Variants in BRCA1/2 in a hospital-based cohort in Chile and national literature review
Fernanda J Martin1,a, Isabel M Saffie2,b, Mabel A Hurtado1,2, Diana Avila-Jaque3,c, Rodrigo A Lagos4,d, Carolina A Selman5, Jonathan Z Huserman6,e, Valentina A Castillo7,8 and Badir J Chahuán1,2,f
1Unidad Asesoramiento Genético Oncológico, Fundación Arturo López Pérez, Santiago 7500921, Chile
2Cirugía de mama, Fundación Arturo López Pérez, Santiago 7500921, Chile
3Sección de Genética, Hospital San Juan de Dios, Santiago 8350488, Chile
4Unidad estadística, Fundación Arturo López Pérez, Santiago 7500921, Chile
5Subdirección Unidades Diagnósticas, Fundación Arturo López Pérez, Santiago 7500921, Chile
6Departamento Genética, Hospital Base San José Osorno, Osorno 5311523, Chile
7Departamento Genética, Hospital Clínico Universidad de Chile, Santiago 8380453, Chile
8Departamento Genética, Hospital Dr. Sótero del Río, Santiago 8150000, Chile
ahttps://orcid.org/0000-0002-7167-8850
bhttps://orcid.org/0000-0002-4723-5750
chttps://orcid.org/0009-0002-7787-6847
dhttps://orcid.org/0000-0002-5806-6227
ehttps://orcid.org/0000-0002-9355-3282
fhttps://orcid.org/0000-0003-3133-6706
Abstract
Purpose: The aim was to assess the diagnostic yield of next generation sequencing (NGS) multi-gene panels for breast and ovarian cancer in a high-complexity cancer centre in Chile. Additionally, our goal was to broaden the genotypic spectrum of BRCA variants already identified in Chilean families.
Methods: Retrospective analysis was conducted on the genetic test results of 722 individuals from Fundación Arturo López Pérez’s genetic counselling unit between 2016 and 2021. A comprehensive literature review encompassing articles analysing the frequency of germinal pathogenic variants in BRCA1/2 within the Chilean population was undertaken.
Results: 23.5% of the panels had positive results, with 60% due to pathogenic variants in the BRCA1/2 genes. Seven previously unreported variants in BRCA1 from Chilean studies were identified.
One or more variants of uncertain significance were detected in 31% of the results, and 11.5% of the families in this cohort presented copy number variants (CNVs) in BRCA1/2.
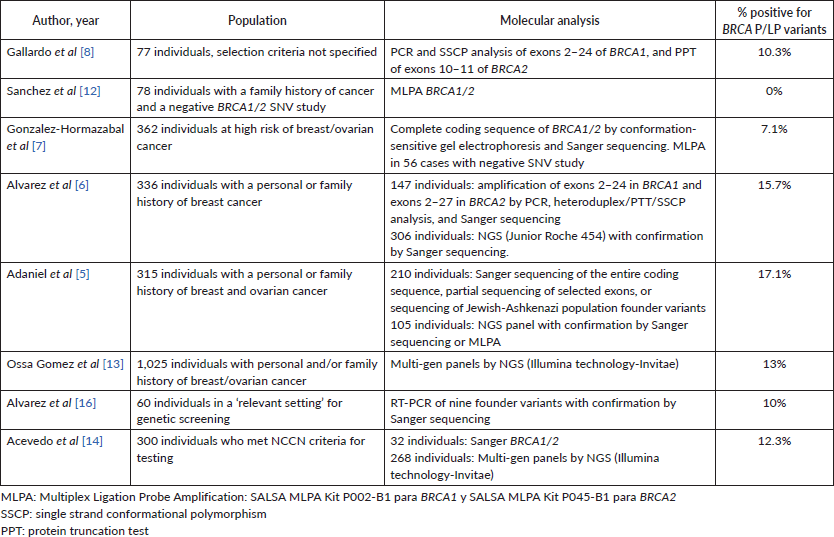
8 studies analysed the frequency of pathogenic variants in BRCA1/2 in the Chilean population between 2006 and 2023, with a frequency between 7.1% and 17.1%.
51 BRCA1 variants in 149 families have been reported in Chile and 38 BRCA2 variants in 132 families. Nine founder pathogenic variants identified by one study were present in 51.9% of the total Chilean families reported.
Conclusion: Our findings advocate for the integration of NGS multi-gene panel testing as a primary strategy within our population. This approach allows for the comprehensive assessment of single nucleotide variants and CNVs in BRCA1/2, alongside other high and moderately penetrant genes associated with breast and ovarian cancer.
Keywords: BRCA1, BRCA2, germline variants, Chile
Correspondence to: Fernanda Martin Merlez
Email: fernanda.martin@falp.org
Published: 21/03/2024
Received: 02/10/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Identifying individuals with hereditary susceptibility to cancer has been a crucial aspect of cancer care acknowledged by the American Society of Clinical Oncology since the 1990s [1]. However, access to genetic counselling and testing remains unequal across ethnic groups and is significantly linked to a country’s level of development [2, 3]. Presently in Chile, genetic testing coverage within health insurance schemes is diverse, particularly limited for sequencing studies. Consequently, its utilisation is inconsistent and heavily reliant on patients’ socioeconomic capabilities [4].
Prior investigations in Chile among individuals with a personal or familial history of breast and ovarian cancer have revealed varying frequencies of pathogenic (P) variants in BRCA1/2, ranging between 7% and 17% [5–14]. One study identified a notable frequency of nine variants identified as founders using haplotype analysis [6]. Our institution has an oncology genetic counselling unit that focuses on high-risk patients for hereditary cancer, maintaining a registry of germline studies since 2016. Through the analysis of this cohort, our aim was to ascertain the diagnostic yield of genetic tests for breast and ovarian cancer within a high-complexity cancer centre in Chile. Furthermore, our objective was to broaden the spectrum of genotypic variants previously identified in BRCA1/2 within Chilean families. This endeavour aims to enrich the genomic understanding of our population and foster the development of strategies that positively impact the health of our patients, their families, and the community.
Methods
We retrospectively analysed the results of the genetic tests of 722 individuals from the genetic counselling unit of Fundación Arturo López Pérez (FALP) between May 2016 and December 2021. Annually, Chile reports approximately 5,331 cases of breast cancer, according to GLOBOCAN, with our institution treating around 400–500 of these cases, constituting roughly 10% of the country’s total cases.
During this period, the unit primarily cared for patients with breast and ovarian cancer, either self-referred or via referrals from other specialists based on their personal and/or family cancer history. The genetic counselling structure involved three primary components: 1) Consultation with a nurse to gather background information and create a three-generational family tree, 2) Pre-test consultation with a genetic counsellor (breast surgeons trained in oncological genetic counselling), and 3) Post-test consultation to deliver results and discuss implications. The test indication was determined based on the evolving criteria of the National Comprehensive Cancer Network.
All reported studies were done by next-generation sequencing (NGS) with copy number variant (CNV) analysis performed by the laboratory Invitae® using Illumina technology. Genomic DNA obtained from peripheral blood or saliva samples was analysed, encompassing coding regions of genes, 20 bp of intronic flanking regions, and specific genomic regions of interest. Laboratory-reported analytical sensitivity and specificity was >99% for single nucleotide variants (SNVs), <15 bp insertions and deletions, as well as exon-level deletions and duplications.
The results were compared to the GRCh37 reference genome. Variant classification was performed using the laboratory’s SHERLOC system following the guidelines established by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology for genetic variant classification [15].
Bivariate statistical analysis was performed in a subgroup of female breast cancer patients to evaluate the relationship between the dichotomized response variable (positive or negative test result) and the variables: age, age <50 years, triple-negative breast cancer, ≥2 primary cancers, positive family history of cancer, known family variant, oestrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status. Results with Pathogenic/Likely pathogenic (P/LP) variants with or without variants of uncertain significance (VUS) were considered positive, and results with VUS-only or no variants were considered negative.
The Kruskal–Wallis test was utilised for continuous variables due to unmet normality and homoscedasticity assumptions. Fisher’s exact test examined relationships between categorical variables. A logistic regression model was performed for the response variable result of the test, and the odds ratio coefficients, the 95% confidence intervals, and the corresponding p-value were calculated for each predictor variable.
A literature search was carried out through Pubmed, SciELO, LILACS, and Google Scholar using combinations of the terms ‘CHILE’, ‘CHILEAN’, ‘BRCA’, ‘GERMINAL’, ‘GERMLINE’, in English and Spanish, with no date limit. The articles that analysed the frequency of germinal P variants in BRCA1/2 in the Chilean population were selected.
Results
During the analysed period, among 891 indicated tests, 722 were conducted primarily on women (91.1%) and patients diagnosed with cancer (75.2%). Of those diagnosed with cancer, 96% were female, with 92.2% having breast and/or ovarian cancer. Multigene panel studies for breast and ovarian cancer accounted for 56.2% of cases (7–36 genes), followed by multicancer panel requests in 22.9% (47–84 genes), and single variant or single gene studies (colon, thyroid, gastric, among others) in 20.7% of cases. Cascade studies of family variants constituted 17.5% of all genetic tests, with a positive result rate of 47.2% (Table 1).
Table 1. FALP study cohort.
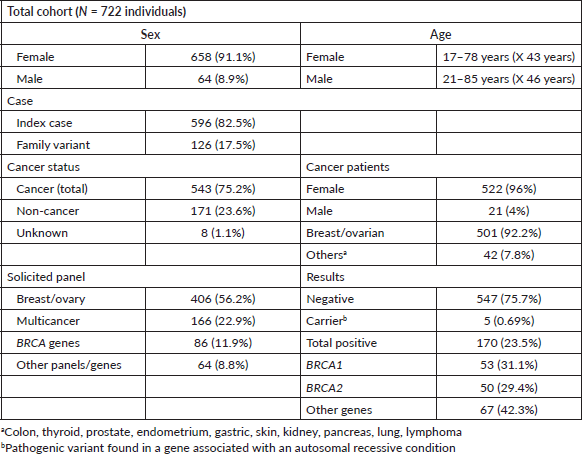
Overall, 23.5% (170 individuals) of panels yielded positive results, with 60% (103 individuals) due to P or LP variants in the BRCA1/2 genes (Figure 1). Additionally, 39.4% of cases (67 individuals) exhibited P/LP variants in other genes: APC, ATM, ATR, AXIN2, CDKN2A, CHEK2, FANCM, MSH2, MSH6, NBN, NF1, PALB2, PTEN, RAD50, RAD51c, RAD51D, RET, and TP53 (see Supplementary Table 1; all supplementary material can be found at https://figshare.com/articles/dataset/Supplementary_material_docx/24942498). Five P variants in genes with an autosomal recessive inheritance mode were regarded as carrier results.
Of the identified BRCA variants, 53 individuals from 31 families had P variants in BRCA1, while 50 individuals from 30 families had P variants in BRCA2. The vast majority (85.4%) with BRCA1/2 variants were women, among whom 63% had cancer. Specifically, 75.3% of BRCA-positive individuals with cancer had breast cancer, 13.8% had ovarian cancer, and 6.1% had both breast and ovarian cancer. Only 2 individuals (3%) presented cancer outside the BRCA1/2 spectrum, with testicular cancer. Both men were studied for the same familial variant (BRCA2: c.6275_6276delTT) identified in a female index case with breast cancer (Table 2).
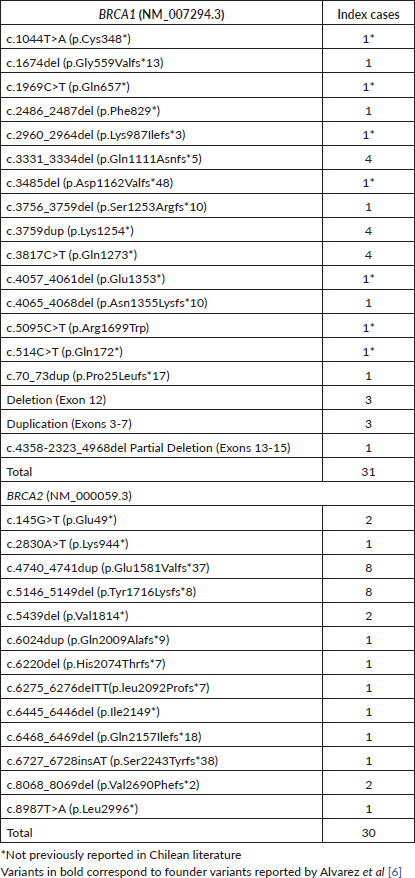
Moreover, seven new variants previously unreported in BRCA1 in Chilean studies were identified, while all variants in BRCA2 had been previously reported (Table 3). The majority of these variants, except for one missense variant (BRCA1: c.5095C>T) were truncating variants (nonsense or frameshift). 11.5% of the families in this cohort (7 families) presented CNVs. Three families presented a pathogenic deletion of exon 12; one presented a deletion of exons 13–15, and three showed a pathogenic duplication of exons 3–7 (Table 3).
Furthermore, 38.8% of positive results for BRCA1/2 variants were from family cascade tests. Relatives up to third degree were tested in 16 families, averaging 2.8 individuals per index case with a positivity rate of 46.6%.
One or more VUS were identified in 31% of the results (224); from these, in 84.8% (190 individuals), only one or more VUS were identified, and in 15.1% (34 individuals), a P/LP variant was identified in conjunction with a VUS. In total, 268 VUS were recognised in 63 genes. 5 VUS were identified in BRCA1 and 14 VUS in BRCA2 (Supplementary Table 2).
Bivariate statistical analysis highlighted a significant difference between a positive and negative test result for individuals with ≥2 primary cancers, a positive family history, and a previously known family variant. However, age, age <50 years, triple-negative breast cancer, ER, PR, and HER2 status showed no statistically significant differences (Supplementary Table 3). A logistic regression model did not yield any statistically significant associations (Supplementary Table 4). To compare the results (positive versus negative as defined above) of the multicancer panel with the breast and ovarian multigene panel, a two-sample test for equality of proportions with continuity correction was used, resulting in a p-value of 0.1026 and a chi-square of 2.6640. Hence, there was no statistically significant difference between them.
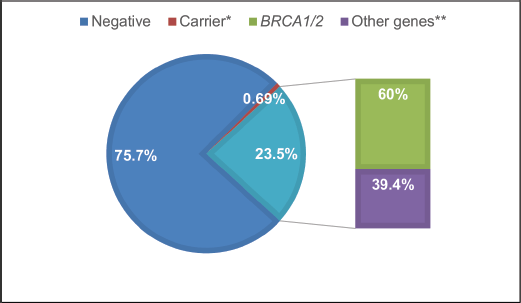
Figure 1. Test results FALP study cohort. *Carrier: PV found in a gene associated with an autosomal recessive condition. **Other genes: PV in cancer predisposition genes other than BRCA1/2.
Table 2. BRCA FALP cohort.
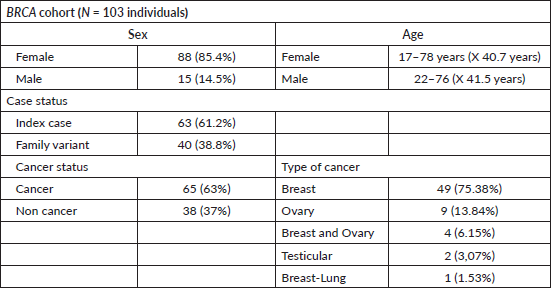
Table 3. P/LP BRCA variants FALP cohort.
In the literature review, from 2006 to 2023, eight studies analysed the frequency of LP/P variants in BRCA1/2 in the Chilean population with cohorts of variable size, inclusion criteria, and technology used for testing [5–8, 12–14, 16] (Table 4). One study [9] was excluded due to variant nomenclature discrepancies, and because the P variants reported were now classified as benign or VUS. The frequency of LP/P variants in BRCA1/2 across studies using Sanger or NGS (all except Sanchez et al [12]) was between 7.1% and 17.1%. The study conducted by Alvarez et al [16] exclusively focused on analysing the frequency of nine previously described variants in BRCA1/2 from a prior study. A partial overlap was observed between the individuals reported in the Ossa Gomez et al [13] cohort and those in our study due to all examinations from our institution being referred to the Invitae laboratory. This temporal overlap occurred between 2016 and 2019 in both studies, hence, individuals were only considered once for the analysis. To avoid redundancy and ensure an accurate representation, we limited our analysis to one individual per family for each variant in this cohort. Similarly, this approach was applied across all studies with adequate information to distinguish index patients from cascade testing, thereby preventing potential overrepresentation of variants. Another study [14] carried out between 2012 and 2021 also utilised Invitae’s services for tests. However, this study did not provide specific variant details for positive cases. Consequently, our analysis considered solely the overall positive test rate from this particular study.
In Chile, 51 BRCA1 variants have been reported among 149 families and 38 BRCA2 variants among 132 families. Most of these variants have been observed in other Latin American populations or globally. However, two BRCA1 variants (c.187_188insA, c.3710_3711del) and three BRCA2 variants (c.-39-?_425+ ?del, c.7397dup, c.8223_8224dup) remain unreported in databases such as ClinVar, human gene mutation database, databases registered in latingen.org (Mexico, Brazil, and Argentina), or in any other published articles to date. Notably, 62.9% (56 variants) were found in single individuals (Supplementary Table 5).
Nine founder variants identified by Alvarez et al [6] in 2017, highlighted in Table 3, were present in 50.8% of the results of this cohort. Considering the total previously reported Chilean families [5–8, 10, 12, 13] along with this cohort, 51.9% carried one of these variants. It’s important to note that 60 families were exclusively studied for these nine variants [6, 16] (Supplementary Table 5).
Table 4. Previous Chilean studies.
Discussion
Our FALP cohort demonstrated a diagnostic yield of 23.5%, akin to recent panel testing studies by Acevedo et al [14], surpassing earlier Chilean reports. This increase was probably due to patient selection criteria and multi-gene panel testing. LP/P variants in BRCA1/2 genes (around 14%) were consistent with recent studies in Chilean and Latin American populations [5, 6, 13, 17], surpassing international rates of 5%–10% in women from more developed countries [17–22]. A review encompassing 33 studies across 13 Central and South American countries in 2015, involving 4,835 individuals, showcased a broad spectrum in BRCA P variant prevalence. Results varied significantly, spanning from 1.2% to 47.8%. However, within unselected breast cancer cohorts, the prevalence ranged between 1.2% and 4.9%, aligning with rates observed in non-Hispanic populations [23]. In that review and the present one, the lack of homogeneity in the selection criteria, population size, and testing methodologies from the different studies could account for some differences in the prevalence of BRCA variants. The limited access to genetic counselling and studies in the region, as well as the lack of coverage of therapeutic, risk reduction, and early detection interventions that have shown cost-effectiveness in people with BRCA1/2 variants, all factors that discourage testing [5, 13, 16, 24], likely contributed to a selection bias towards patients more likely to test positive. Alternate hypotheses suggest that individuals of Latin American or African ancestry might exhibit a higher prevalence of P variants. Additionally, the region’s higher occurrence of triple-negative breast cancer and cases at younger ages might drive more targeted testing practices [24]. While Chile and other Latin American countries demonstrate an average inbreeding coefficient linked to consanguinity higher than that of West European countries and North America but considerably lower than African and Middle Eastern countries [25], the precise relationship between the frequency of BRCA carriers and this coefficient remains unclear. This complexity is further compounded by varying colonisation histories and genetic admixture across American countries, resulting in distinct genetic compositions and BRCA variant spectrums [10]. Estimates indicate that approximately 10.4% of BRCA P variants are shared between Hispanics in the United States and Latin American populations. Within Latin America and the Caribbean, about 8.2% of reported BRCA variants were identified in more than one country [23]. Despite 94.3% of the identified variants having prior records in other countries, most were not shared among families in this cohort. Intriguingly, some variants were exclusively reported outside the Americas, in regions such as China, Korea, Singapore, Australia, Palestine and several European countries (Supplementary Table 5).
There’s a scarcity of data on population-specific risks and inadequate systematic studies on the prevalence of genetic variants associated with breast cancer across Latin American populations. This reflects considerable heterogeneity among countries in the region, emphasising the necessity to consider these differences when implementing precision medicine initiatives. Nations exhibiting a high prevalence of BRCA P variants might derive considerable benefits from adopting more aggressive testing strategies. Conversely, employing recurrent variant panels for testing could offer a cost-effective solution to enhance genetic testing in certain countries, although this might not be universally applicable across all regions.
The statistical analysis undertaken in this study revealed a lack of a straightforward correlation between various patient selection criteria and a positive test result. Notably, there were contradictory results between the bivariate analysis and the regression model. These discrepancies might stem from the limitation of bivariate analysis, which doesn’t account for other potentially influential variables related to those of interest. Sample size could also have influenced these findings. It’s crucial to acknowledge the limitations inherent in these statistical techniques within this study context, alongside considering the NCCN selection criteria filter applied in this specific cohort. Consequently, these results cannot be extrapolated to the general population due to the selection bias in this dataset.
Up to this point, research studies in Chile have primarily cantered around hospitals and selected populations at a heightened risk of presenting genetic variants. This focus has led to a notable gender bias in cohorts of BRCA-mutated individuals in Chile, primarily among women with breast and ovarian cancer. The absence of population-based studies and limited research on cancers beyond breast and ovarian have contributed to this skew in the data.
Estimates of the carrier rate for BRCA1/2 variants in the general population, from various international studies [17, 24, 26–28], range between 0.1% and 0.38%. Considering Chile’s population of approximately 17,500,000 inhabitants (www.censo2017.cl/), this translates to an estimated count of carriers ranging between 17,500 and 66,500 individuals within the country. This highlights the potential presence of undiagnosed carriers in the population, indicating a need for more comprehensive, population-focused research to understand the prevalence of these variants across various demographics and cancer types beyond breast and ovarian.
The findings from Alvarez et al [6] initially suggested a high frequency (77.5%) of nine identified variants, which were determined as founders through short tandem repeat minimal haplotype analysis in a cohort of 453 individuals. However, subsequent studies [5], including the analysis conducted here, observed a lower frequency (51.9%) for these variants reported in Chilean individuals. This discrepancy underscores the need for a more comprehensive approach to genetic testing, especially concerning the identified founder variants.
While the use of panels focusing on these founder variants has been proposed as a cost-effective screening method in Chile [6, 16], it’s essential to recognise its limitations. Such a strategy may overlook the identification of other SNVs or CNVs in BRCA1/2, which, as per this study, are present in approximately half of the cases in Chile. Moreover, restricting testing to these founder variants would also limit the detection of variations in other genes like PALB2, TP53, ATM, and CHEK2, among others, which represent a significant proportion of cases in this and other studies [5, 13, 14] and have established guidelines for management, risk reduction, and early detection strategies [29]. Considering the declining costs of NGS and the demonstrated efficacy of multigene extended panels in the Latin American population [13], a shift towards NGS-based multigene testing is warranted. This approach provides a more comprehensive evaluation of various genes associated with hereditary cancer, especially in populations whose genetic landscape remains incompletely understood, such as in Chile. Alternatively, a staggered testing approach involving the initial screening with the RT-PCR protocol developed by Alvarez et al [16] followed by NGS testing in patients with a negative initial study, could be considered [16]. However, evaluating the cost-effectiveness of such a strategy would necessitate assessing the impact of delayed actionable molecular diagnoses in patients lacking the identified founder variants, alongside the expenses associated with double testing in a significant proportion of patients.
The utilisation of multigene panels raises concerns regarding the detection of VUS. These variants can pose challenges by increasing the workload for clinicians in interpreting test results, potentially leading to misinterpretation, and even causing a negative psychological impact on patients [30]. In populations outside of European or non-Hispanic white groups, such as in Latin America, the incidence of VUS tends to be higher [30]. However, this does not seem to significantly affect the positivity rate of the studies. Additionally, it’s reassuring to note that most of these variants eventually get reclassified as benign [13]. An optimistic aspect is the growing number of individuals undergoing testing, leading to the establishment of local databases that document variants identified within specific populations. This accumulation of data will play a crucial role in facilitating the interpretation of results over time.
The significant percentage of positive results for BRCA1/2 variants (38.8%) within families of this cohort highlights the effectiveness of cascade testing (Table 2). This testing approach has demonstrated its cost-effectiveness by enabling individuals without a history of cancer to recognise their heightened risk for hereditary conditions. It serves as a means to implement preventive measures aimed at reducing risk and facilitating early detection of cancer in those at an increased predisposition due to familial genetic factors [14, 31].
The implementation of genetic counselling for cancer patients with risk factors, as mandated by Chile’s National Cancer Law (www.bcn.cl), faces substantial challenges in practice. Recent studies in Chile [14] reveal that despite clear indications for germline testing, less than 20% of eligible patients undergo this counselling. Several obstacles contribute to this situation, including a shortage of healthcare professionals trained in genetic counselling, limited coverage for most molecular testing within the public health system, disparities in coverage across the private system, lack of public awareness, and insufficient medical referrals [13, 14, 4]. Overcoming these multifaceted challenges is crucial to improving access to genetic counselling and testing for at-risk individuals in Chile.
The inadequate coverage of genetic testing creates missed opportunities to implement prevention guidelines and risk-reducing interventions, particularly for high-risk individuals. Data from Chile’s national health survey reveals concerning statistics regarding mammography screenings, indicating that only 33% and 53% of women over 50 years underwent mammograms within the last year in the public and private healthcare systems, respectively [32]. This represents a significant challenge in prevention, especially for those high-risk women unaware of it.
Prophylactic oophorectomy can reduce the risk of ovarian cancer by 96% and breast cancer by 68% in BRCA-mutated women [33, 34]. Similarly, bilateral prophylactic mastectomy offers an 87% risk reduction for breast cancer in women without a history of cancer and reduces the risk of contralateral breast cancer by 97% in those with previous breast cancer [35, 36]. Surgery is generally performed subcutaneously, prioritising immediate reconstruction to restore the patient’s anatomy, thereby lessening the psychosocial impact of the procedure.
Moreover, knowing a patient’s BRCA mutational status provides opportunities for targeted pharmacological interventions. In metastatic HER2-negative breast cancer patients with BRCA mutations, Poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors have shown significant benefits, offering an effective therapeutic approach [37]. Additionally, in high-risk HER2 negative early breast cancer patients with BRCA mutations, PARP inhibitors have been explored as an adjuvant treatment option [38]. These advancements underscore the importance of genetic testing for targeted therapeutic strategies, optimising treatment outcomes in patients with BRCA mutations.
Indeed, this study’s focus on a specific population at high risk for hereditary cancer predisposition can be perceived as both a strength and a limitation. This targeted approach enhances the diagnostic yield for molecular studies within a resource-constrained setting. Including family and cascade testing aligns with the key objectives of cancer genetic counselling, adding value to the study’s comprehensiveness. However, this targeted population might introduce selection bias, limiting the generalisability of findings to broader populations. Furthermore, the absence of recorded data on the impact of molecular diagnoses on therapeutic and follow-up decisions represents a notable limitation. Future studies should aim to incorporate these elements to comprehensively assess the clinical implications of genetic testing in guiding treatment strategies and patient management.
Conclusion
The utilisation of multi-gene NGS panel testing within a specific cohort of individuals at elevated risk for carrying germline variants has demonstrated a high diagnostic yield for identifying P and LP variants in high and moderate-risk breast and ovarian cancer genes. The BRCA variant spectrum for our population continues to grow with broader testing strategies. The successful implementation of cascade testing has enabled identifying individuals harbouring BRCA mutations, even in the absence of prior cancer history, offering crucial opportunities for preventive measures and risk reduction interventions.
List of abbreviations
CNV, Copy number variant; ER, Oestrogen receptor; LP, Likely pathogenic; NGS, Next generation sequencing; P, Pathogenic; PARP, poly(adenosine diphosphate–ribose) polymerase; PR, Progesterone receptor; SNV, Single nucleotide variant; VUS, Variant of uncertain significance.
Acknowledgments
None.
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Funding
This study received no funding.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Healthcare Ethics Committee of FALP (N° 2022-031-SON-BRS-INT) and carried out in compliance with the current legislation in Chile on Scientific Research in humans of laws 20,120, and 19,628.
Data availability
The main data analysed during the current study has been included in tables and electronic supplementary material. Further datasets collected and analysed in the study are not publicly available due to confidentiality reasons but are available from the corresponding author upon reasonable request. All newly reported variants in this study were submitted to the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/).
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fernanda Martin, Diana Avila-Jaque, Isabel Saffie, Rodrigo Lagos, Mabel Hurtado, Valentina Castillo and Jonathan Huserman. The first draft of the manuscript was written by Fernanda Martin, and all authors commented on later versions. All authors read and approved the final manuscript.
References
1. Robson ME, Bradbury AR, and Arun B, et al (2015) American society of clinical oncology policy statement update: genetic and genomic testing for cancer susceptibility J Clin Oncol 33(31) 3660–3667 https://doi.org/10.1200/JCO.2015.63.0996 PMID: 26324357
2. Zhong A, Darren B, and Loiseau B, et al (2021) Ethical, social, and cultural issues related to clinical genetic testing and counseling in low- and middle-income countries: a systematic review Genet Med [Internet] 23 2270–2280 https://doi.org/10.1038/s41436-018-0090-9
3. Chapman-Davis E, Zhou ZN, and Fields JC, et al (2021) Racial and ethnic disparities in genetic testing at a hereditary breast and ovarian cancer center J Gen Intern Med 36(1) 35–42 https://doi.org/10.1007/s11606-020-06064-x PMCID: 7859010
4. Encina G, Castillo-Laborde C, and Lecaros JA, et al (2019) Rare diseases in Chile: challenges and recommendations in universal health coverage context Orphanet J Rare Dis 14(1) 1–8 https://doi.org/10.1186/s13023-019-1261-8
5. Adaniel C, Salinas F, and Donaire JM, et al (2019) Non-BRCA1/2 variants detected in a high-risk Chilean cohort with a history of breast and/or ovarian cancer J Global Oncol [Internet] 5 1–14 Date accessed: 30/07/23
6. Alvarez C, Tapia T, and Perez-Moreno E, et al (2017) BRCA1 and BRCA2 founder mutations account for 78% of germline carriers among hereditary breast cancer families in Chile Oncotarget [Internet] 8(43) 74233–74243 [www.impactjournals.com/oncotarget] https://doi.org/10.18632/oncotarget.18815 PMID: 29088781 PMCID: 5650336
7. Gonzalez-Hormazabal P, Gutierrez-Enriquez S, and Gaete D, et al (2011) Spectrum of BRCA1/2 point mutations and genomic rearrangements in high-risk breast/ovarian cancer Chilean families Breast Cancer Res Treat 126(3) 705–716 https://doi.org/10.1007/s10549-010-1170-y
8. Gallardo M, Silva A, and Rubio L, et al (2006) Incidence of BRCA1 and BRCA2 mutations in 54 Chilean families with breast/ovarian cancer, genotype-phenotype correlations Breast Cancer Res Treat 95(1) 81–87 https://doi.org/10.1007/s10549-005-9047-1
9. Jara L, Ampuero S, and Santibáñez E, et al (2006) BRCA1 and BRCA2 mutations in a South American population Cancer Genet Cytogenet 166(1) 36–45 https://doi.org/10.1016/j.cancergencyto.2005.08.019 PMID: 16616110
10. Jara L, Morales S, and de Mayo T, et al (2017) Mutations in BRCA1, BRCA2 and other breast and ovarian cancer susceptibility genes in Central and South American populations Biol Res 50 35 https://doi.org/10.1186/s40659-017-0139-2 PMID: 28985766 PMCID: 6389095
11. Ossa CA and Torres D (2016) Founder and recurrent mutations in BRCA1 and BRCA2 genes in Latin American Countries: state of the art and literature review Oncologist 21(7) 832–839 https://doi.org/10.1634/theoncologist.2015-0416 PMID: 27286788 PMCID: 4943386
12. Sanchez A, Faundez P, and Carvallo P (2011) Genomic rearrangements of the BRCA1 gene in Chilean breast cancer families: an MLPA analysis Breast Cancer Res Treat 128(3) 845–853 https://doi.org/10.1007/s10549-011-1382-9 PMID: 21327469
13. Ossa Gomez C, Achatz MI, and Hurtado M, et al (2022) Germline pathogenic variant prevalence among Latin American and US hispanic individuals undergoing testing for hereditary breast and ovarian cancer: a cross-sectional study JCO Global Oncol [Internet] 8 2200104 [https://ascopubs.org/go/authors/open-access]
14. Acevedo F, Walbaum B, and Camus M, et al (2023) Access disparities and underutilization of germline genetic testing in Chilean breast cancer patients Breast Cancer Res Treat [Internet] 199(2) 363–370 https://doi.org/10.1007/s10549-023-06909-z PMID: 36988750
15. Richards S, Aziz N, and Bale S, et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for molecular pathology Genet Med 17(5) 405–424 https://doi.org/10.1038/gim.2015.30 PMID: 25741868 PMCID: 4544753
16. Alvarez C, Ortega-Hernández V, and Cortez A, et al (2022) BRCA1 and BRCA2 screening of nine Chilean founder mutations through allelic-discrimination and real-time PCR in breast/ovarian cancer patients Mol Biol Rep 49(8) 7531–7539 https://doi.org/10.1007/s11033-022-07561-4 PMID: 35596815 PMCID: 9123627
17. Hu C, Hart SN, and Gnanaolivu R, et al (2021) A population-based study of genes previously implicated in breast cancer N Engl J Med 384(5) 440–451 https://doi.org/10.1056/NEJMoa2005936 PMID: 33471974 PMCID: 8127622
18. Couch FJ, Shimelis H, and Hu C, et al (2017) Associations between cancer predisposition testing panel genes and breast cancer JAMA Oncol 3(9) 1190–1196 https://doi.org/10.1001/jamaoncol.2017.0424 PMID: 28418444 PMCID: 5599323
19. Buys SS, Sandbach JF, and Gammon A, et al (2017) A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes Cancer 123(10) 1721–1730 https://doi.org/10.1002/cncr.30498 PMID: 28085182
20. Hauke J, Horvath J, and Groß E, et al (2018) Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: results of the German consortium for hereditary breast and ovarian cancer Cancer Med 7(4) 1349–1358 https://doi.org/10.1002/cam4.1376 PMID: 29522266 PMCID: 5911592
21. Kurian AW, Hughes E, and Handorf EA, et al (2017) Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women JCO Precis Oncol 1 1–12 https://doi.org/10.1200/PO.16.00066 PMID: 35172496
22. Tung N, Battelli C, and Allen B, et al (2015) Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel Cancer 121(1) 25–33 https://doi.org/10.1002/cncr.29010
23. Dutil J, Golubeva VA, and Pacheco-Torres AL, et al (2015) The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: a clinical perspective Breast Cancer Res Treat 154 441–453 https://doi.org/10.1007/s10549-015-3629-3 PMID: 26564481 PMCID: 4661195
24. Herzog JS, Chavarri-Guerra Y, and Castillo D, et al (2021) Genetic epidemiology of BRCA1- and BRCA2-associated cancer across Latin America NPJ Breast Cancer 7(1) 107 https://doi.org/10.1038/s41523-021-00317-6 PMID: 34413315 PMCID: 8377150
25. Saadat M and Saadat I (2010) Correlation between consanguineous marriages and age-standardized mortality rate due to breast cancer, an ecologic study Breast Cancer Res Treat 121 795–797 https://doi.org/10.1007/s10549-009-0711-8 PMID: 20052537
26. Risch HA, McLaughlin JR, and Cole DEC, et al (2006) Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada J Natl Cancer Inst 98(23) 1694–1706 https://doi.org/10.1093/jnci/djj465 PMID: 17148771
27. Dong H, Chandratre K, and Qin Y, et al (2021) Prevalence of BRCA1/BRCA2 pathogenic variation in Chinese Han population J Med Genet 58(8) 565–569 https://doi.org/10.1136/jmedgenet-2020-106970
28. Kraemer D, Azzarello-Burri S, and Steindl K, et al (2019) Prevalence of genetic susceptibility for breast and ovarian cancer in a non-cancer related study population: secondary germline findings from a Swiss single centre cohort Swiss Med Wkly 149 w20092 PMID: 31422574
29. Dwyer M (2023) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic [Internet] [https://www.nccn.org]
30. Kurian AW, Ward KC, and Abrahamse P, et al (2021) Time trends in receipt of germline genetic testing and results for women diagnosed with breast cancer or ovarian cancer, 2012-2019 J Clin Oncol 39 1631–1640 https://doi.org/10.1200/JCO.20.02785 PMID: 33560870 PMCID: 8274804
31. Tuffaha HW, Mitchell A, and Ward RL, et al (2018) Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers Genet Med 20(9) 985–994 https://doi.org/10.1038/gim.2017.231 PMID: 29300376
32. Madariaga B, Mondschein S, and Torres S (2022) Breast cancer trends in Chile: incidence and mortality rates (2007-2018) medRxiv https://doi.org/10.1101/2022.11.04.22281953
33. Oah N, Auff DK, and Aya J, et al (2002) Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation N Engl J Med [Internet] 346(21) 1609–1615 [www.nejm.org] https://doi.org/10.1056/NEJMoa020119
34. Ludwig KK, Neuner J, and Butler A, et al (2016) Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review Am J Surg 212(4) 660–669 https://doi.org/10.1016/j.amjsurg.2016.06.010 PMID: 27649974
35. Rebbeck TR, Friebel T, and Lynch HT, et al (2004) Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE study group J Clin Oncol 22(6) 1055–1062 https://doi.org/10.1200/JCO.2004.04.188 PMID: 14981104
36. Heemskerk-Gerritsen BAM, Rookus MA, and Aalfs CM, et al (2015) Improved overall survival after contralateral risk-reducing mastectomy in brca1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis Int J Cancer 136 668–677 https://doi.org/10.1002/ijc.29032
37. Robson M, Im SA, and Senkus E (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N Engl J Med 377(6) 523–533 https://doi.org/10.1056/NEJMoa1706450 PMID: 28578601
38. Tutt ANJ, Garber JE, and Kaufman B, et al (2021) Adjuvant olaparib for patients with BRCA1 – or BRCA2 -mutated breast cancer N Engl J Med 384(25) 2394–2405 https://doi.org/10.1056/NEJMoa2105215 PMID: 34081848 PMCID: 9126186
Supplementary material
All supplementary files can be found at https://figshare.com/articles/dataset/Supplementary_material_docx/24942498.






