Evaluation of side effects and compliance to chemotherapy in breast cancer patients at a Nigerian tertiary hospital
Sharif Adeniyi Folorunso1, Oyindamola Olajumoke Abiodun2, Abbas Adesina Abdus-Salam3 and Funmilola Olanike Wuraola4
1Department of Radiology, Obafemi Awolowo University Teaching Hospital, Ile Ife, Nigeria 220101
2Department of Pharmacology and Therapeutics, University of Ibadan, Ibadan, Nigeria 200005
3Department of Radiation Oncology, University College Hospital/University of Ibadan, Ibadan, Nigeria 200005
4Department of Surgery, Obafemi Awolowo University/Obafemi Awolowo University Teaching Hospitals Complex, Ile Ife, Nigeria 220101
Abstract
Background: Chemotherapy improves tumour control and survival, but it may be associated with side effects (SEs) which can impair treatment compliance and worsen outcomes. Assessment of patients in routine clinical practice, outside clinical trials, may provide the information on effects of chemotherapy on patients and its impacts on treatment compliance.
Aim: To assess the SE and compliance to chemotherapy in breast cancer patients.
Methodology: A prospective study involving 120 breast cancer patients receiving chemotherapy was carried out at the oncology clinics of the University College Hospital Ibadan. SEs reported were recorded and graded using Common Toxicity Criteria for Adverse Events version 5. Compliance was defined as a receipt of planned cycles of chemotherapy in the planned doses within the planned duration. The data collected were analysed using the Statistical Package for the Social Sciences software version 25.
Results: The patients were all females with a mean age of 51.2 ± 11.8 years. Patients reported between 2 and 13 SE with a median of 8 SE. Forty-two (35.0%) missed at least one course of chemotherapy while 78 (65%) were compliant. The reasons for non-compliance were deranged blood test 17 (14.2%), chemotherapy SE symptoms related 11 (9.1%), financial constraints 10 (8.3%), disease progression 2 (1.7%) and transportation-related 2 (1.7%).
Conclusion: Breast cancer patients encounter multiple SEs from chemotherapy which led to non-compliance with the treatment. Early identification and prompt treatment of these SEs will improve compliance with chemotherapy.
Keywords: breast cancer, chemotherapy, side effects, compliance
Correspondence to: Sharif Adeniyi Folorunso
Email: thenoble20@yahoo.com
Published: 21/04/2023
Received: 28/01/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Globally breast cancer incidence is rising with estimated 2,261,419 new cases of breast cancer and 684,996 deaths resulting from the disease worldwide in 2020 [1]. Breast cancer is the most common female malignancy in Nigeria, with age-standardised incidence rate of 54.4/100,000
[2]. In Nigeria, like other low-middle-income countries, the incidence rate is low compared to high-income countries but it records a high mortality rate. This is due to late presentation, inadequate treatment and other barriers [3]. The higher incidence in developed countries may be due to higher life expectancy, increasing obesity, reduction in fertility rates and rising age for first births [4]. Treatment options for breast cancer include local treatment like surgery and radiotherapy as well as systemic treatment such as chemotherapy, hormonal therapy and targeted therapy. Chemotherapy improves tumour control, increases the chance of cure and prolongs the lives of breast cancer patients [5]. About 80% of breast cancer cases in Nigeria will present at stage III/IV also approximately 40% of patients with breast cancer are triple negative [3]. These patient groups will require systemic therapy.
The choice of chemotherapy regimen for breast cancer has evolved over the years. Previously, cyclophosphamide, methotrexate and 5 fluorouracil (CMF) were the gold standards [6]. Clinical trials in the 1990s, however, showed that anthracycline-based chemotherapy (ABC) (usually a combination of epirubicin or adriamycin with cyclophosphamide) showed superior benefits [7, 8]. More recently, trials have shown taxane (notably docetaxel) or platinum can further improve survival, especially for triple-negative breast cancer [9]. Current treatment guidelines recommend ABC or taxane-based chemotherapy (TBC) as the preferred regimen for breast cancer [5, 10]. In Nigeria, ABC is the most frequently prescribed chemotherapy for breast cancer possibly because it is relatively cheaper as most patients pay out of pocket [11].
Chemotherapeutic agents exert their cytotoxic effects by disrupting the processes in the cell cycle [12]. Cancer cells are rapidly dividing which makes them susceptible to chemotherapy [13]. Rapidly dividing normal cells could also be affected in the process leading to toxicities [14]. Although these agents affect both normal and cancer cells, their therapeutic potential stems from their ability to cause greater damage in cancer cells as opposed to normal cells when received as scheduled [12]. Non-compliance to the schedule can reverse this trend leading to accelerated repopulation of the cancer cells and worsening the outcomes.
Gastrointestinal disorders, bone marrow suppression, neuropathies, hair loss, fatigue and skin disorders are side effects (SEs) often reported by cancer patients [5, 9]. Chemotherapy was described as the most unpleasant cancer treatment and the fear of its SEs can lead to late presentation for cancer treatment [15]. Prompt assessment and treatment of these SEs will improve treatment compliance and reduce hospital admission or treatment discontinuation [14, 16]. Knowledge about SEs often comes from clinical trials which might not really reflect the reality of the SEs in clinical practice as patients with high risk are often excluded from trials, and safety monitoring is more intensive than during routine care [17]. Pragmatic data collection will provide information about the real experience of cancer patients as regards chemotherapy SEs. Previous studies in Nigeria have tried to assess SEs of chemotherapy and their burden on breast cancer patients; however, it was carried out when CMF was the mainstay of treatment [18]. Studies conducted later included all other malignancies [19, 20]. The major limitation of these studies was the wide diversity in the chemotherapy regime used. Each cancer has its own clinical course and is managed with different chemotherapy which influences the SEs experienced by patients. This study aims to assess the SEs and treatment compliance to the chemotherapy regimen for breast cancer.
Methodology
This is a prospective study carried out at the Radiation and Surgical Outpatient Clinics of the University College Hospital, South West Nigeria. The study was conducted for 6 months between June 2021 to December 2021. The study population includes patients with histologically diagnosed breast cancer scheduled to receive chemotherapy at the clinics. Newly diagnosed breast cancer patients and patients with disease recurrence following the previous treatment, receiving either ABC or TBC for breast cancer were included. Patients who switched over from one chemotherapy regimen to another, patients with WHO performance status >2, patients receiving radiotherapy concurrently with chemotherapy and patients who were diagnosed with another type of cancer were excluded from the study. Subsequently, individuals who met these criteria were invited to take part in the study.
Sample size determination
For populations less than 10,000, this formula is used: 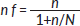
where n is the sample size for populations greater than 10,000 people which is calculated using the formula developed by Cochran as: n = Z2 p q/d2
Z = confidence interval is 1.96
p = prevalence (proportion in target population estimated to have the particular characteristic). Average prevalence of chemotherapy SEs is 0.54 [17].
q = 1−p = 1−0.54 = 0.46
D = precision value is 0.05
n is therefore 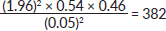
For populations less than 10,000, this formula is used: 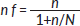
where n (sample size for the population greater than 10,000)
N = study population = 154 (approximate number of women with breast cancer seen in 6 months)
n f = 384 ÷ (1 + 384/154) = 384 ÷ (1 + 2.47) = 110.03
A sample size of 120 participants was selected.
Data collection procedure
One hundred and twenty consecutive breast cancer patients who met the inclusion criteria and gave consent were invited to participate. The study was explained to them and informed consent forms were signed by the patients. The primary data collection included a review of medical records together with patient interviews. Data retrieved from the medical record included patients’ sociodemographic, clinical and treatment information. Patients received chemotherapy in courses usually 3 weeks apart. They were followed up for the first three courses for SEs and compliance with the chemotherapy schedule. Data on chemotherapy SEs were completed before receiving the fourth course of chemotherapy.
Interviewer administered questionnaire was used with items developed from reviewing relevant literature [6, 17, 21]. A pilot test of the instruments was performed among 15 patients with breast cancer in the Radiation Oncology Department, University College Hospital Ibadan (UCH), Ibadan, to determine the reliability, acceptability and clarity of the questionnaire items. The test on 15 items of chemotherapy SEs grading yielded a Cronbach’s alpha of 0.80.
Chemotherapy SEs that are often reported in literature and easy to recognise by patients were included. The SEs reported were graded with the National Cancer Institute Common Toxicity Criteria for Adverse Events version 5. Grade 1 was defined as mild, grade 2 as moderate, grade 3 as severe and grade 4 was life-threatening [23]. Organ systems included were gastrointestinal, general disorders and administration site conditions, nervous system, skin and subcutaneous tissue disorders and urinary disorders. There was space for SEs reported but not included in the developed list.
Data management and analysis
Statistical Package for Social Sciences version 25 was used to analyse the collected data. The sociodemographic data, patients’ clinical characteristics, frequency and grading of the SEs were presented in frequency distribution tables. The number of SEs reported was compared between groups of chemotherapy regimes using Mann–Whitney test. The proportions were compared using the chi-square test. The significance level was set at less than 5%.
Ethical approval
Approval was obtained with registration number UI/EC/21/0289 from the joint institutional ethical review committee of the University of Ibadan and the University College Hospital, Ibadan.
Results
A total of 120 patients who met the selection criteria took part in this study. The sociodemographic, clinical and treatment information of study participants is presented in Table 1. The ages ranged from 22 to 78, with an average of 51.2 years. Sixty-seven (55.8%) patients were in the 41–59-year age category, 25 (20.8%) patients were younger and 28 (23.3%) patients were older. All the patients were female. Most of the cases seen were stage III, 76 (63.3%) followed by stage IV, 28 (23.3%) and stage II 14 (11.7%) (Table 1).
Most of the patients 104 (86.6%) received dual agents, 7(5.8%) were on triple regimen while 9 (7.5%) were on single agents. Of the two-chemotherapy regimen prescribed for breast cancer patients, anthracycline-based is the commonest 82 (68.3%) (Table 1). Epirubicin with cyclophosphamide was the most common ABC prescribed while paclitaxel with platinum was the most common TBC administered (Table 1).
Patients reported between 2 and 13 SEs in the course of their chemotherapy with a median of 8 SEs. A total of 913 SEs was reported by the 120 patients, of which 610 (66.8%) were mild, 292 (32.0%) were moderate and 7 (0.8%) were severe. None of the patients reported life-threatening SEs (Figure 1). The following SEs were most commonly reported: fatigue 113 (94.2%), alopecia 113 (94.2%), loss of appetite 99 (82.5%), nausea 93 (77.5%), nail discolouration 93 (77.5%), skin hyperpigmentation 75 (62.5%) and headache 60 (54.5%), (Table 2). Very few patients had severe SE such as diarrhoea 2 (1.6%), loss of appetite 2 (1.6%), nausea 1 (0.8%), malaise 1 (0.8%) and vomiting 1 (0.8%) (Table 2).
Table 1. Study participants’ sociodemographic, clinical and treatment information.
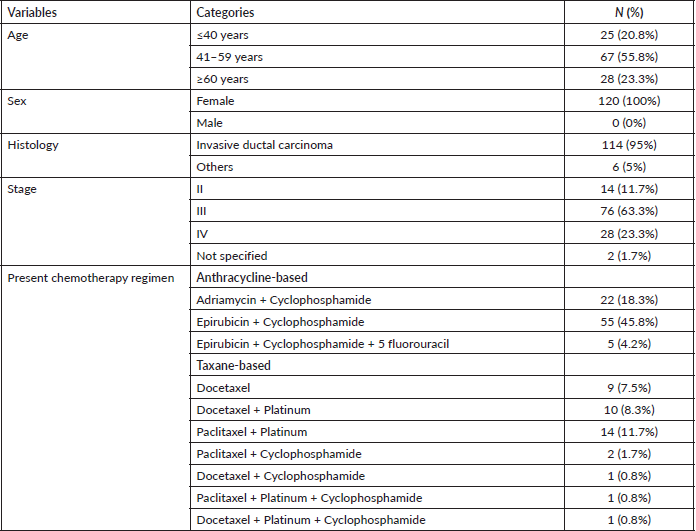
Table 2. Frequency and severity of reported SEs.
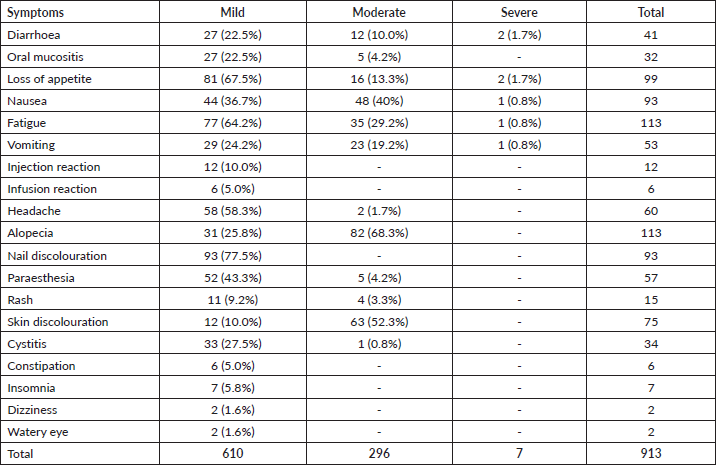
The frequency of SEs in patients that received ABC and TBC was analysed. Patients on ABC reported significantly higher diarrhoea (p = 0.039), vomiting (p = 0.020), headache (p = 0.006), nail discolouration (p = 0.010) and skin hyperpigmentation (p = 0.020) than the patients on TBC (Table 3). The median SEs reported by each patient were also significantly higher in patients on ABC than in patients on TBC, p = 0.044 (Table 3).
Close to two-thirds, 78 (65%) received the chemotherapy as planned (compliance), while 42 (35.0%) had disruption or delay in scheduled chemotherapy (non-compliance). The reason for non-compliance was deranged full blood counts 17 (14.2%), chemotherapy SE symptoms related 11 (9.1%), financial constraints 10 (8.3%), disease progression 2 (1.7%) and transportation-related 2 (1.7%) (Figure 1).
Table 3. Comparison of frequency of SEs between patients taking ABC and TBC.
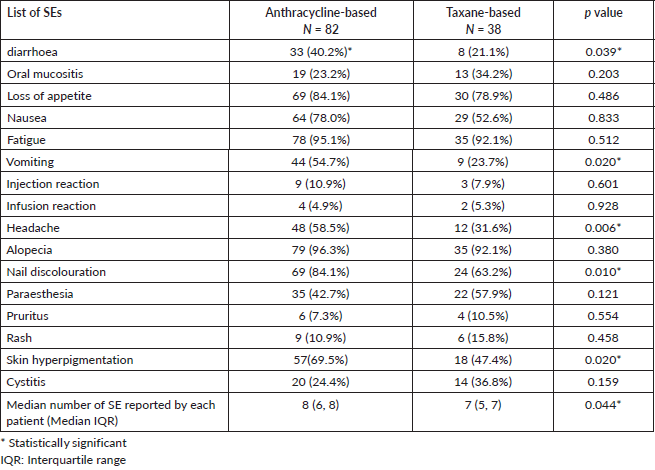
Figure 1. Reasons for non-compliance to chemotherapy.
Discussion
A total of 120 breast cancer patients were recruited to participate in this study. The average age was 50.45 years, with the 40–59 age group in the majority. This study showed that breast cancer occurs primarily in the young and middle-aged groups in this environment. This is similar to the findings of other studies in Nigeria which showed that breast cancer occurs at a younger age compared to Caucasians [4, 24–26]. People in this age group are at the productive age.
All the patients recruited in this study were female. This differs from the findings of a previous study in Lagos that reported 98% female and 2% male. The difference in the findings may be due to differences in the inclusion and exclusion criteria. While the study in Lagos recruited all breast cancer patients in the hospital, this study reviewed patients on chemotherapy and excluded patients on radiotherapy.
Most of the cases seen either have stage III (63.3%) or stage IV, 28 (23.3%). These stages connote advanced disease. Previous studies have demonstrated that cancer patients in Nigeria, often present with advanced diseases [25]. This may be due to aggressive tumour biology in blacks when compared with white [4]. Other possibilities for the late presentation as reported by a previous study are lack of awareness of cancer symptoms, seeking alternative care, fear of diagnosis and treatment and the challenges of distance to available centres of treatment [20].
In this study, the majority of the patients (87%) received combination chemotherapy. The recommended guideline for the treatment of breast cancer is generally the use of two or more agents simultaneously or consecutively. Combination therapy is known to have a higher objective response rate and a longer time to progress than a single agent [27, 28]. The most common chemotherapy regimen used was anthracycline-based. Anthracycline-containing regimen is one of the preferred chemotherapy regimens for breast cancer recommended by treatment guidelines [10]. It is also relatively cheaper than other preferred regimes which further favours its prescription [11]. This is particularly important as most patients in the country pay at their own expense.
Each of the patients reported SEs ranging between 2 and 13, most of which were mild. This may be because the majority of the patients were on multiple agents. As a rule of combination therapy, the selected drug should have non-overlapping toxicity [29]. This allows toxicity to be distributed across multiple organ systems and avoids significant toxicity in individual organs [29]. Patients on ABC reported more SEs when compared with taxane-based (8 versus 7, p = 0.044). This is in agreement with the findings of Nyrop et al [16] (7 versus 5, p = 0.01).
The most commonly reported SEs by participants in this study was fatigue. This is in keeping with the findings of previous studies [16]. A survey of 4,600 cancer patients receiving chemotherapy showed the most frequent SEs was fatigue [30]. Another study revealed that the commonest SE experienced by breast cancer patients receiving chemotherapy in Lagos was nausea [18]. The difference in the findings may be due to the fact that the latter study used CMF-based chemotherapy which is hardly being used again in the treatment of breast cancer.
This study found that close to two-thirds of the patients had good compliance with chemotherapy. This is in keeping with the findings of a previous study in Enugu [31]. Higher compliance was demonstrated by previous studies conducted outside the country [16, 32]. The difference may be due to better health facilities in those countries that could manage chemotherapy SEs better and promptly. Also, out-of-pocket treatment financing of treatment is expected to affect compliance in Nigeria. The most common reasons for non-compliance in this study were chemotherapy SE symptoms related to deranged blood profile and financial constraints. More aggressive pre- and post-treatment medications can reduce the impacts of chemotherapy SEs. Prophylaxis erythropoietin or filgrastim can help reduce the frequency of deranged blood count profile and improve compliance [33] but this will come at an additional cost, especially if the patient will have to pay. Financial constraints are a major barrier to cancer treatment in Nigeria [20]. Health insurance should be expanded to cover all cancer patients in Nigeria.
Limitation
Participants were recruited from a single institution which may not be a perfect representation of the population of breast cancer in Nigeria. A multicentre longitudinal study would have been more appropriate. Though all the patients received pre- and post-medications based on the institutional protocols, this study did not fully explore the pre- and post-treatment medications received by the patients. Nonetheless, our findings have provided insight into the experience of cancer patients as regards chemotherapy SE in routine clinical practice. These have also laid the foundation for future studies and interventions aim at improving treatment compliance and reducing the burden of chemotherapy.
Conclusion
In this study, it was observed that an anthracycline-based regimen was the most commonly prescribed chemotherapy. Breast cancer patients on chemotherapy encountered multiple SEs from the chemotherapy. Close to two-thirds of patients were compliant with the chemotherapy as planned. The commonest reasons for non-compliance in this study were symptoms related to deranged blood profile and financial constraints.
Acknowledgments
We acknowledge the members of staff at the Radiation Oncology Department UCH for their support towards the success of this work.
List of abbreviations
ABC: Anthracycline-based chemotherapy; CMF: Cyclophosphamide, methotrexate and 5 fluorouracil; SE: Side effects; TBC: Taxane-based chemotherapy; UCH: University College Hospital Ibadan.
Ethics approval and consent to participate
Approval for this study was sought from the joint ethical review committee of the University of Ibadan/University College Hospital, Ibadan.
Consent for publication
Consent was taken from the patients with aid of an informed consent form.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
None.
Funding
None.
Author contributions
SAF: Conceptualised the topic and designed the study methodology. He contributed to the data acquisition, data analysis, interpretation of data and wrote the final draft of this work.
OOA: Contributed to the conception, design, data acquisition, data analysis, interpretation of data and the draft of this work.
AAS: Contributed to the conception, design, data acquisition, data analysis, interpretation of data and the draft of this work.
FOW: Contributed to the conception, design, data acquisition, data analysis, interpretation of data and the draft of this work.
They have approved the submitted version and the final draft of the manuscript and take responsibility for this paper.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA: a cancer J Clin [Internet] https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.3322/caac.21660 Date accessed: 07/02/21
2. Jedy-Agba E, Curado MP, and Ogunbiyi O, et al (2012) Cancer incidence in Nigeria: a report from population-based cancer registries Cancer Epidemiol 36(5) e271–e278 https://doi.org/10.1016/j.canep.2012.04.007 PMID: 22621842 PMCID: 3438369
3. Olasehinde O, Alatise O, and Omisore A, et al (2021) Contemporary management of breast cancer in Nigeria: insights from an institutional database Int J Cancer 148(12) 2906–2914 https://doi.org/10.1002/ijc.33484 PMID: 33506499 PMCID: 8394611
4. Abdulrahman GO and Rahman GA (2012) Epidemiology of breast cancer in Europe and Africa J Epidemiol 2012 1–5
5. Korde LA, Somerfield MR, and Carey LA, et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline JCO 28 JCO.20.03399
6. Bayat Mokhtari N, Salek R, and Homaee Shandiz F, et al (2017) Adjuvant chemotherapy of early stage breast cancer in community-based cancer treatment fields: cmf compared with anthracycline/taxane-based regimens Middle East J Cancer 8(2) 83–91
7. Moo TA, Sanford R, and Dang C, et al (2018) Overview of breast cancer therapy Pet Clin 13(3) 339–354 https://doi.org/10.1016/j.cpet.2018.02.006 PMID: 30100074 PMCID: 6092031
8. Dange VN, Shid SJ, and Magdum CS, et al (2017) A review on breast cancer: an overview Asian J Pharm Res 7(1) 49 https://doi.org/10.5958/2231-5691.2017.00008.9
9. Gradishar WJ, Anderson BO, and Balassanian R, et al (2017) NCCN guidelines insights: breast cancer, version 1 J Natl Compr Cancer Netw 15(4) 433–451 https://doi.org/10.6004/jnccn.2017.0044
10. Gradishar WJ, Anderson BO, and Abraham J, et al (2020) Breast cancer, version 3.2020, nccn clinical practice guidelines in oncology J Nat Compr Cancer Netw 18(4) 452–478 https://doi.org/10.6004/jnccn.2020.0016
11. Olatunji T, Sowunmi A, and Ketiku K, et al (2019) Sociodemographic correlates and management of breast cancer in radiotherapy department, lagos university teaching hospital: a 10-year review J Clin Sci 16(4) 111 https://doi.org/10.4103/jcls.jcls_82_18
12. Dickens E and Ahmed S (2018) Principles of cancer treatment by chemotherapy Surgery (Oxford) 36(3) 134–138 https://doi.org/10.1016/j.mpsur.2017.12.002
13. Sun J, Wei Q, and Zhou Y, et al (2017) A systematic analysis of FDA-approved anticancer drugs BMC Syst Biol 11(S5) 87 https://doi.org/10.1186/s12918-017-0464-7 PMID: 28984210 PMCID: 5629554
14. Makin G (2018) Principles of chemotherapy Paediatr Child Health 28(4) 183–188 https://doi.org/10.1016/j.paed.2018.02.002
15. Clegg-Lamptey JNA, Dakubo JCB, and Attobra YN (2009) Psychosocial aspects of breast cancer treatement in Accra, Ghana East Afr Medl J 86(7) 348–353
16. Nyrop KA, Deal AM, and Shachar SS, et al (2019) Patient‐reported toxicities during chemotherapy regimens in current clinical practice for early breast cancer Oncol 24(6) 762–771 https://doi.org/10.1634/theoncologist.2018-0590
17. Pearce A, Haas M, and Viney R, et al (2017) Incidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort study. Ganti AK, editor PLos One 12(10) e0184360 https://doi.org/10.1371/journal.pone.0184360 PMCID: 5634543
18. Ketiku KK and Ajekigbe AT (1990) Chemotherapy of breast cancer in Nigerians: side-effects and quality of life Clin Oncol 2(3) 153–155 https://doi.org/10.1016/S0936-6555(05)80149-9
19. Adenipekun A, Elumelu-Kupoluyi T, and Omoyeni N, et al (2012) Knowledge and experience of cancer patients receiving chemotherapy in a teaching hospital in Nigeria Internet J Pain Symptom Control Palliative Care 9(1) 1–5
20. Oladeji AA, Atalabi OM, and Jimoh MA, et al (2017) Delay in presentation of cancer patients for diagnosis and management: an institutional report Internet J Oncol [Internet] 13(1) http://ispub.com/IJO/13/1/44745 Date accessed: 16/03/21
21. Lorusso D, Bria E, and Costantini A, et al (2017) Patients’ perception of chemotherapy side effects: expectations, doctor–patient communication and impact on quality of life – an Italian survey Eur J Canc Care 26(2) e12618 https://doi.org/10.1111/ecc.12618
22. Youlden DR, Cramb SM, and Yip CH, et al (2014) Incidence and mortality of female breast cancer in the Asia-Pacific region Cancer Biol Med 11(2) 101–105 PMID: 25009752 PMCID: 4069805
23. Common Terminology Criteria for Adverse Events (CTCAE) (2017) (US Department of Health and Human Services) p 155
24. Ntekim A, Nufu FT, and Campbell OB (2009) Breast cancer in young women in Ibadan, Nigeria Afr Health Sci 9(4) 242–246 PMID: 21503175 PMCID: 3074386
25. Vanderpuye V, Grover S, and Hammad N, et al (2017) An update on the management of breast cancer in Africa Infect Agent Cancer [Internet] 12 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5307840/
26. Fregene A and Newman LA (2005) Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer 103(8) 1540–1550 https://doi.org/10.1002/cncr.20978 PMID: 15768434
27. Gradishar WJ, Anderson BO, and Balassanian R, et al (2016) Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology J Natl Compr Cancer Netw 14(3) 324–354 https://doi.org/10.6004/jnccn.2016.0037
28. Hansen EK and Roach M eds (2010) Handbook of Evidence-Based Radiation Oncology 2nd edn (New York: Springer) 786 p
29. Perry MC, Doll DC, and Freter CE eds (2012) Chemotherapy Source Book 5th edn (Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins) 833 p
30. Feyer P, Kleeberg UR, and Steingräber M, et al (2008) Frequency of side effects in outpatient cancer care and their influence on patient satisfaction – a prospective survey using the PASQOC® questionnaire Supportive Care Cancer 16(6) 567–575 https://doi.org/10.1007/s00520-008-0422-4
31. Ingwu JA, Idoko C, Israel CE, Maduakolam I, Madu O. Factors influencing non–adherence to chemotherapy: Perspective of Nigerian breast cancer survivors. Nurs Pract Today [Internet]. 2019 Jan 29 [cited 2022 Aug 3]; Available from: https://publish.kne-publishing.com/index.php/NPT/article/view/392
32. Sabiyanto V, Widiasih R, and Solehati T (2019) Breast cancer patients’ compliance on chemotherapy: a descriptive study J Maternity Care Reprod Health 2 208–222 https://doi.org/10.36780/jmcrh.v2i3.101
33. Chambers P, Jani Y, and Wei L, et al (2019) Patient factors and their impact on neutropenic events: a systematic review and meta-analysis Support Care Cancer 27(7) 2413–2424 https://doi.org/10.1007/s00520-019-04773-6 PMID: 30993453 PMCID: 6541585






