Cost analysis of total neoadjuvant therapy with 5 × 5 Gy radiation therapy versus conventional chemoradiotherapy for locally advanced rectal cancer among Peruvians
José Fernando Robles Díaz1,2 and Henry Olivera Changra1,3
1Regional Institute of Neoplastic Diseases of the Center, Concepción, Junín 12126, Peru
2Los Andes Peruvian University, Huancayo, Junín 12002, Peru
3Continental University, Huancayo, Junín 12000, Peru
Abstract
Background and Objectives: Conventional long-course radiotherapy (LCRT) and a new paradigm of short-course radiotherapy with total neoadjuvant therapy (SCRT-TNT) are used in locally advanced rectal cancer (RC). There are few economic assessment reports available on TNT that focus on cost analysis in a country with limited funding for healthcare systems. The objective of this study was to perform a cost analysis comparing SCRT-TNT versus LCRT.
Materials and Methods: In 2020–2021, a prospective registry was created to document RC patients who received neoadjuvant therapy and the costs of cancer treatments, transportation and the time patients and family members spent in the hospital. This registry outlined the direct and indirect costs of LCRT versus SCRT-TNT.
Results: LCRT and SCRT-TNT regimens have direct costs that range from S/.5,993.30 to S/.27,928.36 and from S/.3,409.81 to S/.18,159.42, respectively. FOLFOX regimens are the most expensive. Administering radiotherapy in 28 3D sessions and 5 sessions of intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT) sessions costs S/.2,603.88, S/.1,277.19 and S/.1,027.77, respectively. The indirect cost of FOLFOX regimens is twice that of the similar modality that combines irradiation and Oxaliplatin IV and Capecitabine VO (CAPOX). SCRT-TNT regimens with CAPOX reduce costs by at least 50%, while SCRT-TNT regimens with FOLFOX reduce costs by 32%.
Conclusion: Despite using IMRT/VMAT, SCRT-TNT is a less expensive approach for patients with RC when compared to LCRT. The costs to patients using SCRT-TNT are much lower, but it is also a better option because it saves hospital resources.
Keywords: costs and cost analysis, radiation dose hypofractionation, neoadjuvant therapy, rectal neoplasms, chemoradiotherapy, short-course radiation therapy
Correspondence to: José Fernando Robles Díaz
Email: bayern014@hotmail.com
Published: 07/06/2022
Received: 05/01/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
According to GLOBOCAN 2020, colorectal cancer is the third most common type of cancer globally and the fourth most common in Peru, with incidence rates of 10% and 6.6%, respectively. Incidence-based mortality rates of colorectal cancer in Latin America and Peru are 16.6–8.2 and 11.4–5.6 per 100,000 people, respectively [1]. These rates make colorectal cancer one of the costliest cancers for healthcare systems.
It has been proven in the treatment of locally advanced rectal cancer (RC) that radiation therapy (RT) before total mesorectal excision results in low rates of pelvic recurrence [2–6]. Two radiation treatment paradigms have emerged with comparable results in overall survival and recurrence-free periods. The first paradigm is long-course chemoradiotherapy (LC-CRT), which consists of 50.4 Gy in 28 fractions with concomitant capecitabine-based chemotherapy, followed by delayed total mesorectal excision. The second paradigm is short-course radiotherapy (SCRT), which consists of 25 Gy in five sessions, followed by immediate or delayed surgical resection [7].
SCRT advocates its convenience for patients, its low cost and less acute radiation toxicities. The Trans-Tasman Radiation Oncology Group [4] and Bujko et al[6] have not reported significant differences in delayed toxicity between LC-CRT and SCRT. SCRT with and without pre-surgical delay were compared (surgery occurring the next week versus after a 4–8-week delay) and showed similar oncological results. But delaying surgery resulted in fewer surgical and post-operative complications [7]. This delay opens a window to provide an effective systemic therapy early in treatment that prevents the development of distant metastasis. This finding is very important as randomised trials consistently show an incidence of distant metastasis in around 30% of the patients treated for RC [3]. This new paradigm signifies progress towards a useful systemic therapy with oxaliplatin-based CTx to prevent the development of distant metastasis. Being able to administer all adjuvant therapies preoperatively would, therefore, be ideal. This concept is known as total neoadjuvant therapy (TNT). The main objective of TNT is to prevent distant metastasis by instructing patients to use an effective systemic therapy in the first stages of the disease. Other TNT benefits are a better local response, which may appear as both clinical and complete pathological responses, better tolerance of and compliance with treatment, reduced overall time required to complete treatment and earlier stoma closure [8, 9].
There have been few economic assessment reports on TNT to date. Examining the optimal neoadjuvant regimens for patients with RC is thus important, as is considering cost analysis in a country where healthcare system funding is limited. The objective of this study was, therefore, to perform a cost analysis to compare SCRT-TNT versus LCRT in patients with locally advanced rectal cancer.
Materials and methods
In 2020–2021, the clinical features of RC patients who received neoadjuvant therapy were prospectively recorded using medical histories. Transportation expenses were collected using patient questionnaires on their daily expenses for travelling to the institute. Medical costs were obtained from medical histories and pharmacy records. The medications and medical devices used to prepare and administer chemotherapy and radiation treatments were also identified during this process and the involved healthcare professionals were interviewed to determine the duration. This study was approved by the Institutional Ethics Committee in advance and only analysed patients who provided complete data.
Estimated cost
A spread sheet template was created using the averages of patients who received neoadjuvant therapy, to outline the cost of LCRT and SCRT-TNT treatments. The template included the direct and indirect medical costs associated with the time healthcare professionals worked, equipment and infrastructure, and the medications and supplies used in Peruvian currency (Peruvian Nuevo Sol, S/.). The template also included non-medical costs associated with the time patients and relatives spent in the facility, including travel time from their homes.
Data analysis was carried out for patients treated at the institute with tumour staging (≥3) NxM0, who completed treatment and were granted full access to costs. If radiation was part of the LC-CRT, the three-dimensional (3D) technique was used, but if radiation was part of SCRT, inverse planning was used (intensity-modulated radiation therapy (IMRT)/volumetric-modulated arc therapy (VMAT)).
Regimens
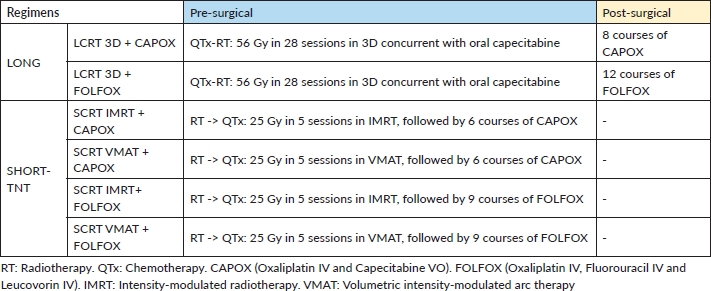
This study compared these treatment regimens (Table 1): 1) CTx-RT in 28 sessions with 3D techniques, oral capecitabine and 8 courses of adjuvant Oxaliplatin IV and Capecitabine VO (CAPOX) therapy (LCRT 3D + CAPOX); 2) CTx-RT in 28 sessions with 3D techniques, oral capecitabine and 12 courses of adjuvant FOLFOX therapy (LCRT 3D + FOLFOX); 3) RT with IMRT in 5 sessions, followed by 6 courses of CAPOX (SCRT IMRT + CAPOX); 4) RT with VMAT in 5 sessions, followed by 6 courses of CAPOX (SCRT VMAT + CAPOX); 5) RT with IMRT in 5 sessions, followed by 9 courses of FOLFOX (SCRT IMRT + FOLFOX); 6) RT with VMAT in 5 sessions VMAT, followed by 9 courses of FOLFOX (SCRT VMAT + FOLFOX). Notably, FOLFOX involves 4 days in hospital per course, while CAPOX involves 6 hours of chemotherapy in a day hospital.
Direct cost
Direct costs were calculated using medication costs shared by the institutional pharmacy cost centre and the costs of equipment and infrastructure use and procedures for consultations, simulations, target volume delineation and treatment planning. Costs related to healthcare staffing were determined using the healthcare professionals’ monthly payroll, calculating per-minute values. The cost of mesorectal excision was not analysed as it did not vary between LCRT versus SCRT-TNT nor were toxic effects analysed as there were no significant adverse effects in most cases.
Indirect cost
Indirect costs to patients included those associated with their hospital stays, calculated from their admission to their discharge. Costs related to transportation were determined using average patient costs for travelling from their homes to the hospital. Our analysis included costs associated with companions who accompanied patients because the therapies study participants received normally affect patients independence. A population-based, per-hour-worked reference value for the central macro-region was used to calculate patient or household loss of productivity due to being in hospital to either receiving cancer treatments or as companions, respectively, and therefore missing work [10].
Table 1. Description of treatment regimens.
Statistical analysis
All data and statistical analyses were carried out using SPSS version 21. Descriptive statistics are presented as means or proportions.
Results
After reviewing the 2020–2021 case histories, 38 were found for patients with neoadjuvant regimens that contained complete information for the study. Table 2 shows patient characteristics and Table 3 outlines the unit cost of the resources used for comparative projections.
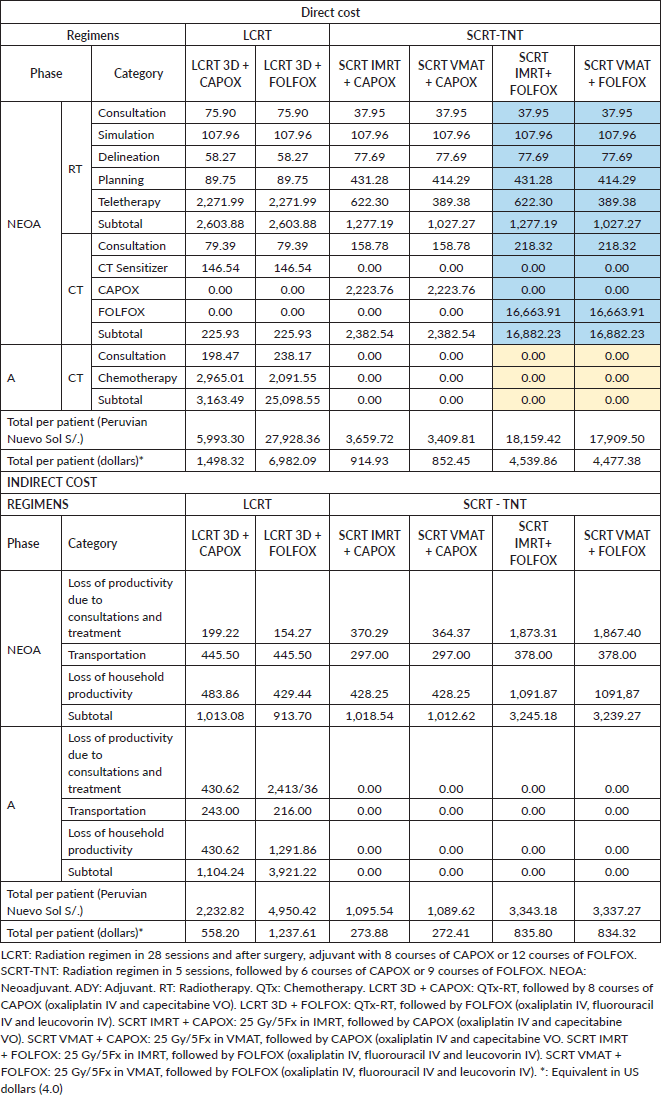
The LCRT 3D + CAPOX, LCRT 3D + FOLFOX, SCRT IMRT + CAPOX, SCRT VMT + CAPOX, SCRT IMRT + FOLFOX and SCRT VAMT + FOLFOX regimens have direct costs of S/.5,993.30, S/.27,928.36, S/.3,659.72, S/.3,409.81, S/.18,159.42 and S/.17,909.50, respectively. FOLFOX regimens are the most expensive. Administering radiotherapy in 28 3D sessions and 5 IMRT or VMAT sessions costs S/.2,603.88, S/.1,277.19 and S/.1,027.77, respectively. Planning treatment thus has an increased value over 3D of S/.324.54 for VMAT and S/.341.53 for IMRT. Likewise, the cost of all 3D teletherapy sessions (28) is triple the cost of 5 IMRT sessions and 5 times that of 5 VMAT sessions (Table 4).
The indirect cost of regimens that include FOLFOX is higher, amounting to twice that of regimens that use the same radiation technique but include CAPOX. SCRT-TNT regimens with CAPOX reduce costs by at least 50%, while SCRT-TNT regimens with FOLFOX reduce costs by 32%. The cost of lost household productivity for LCRT 3D + CAPOX, LCRT 3D + FOLFOX, SCRT IMRT + CAPOX, SCRT VMT + CAPOX, SCRT IMRT + FOLFOX and SCRT VAMT + FOLFOX is S/.914.48, S/.1,721.3, S/.428.25, S/.428.25, S/.1,091.87 and S/.1,091.87, respectively (Table 4).
Patients spend over 850 hours in the hospital for FOLFOX regimens versus less than 100 hours for CAPOX regimens. Moreover, SCRT VMAT + CAPOX is the shortest of the SCRT-TNT regimens, with 61.6 hours in the hospital (Figure 1).
The distribution of these processes shows that SCRT-TNT regimens only take around 3 hours to administer the total prescribed radiation, while LCRT regimens take a minimum of 14 hours. Similarly, full-dose FOLFOX chemotherapy in the SCRT-TNT regimen takes 756 hours versus 1,008 hours in the LCRT regimen. CAPOX chemotherapy takes 36 hours in the SCRT-TNT regimen versus 48 hours in the LCRT regimens (Figure 2).
Table 2. Characteristics of patients and companions.
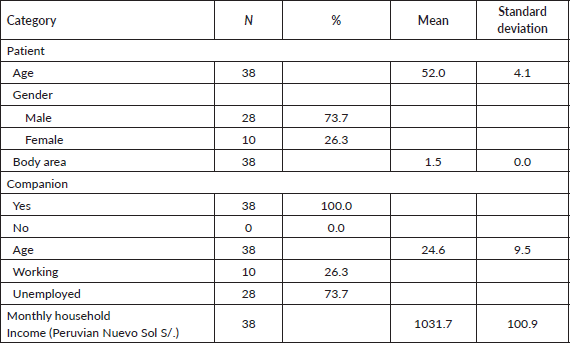
Table 3. Unit costs associated with radiotherapy and chemotherapy.
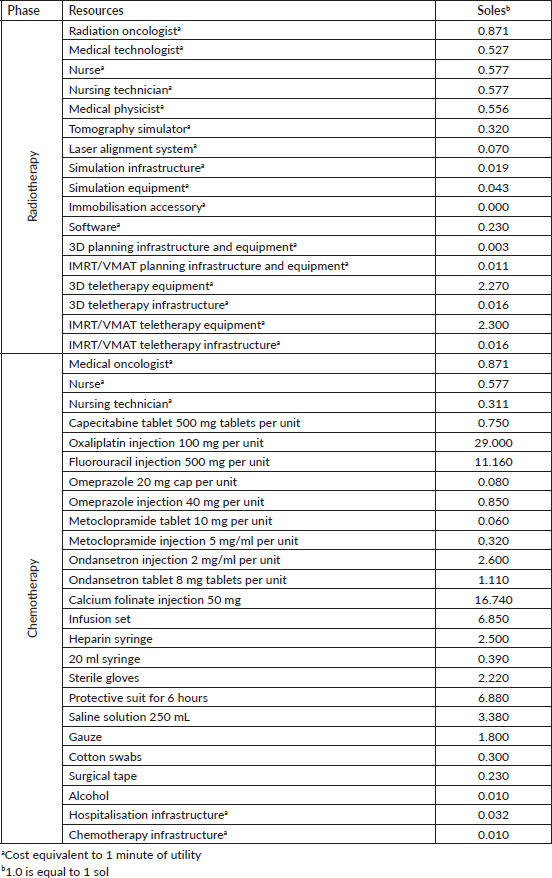
Table 4. Comparison of direct and indirect costs by treatment regimens.
Discussion
The institute is a public facility located 284 km from the national capital and covers most of the central Peruvian Andes. Patients with rectal cancer have comprehensive health insurance, which subsidises the direct costs of medical care. For neoadjuvant therapy, the Peruvian government pays the cost of medication, associated supplies, equipment and infrastructure and the fee of healthcare professionals. However, funding for neoadjuvant therapy is limited and requires making decisions. These choices must reflect the efficacy of treatments and the direct and indirect costs associated with the patient’s recovery to make more cancer treatments available [11, 12].
We have known, since the Gastrointestinal Tumour Study Group research was published in the New England Journal of Medicine in 1985, that surgery is insufficient for locally advanced RC. This study showed a clear benefit to adding RT and concomitant CTx around 5 weeks after surgery (adjuvant) [13]. A 1997 study in Sweden compared a preoperative (neoadjuvant) 25 Gy dose of RT administered in 5 sessions (SCRT), followed by surgery within 1 week versus surgery alone. The study found that SCRT led to better local control and greater overall survival [14].
It seems counterintuitive that SCRT with 25 Gy is equivalent to CTx-RT with twice the dose. But a radiobiological analysis shows that these doses are equivalent as the 25 Gy is administered in doses of 5 Gy per session versus the conventional 1.8–2.0 Gy per session. Furthermore, completing SCRT treatment requires only 1 week versus 5–5.5 weeks.
Analysis that uses a linear quadratic model and accounts for total treatment time shows that SCRT delivers the equivalent of 35.7 Gy versus 34.4 Gy in LCRT treatment [9, 15].
To date, we have understood that both modalities (SCRT and LCRT) provide comparable local results. Before the TNT concept emerged, treatments combined CTx, which used induction and/or consolidation cycles, with standard CTx-RT before surgery to increase pathological degradation and act on occult micrometastatic diseases [16]. The PRODIGE23 study confirmed this approach, by finding a 3-year metastasis-free survival rate of 78% [17]. Finally, the RAPIDO study combined SCRT with high-dose CTx before mesorectal excision. RAPIDO randomised patients with high-risk characteristics to TNT with oxaliplatin for 18 weeks versus CTx-RT adjuvant oxaliplatin treatment for 24 weeks. The treatment was well tolerated in the TNT arm, with these adverse events of grade 3 or higher: diarrhoea in 17.6% of the group; vascular disorders in 8.5%; and all other adverse events affecting lesser than 5%. The primary focus was treatment-related failure, which was 23.7% in the group receiving TNT versus 30.4% in the control group (p = 0.019). The complete pathological response was duplicated in the TNT group, reaching 28.4% versus 14.3% (p < 0.001). Distant metastasis occurred in 20% of the patients in the TNT group versus 26.8% of the patients in the CTx-RT group (p = 0.005) [8]. The institute, given proof of better clinical results and a better toxicity profile [8, 9, 16–18], has since 2021 opted to follow RAPIDO study guidelines and manage patients using TNT treatment regimens, such as SCRT-TNT, and to abandon the LCRT regimen.
Many published studies have examined preoperative CTx-RT regimens that have been used widely in clinical practice. However, research on economic analysis is rarely reported. This is the first Latin American study to analyse the costs of TNT (SCRT with CTx) and LCRT for patients with advanced RC.
Our data show that SCRT-TNT regimens are less expensive than LCRT. The SCRT IMRT/VMAT + CAPOX treatment plan results in the lowest direct costs. Teletherapy reduces the cost by four-fifths (from 28 to 5 sessions) and a course of CAPOX treatment is administered in about 6 hours in an outpatient setting. On the contrary, FOLFOX requires hospitalising the patient for 4 days per course, raising its cost. However, unsupervised outpatient CTx with capecitabine involves a risk of irregular treatment and can be inappropriate for some patients, leading to worse outcomes [19–21]. Administering SCRT requires specialised techniques, such as IMRT or VMAT, which costs less, and there is no marked difference between them. Raldow et al [22] found that SCRT was more cost-effective than LCRT. But the study also analysed the location and found that LCRT was more cost-effective in tumours in the distal third of the rectum. However, in this study, patients underwent surgery after receiving RT. It is likely that the interval after radiotherapy, which we provide our patients before surgery under the RAPIDO oxaliplatin protocol, would be more favourable even in distal tumours, comparable to cost-effectiveness in other sites [8]. Wang et al [23] compared SCRT followed by CTx (TNT) to LCRT, and found that the general costs of SCRT-TNT and LC-CRT were $78,937 and $38,140, with an efficacy of 29.92 and 22.99 quality-adjusted life months (QALM), respectively. SCRT is thus a rather cost-effective strategy. Moreover, the study found that CTx costs were always higher in both SCRT-TNT and LCRT ($7,064.01 and $5,670.94, respectively), while RT costs were $3,693.70 and $5,589.70, respectively. These findings are consistent with our results, showing that the costs of CT are up to 2.3 or up to 16 times higher when using CAPOX or FOLFOX, respectively (Table 4).
Our study shows that indirect costs are lower for all TNT modalities. The difference in the loss of productivity and the use of transportation is notable (Table 4). This is because patients need fewer hospital visits, and thus spend fewer total hours in teletherapy or chemotherapy (Figure 1). Results from comparing CAPOX and FOLFOX are consistent with those of Lin et al [24], which found that FOLFOX generates higher indirect costs due to the loss of productivity for the patient and the relatives who accompany them. An evaluation of only these two types of indirect costs found that they represent 36% and 8% of the annual household income (S/.13,628) [10] with LC-CRT 3D + FOLFOX and SCRT IMRT/VMAT + CAPOX regimens, respectively (Table 4). This result is worrisome, due to the risk of incurring catastrophic costs and increasing barriers to treatment compliance [25, 26].
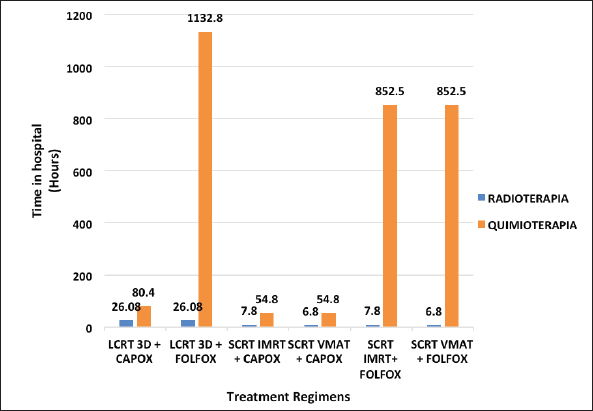
Figure 1. Time that patients spend in hospital for each regimen.
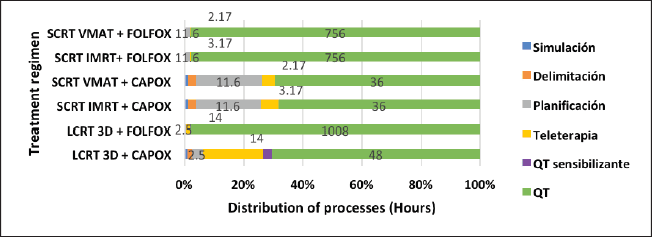
Figure 2. Comparison of time for each process by regimen. Sensitising QTx: Capecitabine VO-based chemotherapy, used during QTx-RT. QTx: Neoadjuvant or adjuvant chemotherapy regimen in the form of CAPOX or FOLFOX.
Hanly et al [27] showed that radiation therapy cost between €2,080 (5-fraction cycle) and €3,609 (25-fraction cycle) per patient. Costs were higher during the treatment planning phase for a short course (€1,217; 58% of the total cost), but were still higher in the long-cycle teletherapy phase (€1,974; 60% of the total cost). These results are consistent with our distribution analysis for hours involved professionals worked. They also show how complex the planning process is, but with fewer hours of teletherapy in the SCRT regimen (Figure 2). The study accounted for simultaneous variations in treatment time, capacity utilisation rates and the number of linear accelerator personnel, and found that the baseline cost fell by 20% for 5 fractions (€1,660) and 35% for 25 fractions (€2,354). In our analysis, the minimum required number of personnel for the Elekta® Synergy full linear accelerator (LINAC) was used: two medical technologists. Staffing costs could thus not have been reduced further. Perhaps using another LINAC that optimises treatment speed through gantry mobility and MLC motion, such as the Varian® Halcyon [28, 29], could significantly lower teletherapy costs.
Our analysis has limitations. First, we made some simplifying assumptions regarding the natural history and treatment of the disease. Second, it is possible we did not account for differences in delayed toxic effects as follow-up data are limited. However, the absolute rate of these effects seems lower for SCRT-TNT according to clinical trials [7, 8, 17]. Third, adverse events were underreported.
Our study is one of the few to evaluate the costs of LCRT and SCRT-TNT treatments that have incorporated patient weight, direct costs and the indirect cost of time to patients and their households. Moreover, this is the first report that includes clinical and economic oncologic data from a Peruvian public hospital.
Conclusion
In patients with locally advanced RC, SCRT-TNT is a less expensive approach than LCRT, despite using IMRT/VMAT. Using SCRT-TNT is also preferable because hospitals save resources and patients incur much lower indirect costs. Among the SCRT-TNT modalities, the use of CAPOX is much less expensive because it is an outpatient treatment. However, its usefulness has to be assessed to reflect the patient’s profile.
Acknowledgments
The authors thank the Junín Regional Health Authority and the Regional Institute for Neoplastic Diseases (Central Region).
Funding
No funding was received.
Conflicts of interest
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Sauer R, Becker H, and Hohenberger W, et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer N Engl J Med 351(17) 1731–1740 https://doi.org/10.1056/NEJMoa040694 PMID: 15496622
3. Sauer R, Liersch T, and Merkel S, et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomised phase III trial after a median follow-up of 11 years J Clin Oncol 30(16) 1926–1933 https://doi.org/10.1200/JCO.2011.40.1836 PMID: 22529255
4. Ngan SY, Burmeister B, and Fisher RJ, et al (2012) Randomised trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04 J Clin Oncol 30(31) 3827–3833 https://doi.org/10.1200/JCO.2012.42.9597 PMID: 23008301
5. Bujko K, Nowacki MP, and Nasierowska-Guttmejer A, et al (2004) Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy Radiother Oncol 72(1) 15–24 https://doi.org/10.1016/j.radonc.2003.12.006 PMID: 15236870
6. Bujko K, Nowacki MP, and Nasierowska-Guttmejer A, et al (2006) Long-term results of a randomised trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer Br J Surg 93(10) 1215–1223 https://doi.org/10.1002/bjs.5506 PMID: 16983741
7. Erlandsson J, Holm T, and Pettersson D, et al (2017) Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial Lancet Oncol 18(3) 336–346 https://doi.org/10.1016/S1470-2045(17)30086-4 PMID: 28190762
8. Bahadoer RR, Dijkstra EA, and van Etten B, et al (2021) Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial Lancet Oncol 22(1) 29–42 https://doi.org/10.1016/S1470-2045(20)30555-6
9. Solé S, Baeza R, and Gabler C, et al (2020) New standard in locally advanced rectal cancer World J Clin Oncol 11(12) 990–995 https://doi.org/10.5306/wjco.v11.i12.990
10. Perú, Instituto Nacional de Estadística e Informática (INEI) (2018) Evolución de los indicadores de empleo e ingresos por departamento, 2007-2017 [Internet] (Lima: INEI) 2018 Citado el 26 de diciembre del 2021
11. Cañizares FW (2017) Evolución del sistema de salud de Perú: buenas prácticas y desafíos en su construcción. Década 2005–2014 An Fac Med 78(4) 445–451 https://doi.org/10.15381/anales.v78i4.14269
12. Olivera Changra H and Robles Díaz JF (2021) Costs of intravenous vs. subcutaneous administration of trastuzumab in peruvian patients with HER2-positive breast cancer - An observational analysis of direct and indirect costs J Healthc Qual Res S2603-6479(21)00106-8
13. Gastrointestinal Tumour Study Group (1985) Prolongation of the disease-free interval in surgically treated rectal carcinoma N Engl J Med 312 1465–1472 https://doi.org/10.1056/NEJM198506063122301 PMID: 2859523
14. Swedish Rectal Cancer Trial, Cedermark B, and Dahlberg M, et al (1997) Improved survival with preoperative radiotherapy in resectable rectal cancer N Engl J Med 336(14) 980–987 https://doi.org/10.1056/NEJM199704033361402 PMID: 9091798
15. Guckenberger M, Wulf J, and Thalheimer A, et al (2009) Prospective phase II study of preoperative short-course radiotherapy for rectal cancer with twice daily fractions of 2.9 Gy to a total dose of 29 Gy--long-term results Radiat Oncol 4 67 https://doi.org/10.1186/1748-717X-4-67 PMID: 20025752 PMCID: 2806295
16. Petrelli F, Trevisan F, and Cabiddu M, et al (2020) Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes Ann Surg 271(3) 440–448 https://doi.org/10.1097/SLA.0000000000003471
17. Conroy T, Bosset JF, and Etienne PL, et al (2021) Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial Lancet Oncol 22(5) 702–715 https://doi.org/10.1016/S1470-2045(21)00079-6 PMID: 33862000
18. Garcia-Aguilar J, Patil S, and Kim JK, et al (2020) Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial J Clin Oncol 38 (Suppl) 4008 https://doi.org/10.1200/JCO.2020.38.15_suppl.4008
19. Hattori N, Nakayama G, and Uehara K, et al (2020) Phase II study of capecitabine plus oxaliplatin (CapOX) as adjuvant chemotherapy for locally advanced rectal cancer (CORONA II) Int J Clin Oncol 25(1) 118–125 https://doi.org/10.1007/s10147-019-01546-3 PMCID: 6946745
20. Nishimura J, Hasegawa J, and Kato T, et al (2018) Phase II trial of capecitabine plus oxaliplatin (CAPOX) as perioperative therapy for locally advanced rectal cancer Cancer Chemother Pharmacol 82(4) 707–716 https://doi.org/10.1007/s00280-018-3663-z PMID: 30078098
21. Deng Y, Chi P, and Lan P, et al (2019) Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial J Clin Oncol 37(34) 3223–3233 https://doi.org/10.1200/JCO.18.02309 PMID: 31557064 PMCID: 6881102
22. Raldow AC, Chen AB, and Russell M, et al (2019) Cost-effectiveness of short-course radiation therapy vs long-course chemoradiation for locally advanced rectal cancer JAMA Netw Open 2(4) e192249 https://doi.org/10.1001/jamanetworkopen.2019.2249 PMID: 30977859 PMCID: 6481445
23. Wang S, Wen F, and Zhang P, et al (2019) Cost-effectiveness analysis of long-course oxaliplatin and bolus of fluorouracil based preoperative chemoradiotherapy vs. 5x5Gy radiation plus FOLFOX4 for locally advanced resectable rectal cancer Radiat Oncol 14(1) 113 https://doi.org/10.1186/s13014-019-1319-8 PMID: 31234886 PMCID: 6591876
24. Lin JK, Tan EC, and Yang MC (2015) Comparing the effectiveness of capecitabine versus 5-fluorouracil/leucovorin therapy for elderly Taiwanese stage III colorectal cancer patients based on quality-of-life measures (QLQ-C30 and QLQ-CR38) and a new cost assessment tool. Health Qual Life Outcomes 13 61
25. Gigli A, Francisci S, and Capodaglio G, et al (2021) The economic impact of rectal cancer: a population-based study in Italy Int J Environ Res Public Health 18(2) 474 https://doi.org/10.3390/ijerph18020474 PMCID: 7827442
26. CROCODILE study group (2021) Catastrophic expenditure rates and barriers for treatment adherence in patients with colorectal cancer in India: the CROCODILE study protocol Colorectal Dis 23(8) 2161–2172 https://doi.org/10.1111/codi.15674 PMID: 33848062
27. Hanly P, Céilleachair AÓ, and Skally M, et al (2015) Direct costs of radiotherapy for rectal cancer: a microcosting study BMC Health Serv Res 15 184 https://doi.org/10.1186/s12913-015-0845-9 PMID: 25934169 PMCID: 4494796
28. Li C, Chen J, and Zhu J, et al (2019) Plan quality comparison for cervical carcinoma treated with Halcyon and Trilogy intensity- modulated radiotherapy J Cancer 10(24) 6135–6141 https://doi.org/10.7150/jca.32500 PMID: 31762823 PMCID: 6856582
29. Pawlicki T, Atwood T, and McConnell K, et al (2019) Clinical safety assessment of the Halcyon system Med Phys 46(10) 4340–4345 https://doi.org/10.1002/mp.13736 PMID: 31350914






