chemotherapy
Correspondence to: Saqib Raza Khan
Email: saqibrazakhan1994@gmail.com
Published: 21/05/2024
Received: 16/01/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastric and esophageal adenocarcinomas are some of the leading causes of cancer-related deaths worldwide [1]. Esophagogastric adenocarcinoma includes adenocarcinomas of the stomach (gastric cancer), the distal oesophagus and the gastroesophageal junction (GEJ) [2]. The incidence of gastric cancer in the past couple of years has been declining over time, while gastroesophageal cancer has been on a rising trend [3]. Gastric cancer, as per the literature, is the fifth most common cause of cancer around the globe and the third most common cause of cancer-related deaths, with 951,600 new cases globally recorded in 2012 [2]. In Pakistan, gastric cancers ranked seventh as per the GLOBOCAN 2020 data with a 5-year prevalence of 4.00 per 100,000 [4].
The increase in survival and decrease in mortality of gastric cancer can be attributed to a combination of early detection, better access to care and improved treatment options available [5]. Another important factor is a change in lifestyle and environmental exposures over succeeding generations. The increase in the incidence of gastroesophageal cancer can be attributed to the rise in the prevalence of gastroesophageal reflux disease, Barrett's oesophagus, abdominal obesity and reduced intake of fruits, vegetables and low-fibre diet, among many causes [6]. Symptoms include weight loss, dysphagia, dyspepsia, vomiting, early satiety, or iron deficiency anaemia. Surgical resection is by far the only curative option available for early-stage disease without suspected lymph nodes. Locally advanced and node-positive diseases should be treated via a multi-disciplinary approach [7]. Similarly, early-stage esophageal cancer can be treated with chemoradiation alone. Despite significant advances in the multimodal approach to locally advanced esophageal and esophageal junction cancer, the 5-year survival of patients treated with curative intent remains poor, in between 30% and 47% in the recent series [8].
Neoadjuvant treatment, either with chemotherapy or a combination of chemotherapy with radiation followed by surgery and adjuvant treatment, is now the current standard of care, rather than surgery alone, based on randomised clinical trials [9]. Neoadjuvant treatment aims to increase resection rates, including complete resection, decrease local and systemic recurrence and increase overall survival (OS) rates [9]. The survival benefit of perioperative treatment in gastric and GEJ cancer has also been demonstrated in clinical trials [10].
The need for a balance between a neoadjuvant treatment and dealing with acceptable toxicities remains a significant challenge for patients with resectable gastric and gastroesophageal cancers. We aim to investigate pathological response rates after neoadjuvant systemic therapy in our population. As per our knowledge, no similar study has been conducted in this part of the world. Our analysis is of utmost importance in providing valuable data regarding treatment with the neoadjuvant docetaxel, oxaliplatin, leucovorin and 5-fluorouracil (FLOT) regimen, which is easier to administer and offers better response rates than the conventional treatment options.
Methods
This retrospective study was conducted in the Department of Medical Oncology at a large tertiary care hospital in Karachi, Pakistan, from March 2018 to December 2020. It was a descriptive observational study. Forty-one patients with biopsy-proven locally advanced gastric or gastroesophageal adenocarcinoma who were being treated with curative intent were included, however, six patients were lost to follow-up after the initial presentation, and no complete data was available. A total of 35 patients were therefore included in our study analysis. The patients included in the study were between the age group of 18–75 years, with a mean age of 59.8 ± 9.9, with biopsy-proven locally advanced resectable gastric or gastroesophageal adenocarcinoma. The study did not include patients who had distant metastasis, peripheral neuropathy grade II, cardiac insufficiency, Eastern Cooperative Oncology Group (ECOG) 3-4, uncontrolled medical illness, acute infection, or history of other malignancies within the past 5 years.
Data collection and treatment
ERC approval was obtained, and all the patients who fulfilled the eligibility criteria were included in the study. All the patients were being treated per the standard of care and at the primary physician's discretion. No intervention was performed as part of this study. Patient demographic data was collected along with clinical outcomes. Baseline laboratories were checked before administration of chemotherapy, and if they were fine, chemotherapy was proceeded with. They received a preoperative systemic chemotherapy regimen with Docetaxel 50 mg/m2, Oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, all on day 1 and 5-FU 2,600 mg/m2 as a 24-hour infusion on a 2-weekly interval. The response was evaluated after completion of neoadjuvant treatment with a computerised tomography scan of the chest, abdomen and pelvis or positron emission tomography scan. Then, the pathological stage was determined via the histological outcome after surgery. Patients received adjuvant FLOT 4–6 months after surgery and were on close follow-up with history and physical examination every 3–6 months for 2 years and then 6–12 months after that, along with a CT scan every 6 months for 2 years and then annually. The patients were being followed up for a duration of 4 years.
Dose modification for toxicities
In the study cohort, eight (n = 8, 22.8%) patients needed dose adjustments in the regimen (Table 1). Out of which, two patients needed dose adjustment of the entire regimen secondary to grade III neutropenia, grade II neuropathy and grade III diarrhoea. Two patients needed dose adjustment in docetaxel infusion secondary to fatigue and grade II neuropathy. Two patients needed dose adjustment in 5-FU secondary to grade III diarrhoea and severe (Grade III-IV) oral mucositis. Two patients had to be switched to another regimen in the last cycle secondary to a decline in performance status and poor overall tolerance to the regimen. Furthermore, the majority (97%) of patients received at least three cycles of neoadjuvant FLOT chemotherapy, and 77% of patients received at least three cycles of FLOT in the adjuvant setting. Four patients received no adjuvant treatment. Four patients received another chemotherapy regimen including oral capecitabine or FOLFOX in the adjuvant setting.
Statistical analysis
SPSS version 22 was used for data analysis. Frequencies and percentages were calculated for categorical variables such as gender, ECOG status, co-morbid (DM/Hypertension/IHD/CVA), pre-treatment clinical stage (stage I–IV), dose modifications in neoadjuvant regimen, the reason for dose modification of neoadjuvant regimen (no response/suboptimal response, disease progression/ intolerance), surgery performed (yes/no), pathological stage (stage I–IV), pathological grade (grade-I–III) and pathological response (complete/partial). Values were presented as mean ± SD for continuous variables like age and number of chemotherapy cycles received. The chi-square test was used to determine the difference between categorical variables. A p-value of ≤0.05 was considered the level of significance. Kaplan-Meier curves were used to calculate survival analysis.
Results
A total of 35 patients with locally advanced resectable gastric or gastroesophageal adenocarcinoma were included in our study. The mean age was 59.875 ± 9.908 years. The descriptive statistic of age is presented in Table 1. Most patients (n = 33, 94.3%) were males and had an ECOG status of 1 in 33 (94.3%), as shown in Table 1. The pre-treatment clinical stage was stage 1 in 1 patient (2.9%), stage II in 10 (28.6%), stage III in 24 (68.5%). Histological subtypes include gastric adenocarcinoma (n = 30, 85%) and GEJ adenocarcinoma (n = 5, 15%). Among these, further histological sub-types consist of; well differentiated (n = 5, 14.3%), moderately differentiated (n = 12, 34.3%), poorly differentiated (n = 11, 31.4%), poorly differentiated with signet ring cell morphology (n = 6, 17.1%) and mucinous adenocarcinoma (n = 1, 2.9%). The median number of chemotherapy cycles received was 4 (IQR, 1–6). Baseline characteristics are shown in Table 1.
The surgery was performed on 33 (94.28%) patients (Table 1). In the study cohort, majority (51.42%) of the patients underwent total gastrectomy + D2 resection. Partial gastrectomy + D2 resection was performed in 22.8% of the patients. Two-staged esophagectomy and distal gastrectomy were performed in 14.28% and 2.8% of the patients, respectively. Surgery was not done for two patients as one patient was not medically fit for surgery because of a decline in performance status and the other patient developed metastatic disease on scans post neoadjuvant chemotherapy. Moreover, incomplete surgery was performed in one as the patient was found to have metastatic disease with peritoneal deposits during surgery. The pathological stage was stage I in 10 (30.30%) patients, stage II in 9 (27.27%) patients and stage III in 14 (42.42) patients, as shown in Table 2 The pathological grade was grade I in 7 (20%), grade II in 11 (31.4%) and grade III in 17 (48.6%). The pathological response achieved was 77% (n = 27), out of which complete pathological response (PCR) was observed in 4 (11.4%) and partial response was seen in 23 (66.7%), as shown in Table 2 (Figure 1a–d).
Table 1. Baseline characteristics of participants receiving FLOT regimen in the neoadjuvant setting for esophagogastric and gastric cancer.
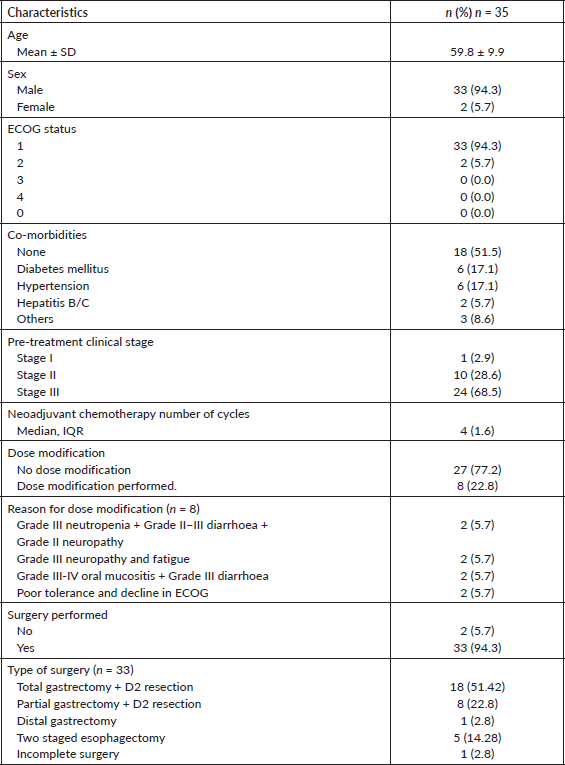
Table 2. Disease characteristics and response rates.
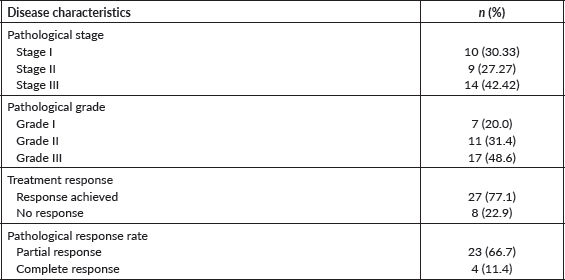
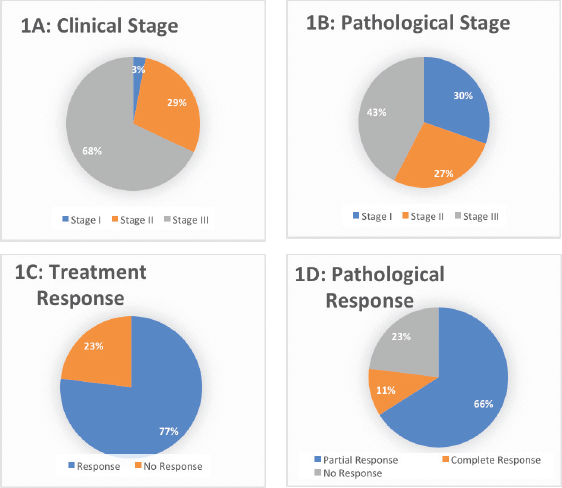
Figure 1. Clinical (1A) and pathological (1B) stage and the overall treatment response (1C-D) in the entire cohort.
In our study, there is a significant association of pathological response with age, gender, stage, grade, co-morbid and number of chemotherapy cycles received (Table 3).
In our study, the OS for 2 years was 80% with a mean OS of 2.6 years (31 months) (Figure 2). Among the cohort of 35 patients, 6 patients experienced mortality. One patient died within the first year following the initiation of FLOT therapy due to cardiopulmonary arrest. Another patient after the first year due to complicated pneumonia, and the remaining four patients at 2, 3 and 4 years, respectively, after initiation of the therapeutic intervention.
Table 3. Pathological response stratified based on patients and disease characteristics.
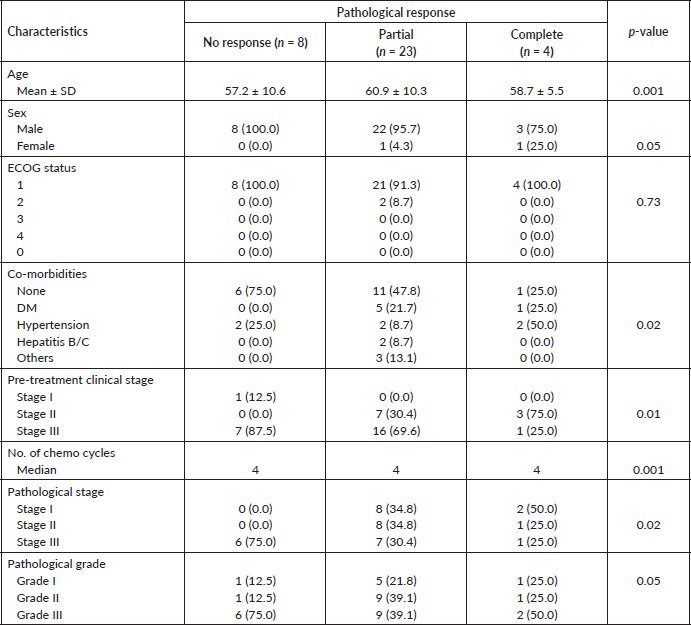
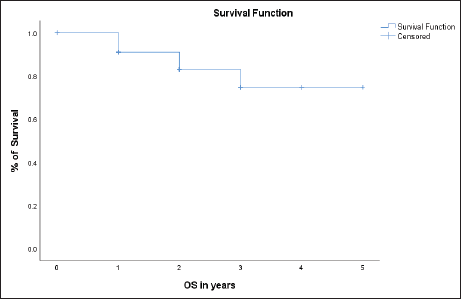
Figure 2. Overall survival (OS) in the entire population.
Discussion
The incidence of gastric cancer has been declining; however, it remains a major cause of cancer death, globally. Attempts to enhance treatment outcomes beyond those achieved through surgery alone have involved both adjuvant (postoperative) and neoadjuvant (preoperative) strategies. Over time, the beneficial effects of neoadjuvant chemotherapy on the survival of individuals with locally advanced gastric adenocarcinoma have become increasingly evident. Nevertheless, there remains a lack of consensus regarding the optimal approach. Determining the most effective chemotherapy regimen for neoadjuvant therapy remains elusive, with practice varying considerably. While the FLOT regimen has shown superior 5-year survival and disease-free survival (DFS) rates compared to previous treatments for gastric cancer and GEJ cancer [11–13], it remains unclear whether similar outcomes can be achieved in our patient population.
In our study, the overall pathological response rate after neoadjuvant FLOT in Gastric cancer (GC) and GEJ cancer was 77%. The CR was achieved in 11.4% of patients, and a partial response was seen in 66.7%. Our results are similar to most of the studies published in the literature [11–15]. Moreover, in the published literature, as compared to the Neo-FLOT study, PCR with the FLOT regimen reached 20%, and partial response reached 40% [15, 3].
The role of radiation therapy concurrent with chemotherapy in GC and GEJ cancer has been extensively investigated in multiple clinical trials. Perioperative chemoradiation is associated with improved DFS, OS and PCR compared with chemotherapy or surgery alone in early-stage cancer. Results from the multicenter phase 3 CROSS trial showed that perioperative chemoradiation with carboplatin and paclitaxel significantly improved DFS and OS with surgery alone in patients with resectable GEJ cancer. The R0 resection rate was higher in the perioperative chemoradiation arm compared to the surgery alone arm (92% versus 69%; p < 0.001). Median OS was 49 months in the chemoradiation arm versus 24 months in the surgery-alone arm [8].
The survival benefit of perioperative chemotherapy in GEJ cancer was first demonstrated in a landmark phase 3 MAGIC trial. This study, which compared perioperative epirubicin, cisplatin and fluorouracil (ECF) to surgery alone, established that perioperative ECF improves DFS and OS [16–18]. However, in the FLOT4 trial, PCR reached 15% and 16% in gastric and GEJ cancers, respectively. The FLOT4 study compared the FLOT regimen that is Docetaxel 50 mg/m2, Oxaliplatin 85 mg/m2, leucovorin 200 mg/m2 all on day 1 and 5-FU 2,600 mg/m2 as a 24-hour infusion four cycles preoperatively followed by surgery then four cycles postoperatively to ECX/ECF three cycles preoperatively followed by three cycles after surgery showed that the median progression-free survival was 30 months in the FLOT group versus 18 months in the ECF arm. The OS was also better in the FLOT arm, which is 50 months versus 35 months [17–19]. Our study also showed a significant survival benefit of neoadjuvant FLOT, with mean OS reaching 31 months. In our study, moreover, because of the small sample size as well as possible differences in the protoplasm/genetic variation, might be one of the reasons apart from treatment adherence and completion of treatment that leads to the relatively lower survival rate. A propensity score-matched retrospective study from China also suggested that the patients with neoadjuvant FLOT had improved OS compared with surgery first. The results of these studies indicated that the FLOT was beneficial to locally advanced gastric cancer (LAGC) in terms of pathological regression and survival [13, 15, 3, 16–19]. Hence, perioperative systemic treatment is now the standard of care for resectable gastric and gastroesophageal cancer.
In the era of immunotherapy and targeted therapy, progress has been made in personalised treatment for advanced gastric and esophagogastric cancers. The current revised 2010 histologic classification of gastric cancer by the World Health Organisation in 2019 did not consider the molecular profiling of gastric cancer [20]. The cancer genome atlas (TCGA) proposed a classification based on molecular profiling into four sub-groups, namely, Epstein bar virus tumours (9%), microsatellite instability (MSI) tumours (21%), gnomically stable tumours (20%) and chromosomal instability tumours (50%) [21]. Following this, the Asian Cancer Research Group conducted a study built on TCGA molecular classification and co-relate it with clinical outcomes and identified four distinct subtypes, including MSI-High, Microsatellite stability (MSS)/epithelial-mesenchymal transition, MSS/TP53 intact and MSS/TP53 loss [22]. These molecular mechanics have a significant role in identifying novel targeted treatments. Currently, Her-2, also known as ERBB2, and combined positive score/tumour proportion score have been identified as therapy targets in advanced-stage disease via Trastuzumab and Immunotherapy including Pembrolizumab, Nivolumab and Camrelizumab, respectively [23–27]. Nevertheless, exciting immunotherapy results in neoadjuvant settings from phase II data, which evaluates Nivolumab and Ipilimumab in dMMR gastric and GEJ cancer, have shown that 59% of patients had PCR [28]. A phase II study also explored spartalizumab in combination with FLOT in a GASPAR trial as a perioperative treatment for gastric and GEJ adenocarcinoma to improve treatment efficacy and overall outcomes [14]. Additional follow-up studies and randomised trials are required to prove this claim.
Although with certain limitations of a single institutional study and a smaller sample size, our study demonstrated that preoperative FLOT regimen chemotherapy responds well in patients with LAGC. The use of the FLOT regimen as neoadjuvant chemotherapy should be taken into consideration during the comprehensive treatment of patients with LAGC. Rigorous randomised studies are needed to determine the role of FLOT and optimal patient selection in the Pakistani population.
Conclusion
Our study underscores the favourable impact of neoadjuvant FLOT regimen chemotherapy on locally advanced gastric and esophagogastric cancer within our local population. Notably, we observed a substantial overall pathological response rate of 77%, with a partial response recorded in 66% of cases. These findings align cohesively with existing literature, reinforcing the efficacy of the FLOT regimen and its potential to yield promising outcomes. Furthermore, our results contribute significantly to the expanding body of evidence emphasising the survival advantages associated with perioperative systemic treatments, particularly FLOT, in resectable gastric and gastroesophageal cancer. We advocate that all eligible patients stand to benefit from this approach. As we transition into an era marked by molecular and targeted treatments, ongoing trials exploring immunotherapy in the neoadjuvant setting exhibit promising signs regarding pathological response. However, a more comprehensive dataset, particularly concerning OS benefits, is crucial before considering a shift in the standard of care.
List of abbreviations
CPS, Combined positive score; DFS, Disease-free survival; ECOG, Eastern Cooperative Oncology Group; FLOT, 5 FU, leucovorin, oxaliplatin and docetaxel; GEJ, Gastroesophageal junction; LAGC, Locally advanced gastric cancer; OS, Overall survival; PCR, Pathological complete response and PET-CT, Positron emission tomography.
Acknowledgments
The authors would like to acknowledge Dr. Nawazish Zehra, senior instructor in the Department of Medical Oncology at the Aga Khan University Hospital, Karachi, Pakistan, for providing kind help with paper editing and statistics.
Conflicts of interest
All the authors declare no competing interest.
Funding
The author(s) received no funding for the research and/or publication of this article.
Ethical approval
The Aga Khan University Hospital Ethical Review Committee (AKUH-ERC) approved our study.
Data availability
The data that has been used is confidential.
Author contributions
TD: Conceptualisation, writing, perform the experiment, contributed reagents, analysed and interpret the data.
YR: Conceptualisation. Perform the experiment.
SRK: Writing, analysed and interpret the data.
AJ, NZ, MM: Conceived and designed the experiments.
References
1. Samalin E and Ychou M (2016) Neoadjuvant therapy for gastroesophageal adenocarcinoma World J Clin Oncol 7(3) 284 https://doi.org/10.5306/wjco.v7.i3.284 PMID: 27298768 PMCID: 4896896
2. Bose K, Franck C, and Müller MN, et al (2017) Perioperative therapy of oesophagogastric adenocarcinoma: mainstay and future directions Gastroenterol Res Pract 2017 5651903 https://doi.org/10.1155/2017/5651903 PMID: 28785280 PMCID: 5530426
3. Schulz C, Kullmann F, and Kunzmann V, et al (2015) NeoFLOT: multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma – very good response predominantly in patients with intestinal type tumors Int J Cancer 137(3) 678–685 https://doi.org/10.1002/ijc.29403
4. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA: A Cancer J Clin 71(3) 209–249
5. Amiri M, Janssen F, and Kunst AE (2011) The decline in stomach cancer mortality: exploration of future trends in seven European countries Eur J Epidemiol 26 23–28 https://doi.org/10.1007/s10654-010-9522-9 PMCID: 3018592
6. Buas MF and Vaughan TL (2013) Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease Semin Radiat Oncol 23(1) 3–9 https://doi.org/10.1016/j.semradonc.2012.09.008 PMCID: 3535292
7. Homann N, Pauligk C, and Luley K, et al (2012) Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5‐fluorouracil, oxaliplatin and Docetaxel Int J Cancer 130(7) 1706–1713 https://doi.org/10.1002/ijc.26180
8. Donohoe CL and Reynolds JV (2017) Neoadjuvant treatment of locally advanced oesophagal and junctional cancer: the evidence-base, current key questions and clinical trials J Thorac Dis 9(Suppl 8) S697 https://doi.org/10.21037/jtd.2017.03.159 PMID: 28815065 PMCID: 5538972
9. Al-Batran SE, Homann N, and Schmalenberg H, et al (2017) Perioperative chemotherapy with Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial J Clin Oncol 35 4004 https://doi.org/10.1200/JCO.2017.35.15_suppl.4004
10. Cordin J, Lehmann K, and Schneider PM (2010) Clinical staging of adenocarcinoma of the esophagogastric junction Recent Results Cancer Res 182 73–83 https://doi.org/10.1007/978-3-540-70579-6_6 PMID: 20676872
11. Paszt A, Simonka Z, and Budai K, et al (2023) Impact of neoadjuvant FLOT treatment of advanced gastric and gastroesophageal junction cancer following surgical therapy Front Surg 10 1148984 https://doi.org/10.3389/fsurg.2023.1148984 PMID: 37077865 PMCID: 10106678
12. Sisic L, Crnovrsanin N, and Nienhueser H, et al (2023) Perioperative chemotherapy with 5-FU, leucovorin, oxaliplatin, and Docetaxel (FLOT) for esophagogastric adenocarcinoma: ten years real-life experience from a surgical perspective Langenbeck's Arch Surg 408(1) 81 https://doi.org/10.1007/s00423-023-02822-7
13. Wang K, Ren Y, and Ma Z, et al (2019) Docetaxel, oxaliplatin, leucovorin, and 5-fluorouracil (FLOT) as preoperative and postoperative chemotherapy compared with surgery followed by chemotherapy for patients with locally advanced gastric cancer: a propensity score-based analysis Cancer Manag Res 11 3009–3020 https://doi.org/10.2147/CMAR.S200883 PMID: 31114348 PMCID: 6489649
14. Dos Santos M, Lequesne J, and Leconte A, et al (2022) Perioperative treatment in resectable gastric cancer with spartalizumab in combination with fluorouracil, leucovorin, oxaliplatin and Docetaxel (FLOT): a phase II study (GASPAR) BMC Cancer 22(1) 537 https://doi.org/10.1186/s12885-022-09623-z PMID: 35549674 PMCID: 9097175
15. Al-Batran SE, Hofheinz RD, and Pauligk C, et al (2016) Histopathological regression after neoadjuvant Docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomized phase 2/3 trial Lancet Oncol 17(12) 1697–1708 https://doi.org/10.1016/S1470-2045(16)30531-9 PMID: 27776843
16. Adenis A, Samalin E, and Mazard T, et al (2020) Does the FLOT regimen a new standard of perioperative chemotherapy for localized gastric cancer? Bull Cancer 107(1) 54–60 https://doi.org/10.1016/j.bulcan.2019.12.005 PMID: 31980145
17. Moussa O, Bhogal RH, and Malietzis G, et al (2022) Effect of perioperative FLOT versus ECF/ECX on short-term outcomes after surgery for resectable oesophagogastric adenocarcinoma: propensity score-matched study BJS Open 6(1) zrac003 https://doi.org/10.1093/bjsopen/zrac003 PMID: 35195263 PMCID: 8864466
18. Chua YJ and Cunningham D (2007) The UK NCRI MAGIC trial of perioperative chemotherapy in resectable gastric cancer: implications for clinical practice Ann Surg Oncol 14 2687–2690 https://doi.org/10.1245/s10434-007-9423-7 PMID: 17653804
19. Reece‐Smith AM, Saha S, and Cunnell ML, et al (2012) MAGIC in practice: experience of perioperative ECF/X chemotherapy in gastroesophageal adenocarcinomas J Surg Oncol 106(6) 748–752 https://doi.org/10.1002/jso.23187
20. Bosman FT, Carneiro F, and Hruban RH, et al (2010) WHO Classification of Tumours of the Digestive System (Geneva: World Health Organization)
21. Bass AJ, Thorsson V, and Shmulevich I, et al (2014) Cancer genome atlas research network: comprehensive molecular characterization of gastric adenocarcinoma Nature 513 202–209 https://doi.org/10.1038/nature13480
22. Cristescu R, Lee J, and Nebozhyn M, et al (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes Nature Med 21(5) 449–456 https://doi.org/10.1038/nm.3850 PMID: 25894828
23. Xu RH, Luo H, and Lu J, et al (2021) ESCORT-1st: a randomized, double-blind, placebo-controlled, phase 3 trial of camrelizumab plus chemotherapy versus chemotherapy in patients with untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC) J Clin Oncol 39 4000 https://doi.org/10.1200/JCO.2021.39.15_suppl.4000
24. Ajani JA, Kato K, and Doki Y, et al (2018) CheckMate 648: a randomized phase 3 study of nivolumab plus ipilimumab or nivolumab combined with fluorouracil plus cisplatin versus fluorouracil plus cisplatin in patients with unresectable advanced, recurrent, or metastatic previously untreated esophageal squamous cell carcinoma J Clin Oncol 36 TPS193 https://doi.org/10.1200/JCO.2018.36.4_suppl.TPS193
25. Sun JM, Shen L, and Shah MA, et al (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomized, placebo-controlled, phase 3 study Lancet 398(10302) 759–771 https://doi.org/10.1016/S0140-6736(21)01234-4 PMID: 34454674
26. Janjigian YY, Kawazoe A, and Yanez PE, et al (2021) Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: initial findings of the global Phase 3 KEYNOTE-811 study J Clin Oncol 39 4013 https://doi.org/10.1200/JCO.2021.39.15_suppl.4013
27. Bang YJ, Van Cutsem E, and Feyereislova A, et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial Lancet 376(9742) 687–697 https://doi.org/10.1016/S0140-6736(10)61121-X PMID: 20728210
28. André T, Tougeron D, and Piessen G, et al (2023) Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability–high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study J Clin Oncol 41(2) 255 https://doi.org/10.1200/JCO.22.00686






