Bilateral adrenalectomy in the context of primary adrenal insufficiency due to colorectal cancer metastasis
Joaquin Fernandez Alberti1, Walter S Nardi2, Maricel Recalde3 and Sergio D Quildrian2
1Department of General Surgery, Buenos Aires British Hospital, Perdriel 74, Buenos Aires C1280AEB, Argentina
2Retroperitoneal, Pelvic and Adrenal Unit, Department of General Surgery, Buenos Aires British Hospital, Perdriel 74, Buenos Aires C1280AEB, Argentina
3Department of Endocrinology, Buenos Aires British Hospital, Perdriel 74, Buenos Aires C1280AEB, Argentina
Abstract
Introduction: Adrenal glands are a common site of metastasis for several types of malignancies. Nevertheless, bilateral metastasis leading to adrenal insufficiency is a very rare presentation.
Presentation of case: We present a 62-year-old woman with previous history of colorectal cancer and bilateral adrenal metastasis associated with primary adrenal insufficiency. The patient underwent bilateral open adrenalectomy after a multidisciplinary tumour board evaluation.
Conclusion: The incidence of adrenal insufficiency may be underestimated in patients with a history of cancer. Adrenal function must be evaluated in those patients presenting with bilateral adrenal masses and hormonal replacement therapy should be considered, if appropriate. In selected cases, bilateral adrenalectomy can give a possible therapeutic option for patients with confined disease to the adrenal glands.
Keywords: adrenal insufficiency, bilateral metastasis, adrenalectomy, surgical oncology, colorectal cancer
Correspondence to: Sergio D Quildrian
Email: squildrian@intramed.net
Published: 23/05/2022
Received: 07/02/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The adrenal glands are commonly infiltrated with metastasis in patients with a variety of neoplastic diseases. The most frequent primary cancers that metastasise to the adrenals are lung (42%), breast (58%), melanoma (50%), gastric (16%), colorectal (14%) and oesophageal (10.3%) among others [1].
Although bilateral metastases are more common than unilateral, in most cases they do not lead to adrenal insufficiency. It develops when more than 90% of the adrenal tissue is destroyed, resulting in near to complete glucocorticoid deficiency often accompanied by a mineralocorticoid deficiency [2]. Moreover, it is rare for a patient to present with primary adrenal insufficiency secondary to bilateral adrenal gland metastases from a colorectal tumour; in fact, less than ten cases have been published in the English literature [3–8]. Failure to recognise chronic or acute adrenal insufficiency or epinephrine excess can cause significant morbidity or mortality [9].
Case presentation
A 62-year-old woman with a previous history of colorectal cancer was referred to our surgical department for presenting bilateral adrenal masses found on an abdominal CT scan at month 9 follow-up (Figure 1), associated with increasing levels of adrenocorticotropic hormone (ACTH) (last value: 320 pg/ml; normal value: <54 pg/ml) and low levels of dehydroepiandrosterone (86 ng/ml; normal value: 336–2,490 ng/ml). Her medical history revealed a laparoscopic right hemicolectomy for a colorectal cancer (pT4 N0 M0, pStage II) without requiring any adjuvant therapy.
Physical examination evidenced morbid obesity (body mass index (BMI): 49 kg/m2) and skin hyperpigmentation. She also referred long-time tiredness and weight gain. Laboratory tests evidenced a primary adrenal insufficiency associated with elevated tumour markers (carcinoembryonic antigen (CEA): 40.9 ng/ml; normal value: 1–5.2 ng/ml). Oral corticosteroid replacement was initiated (hydrotisone 20 mg and fludrocortisone 0.05 mg daily) showing immediate symptom improvement. Also, a central nervous magnetic resonance imaging (MRI) was requested which showed no abnormalities in the pituitary gland and pheochromocytoma was ruled out in laboratory tests. Abdominal MRI is shown in Figure 2a and b. PET-CT was also performed showing hypermetabolic bilateral adrenal masses with no systemic disease (Figure 2c and d).
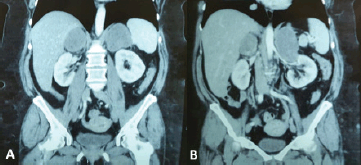
Figure 1. (a and b) Coronal sections on computed tomography (CT) scans showing bilateral adrenal masses (right of 66 mm and left of 106 mm).
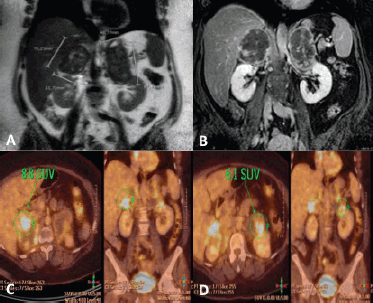
Figure 2. (a) Coronal section on MRI in T2-weighted sequence showing bilateral adrenal masses (right of 75 × 52 mm and left of 98 × 49 mm). (b) Similar section in T1-weighted with fat suppression and Gadolinium sequence. (c and d) PET-CT showing peripheral metabolic increase in nodular formations in right and left adrenal glands, with 8.8 and 6.1 SUV, respectively.
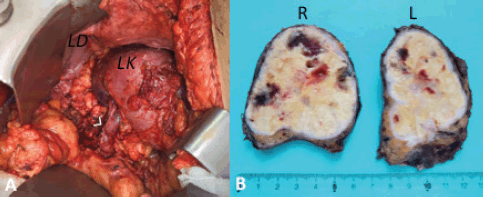
Figure 3. (a) Left surgical site post-adrenalectomy. Arrow: left renal vein; LK: left kidney; LD: left diaphragm. (b) Right (R) and left (L) adrenal glands sectioned.
After discussing the case in a multidisciplinary tumour (MDT) meeting, multiple CT-guided core biopsies were performed with a 14G needle using a posterior approach. Because of the impossibility to establish a definitive diagnosis on an imaging basis only, pathological assessment remains the gold standard of diagnosis. Differential diagnosis includes lymphoma, hyperplasia, hematoma, histoplasmosis and pheochromocytoma among others. As mentioned, a percutaneous CT-guided core needle biopsy was performed using the retroperitoneal approach. This route shows a minimal risk of seeding by preserving the posterior parietal peritoneum. Histopathology findings were primarily related to colorectal lineage, associated with patient’s cancer history. Based on this result, the decision was made for complete surgical resection. The patient underwent open bilateral adrenalectomy with two subcostal incisions (Figure 3). We chose to go with an open approach because of the size of both tumours and their relationship with renal vessels. She was discharged on post-operative day 6 without complications. Final histopathological examination confirmed a massive involvement by adenocarcinoma with multiple sites of necrosis in both surgical specimens.
In a new MDT meeting, it was decided to complete treatment with six sessions of chemotherapy (Capecitabine). At 1-year follow-up, she had normal CEA levels and abdominal CT scan and PET-CT without evidence of disease recurrence.
Discussion
Colorectal cancer is one of the most frequent cancers worldwide. One of the first steps to initial staging includes abdominal/pelvis and chest computed tomography (CT) to evaluate for metastatic disease which occurs in approximately 20% of the patients at the time of diagnosis [10]. The most common sites of metastases are the lungs, liver and peritoneum [10]. Adrenal metastases can be seen in up to 14% of the patients with advanced colorectal cancer [3], indicating widespread disease; and, hence, patients with a solitary adrenal metastasis from colorectal carcinoma are rare, especially coupled with metachronous contralateral adrenal metastasis. Bilateral metastases are more commonly seen than unilateral ones and, in most cases, they do not lead to adrenal insufficiency. In order for this to happen more than 90% of the adrenal tissue needs to be destroyed. Adrenal insufficiency secondary to any type of metastatic cancer has been reported in fewer than 100 cases in the literature [11].
A metastatic origin should always be considered in a patient presenting with an adrenal mass and a history of malignancy. Evaluation of patients must include imaging with abdominal CT scan or MRI and be compared with a previous scan. In addition, a biochemical assessment to rule out a functional adrenal tumour needs to be performed. There are imaging features to be considered such as size >4 cm, growth in size >1 cm on serial imaging (within 1 year), irregular shape, heterogeneous and poorly defined margins, necrosis, Hounsfield unit >10 on non-contrasted imaging, <60% washout on CT imaging and hyperintense on MRI T2-weighted imaging [12].
Serum CEA is thought to be essential in predicting the presence of metastatic disease in the post-operative period of colorectal cancer [13]. In the case presented, imaging findings were associated with elevated serum CEA levels, making the scenario highly suspicious of bilateral metastasis. However, the multidisciplinary committee agreed to confirm the diagnosis with a percutaneous CT-guided biopsy. This practice is recommended for selected cases if the expected findings are likely to alter the management of the individual patient and after biochemical exclusion of catecholamine-producing tumours to help prevent potentially life-threatening complications [14].
Although prospective data are generally lacking, multiple retrospective investigations have demonstrated that adrenalectomy in highly selected patients with isolated or oligometastatic disease from primary sites, including the lungs, melanoma and kidney, can result in improved overall survival (OS) compared with similar patients who do not undergo adrenalectomy [12]. Several factors associated with poor survival outcome have been identified, such as the presence of active disease at the time of adrenalectomy, type of primary, disease-free interval, size of metastases and adrenalectomy bed recurrence [15–17]. In terms of OS, it is important to highlight the improvement observed in the past decades by the group of Memorial Sloan Kettering. They reported a 5-year OS of 24%, 29%, 31% and 40% in four consecutive series published between 1998 and 2018 [15, 18, 19]. These results probably represent better patient selection and improvements in systemic therapy.
Other alternatives to surgery have been described such as radiofrequency ablation mainly in patients who are poor surgical candidates. This treatment modality is used to ablate tumours (both benign and malignant) in a variety of tissues, including liver, lungs, bone, breast or kidney, and proved to be effective, safe and technically feasible. Mayo-Smith and Dupuy [20] presented the results from percutaneous ablation in 11 patients with metastatic lesions to the adrenal glands with a mean tumour size of 3.9 cm. Nonetheless, there are few reports of its application to adrenal tissue in the clinical scenario presented in our case report with bilateral and large tumours. No guidelines for adrenal ablation are established in the American or European literature.
The survival rate of patients with cancer is increasing, and improvements in imaging techniques have resulted in increased promptly recognition of adrenal gland metastases. However, the incidence of adrenal insufficiency may be underestimated, especially in patients with multiple metastases. In agreement with Crisci et al [7], we think that patients with multiple metastases from a known primary that complains of systemic symptoms must be evaluated for adrenal function and provided with a hormonal replacement therapy, if necessary.
Conclusion
In conclusion, it seems reasonable to offer adrenalectomy to carefully selected patients with solid tumours and adrenal metastases, mainly patients with metachronous presentation (>6 months). The indication of LA must be made on a case-by-case evaluation in the context of a MDT board.
Conflicts of interest
None declared.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Take home messages
• It is important to suspect potential adrenal malignancy in patients with oncological background. CT scanning and serum markers are useful tools in the postoperative period.
• Patients with multiple metastases from a known primary that complains of systemic symptoms must be evaluated for adrenal function and provided with a hormonal replacement therapy, if necessary. Adrenal insufficiency should not be underestimated.
• Laparoscopic adrenalectomy in these scenarios can be offered to selected patients after a MDT board discussion.
Patient’s perspective
Oncologists, surgeons and endocrinologists meet with me every 3 months for my follow-up as a multidisciplinary team. I have direct communication with them in case of any need or doubt. I have felt very comfortable and secure during the treatment and follow-up so far.
References
1. Seidenwurm DJ, Elmer EB, and Kaplan LM, et al (1984) Metastases to the adrenal glands and the development of Addison’s disease Cancer 54(3) 552–557 https://doi.org/10.1002/1097-0142(19840801)54:3<552::AID-CNCR2820540328>3.0.CO;2-R PMID: 6733685
2. Herndon J, Nadeau AM, and Davidge-Pitts CJ, et al (2018) Primary adrenal insufficiency due to bilateral infiltrative disease Endocrine 62(3) 721–728 https://doi.org/10.1007/s12020-018-1737-7 PMID: 30178435
3. Cedermark BJ, Blumenson LE, and Pickren JW (1977) The significance of metastases to the adrenal glands in adenocarcinoma of the colon and rectum Surg Gynecol Obstet 144(4) 537–546 PMID: 847609
4. Black RM, Daniels GH, and Coggins CH, et al (1981) Adrenal insufficiency from metastatic colonic carcinoma masquerading as isolated aldosterone deficiency. Report of a case and review of the literature Acta Endocrinol (Copenh) 98(4) 586–691 https://doi.org/10.1530/acta.0.0980586
5. Khristov V, Kolebinov N, and Tsanev A, et al (1990) Bilateral adrenal metastases with the clinical manifestations of hypocorticism in rectal cancer Vutr Boles 29(4) 97–101 PMID: 2177937
6. Omoigui NA, Cave Jr WT, and Chang AY (1987) Adrenal insufficiency. A rare initial sign of metastatic colon carcinoma J Clin Gastroenterol 9(4) 470–474 https://doi.org/10.1097/00004836-198708000-00024 PMID: 3309024
7. Crisci A, Cartei G, and De Antoni P, et al (2001) Surgical management of isolated bilateral adrenal metastases from colon carcinoma causing adrenal insufficiency Urol Int 67(1) 113–116 https://doi.org/10.1159/000050963 PMID: 11464135
8. Imaoka Y, Kuranishi F, and Ogawa Y, et al (2017) Adrenal failure due to bilateral adrenal metastasis of rectal cancer: a case report Int J Surg Case Rep 31 1–4 https://doi.org/10.1016/j.ijscr.2016.12.011 PMID: 28073054 PMCID: 5222944
9. Taub YR and Wolford RW (2009) Adrenal insufficiency and other adrenal oncologic emergencies Emerg Med Clin North Am 27(2) 271–282 https://doi.org/10.1016/j.emc.2009.01.008 PMID: 19447311
10. van der Geest LGM, Lam-Boer J, and Koopman M, et al (2015) Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases Clin Exp Metastasis 32(5) 457–465 https://doi.org/10.1007/s10585-015-9719-0 PMID: 25899064
11. Ross IL, Marais S, and Raubenheimer P, et al (2003) Overt hypoadrenalism is uncommon in patients with stage 3 and 4 bronchogenic carcinoma S Afr Med J 93(9) 695–699 PMID: 14635559
12. Kiernan CM and Lee JE (2019) Minimally invasive surgery for primary and metastatic adrenal malignancy Surg Oncol Clin N Am 28(2) 309–326 https://doi.org/10.1016/j.soc.2018.11.011 PMID: 30851831
13. Katayama A, Mafune K, and Makuuchi M (2000) Adrenalectomy for solitary adrenal metastasis from colorectal carcinoma Jpn J Clin Oncol 30(9) 414–416 https://doi.org/10.1093/jjco/hyd104 PMID: 11095141
14. Bancos I, Tamhane S, and Shah M, et al (2016) Diagnosis of endocrine disease: the diagnostic performance of adrenal biopsy: a systematic review and meta-analysis Eur J Endocrinol 175(2) R65–R80 https://doi.org/10.1530/EJE-16-0297 PMID: 27257146
15. Russo AE, Untch BR, and Kris MG, et al (2019) Adrenal metastasectomy in the presence and absence of extraadrenal metastatic disease Ann Surg 270(2) 373–377 https://doi.org/10.1097/SLA.0000000000002749
16. Drake FT, Beninato T, and Xiong MX, et al (2019) Laparoscopic adrenalectomy for metastatic disease: retrospective cohort with long-term, comprehensive follow-up Surgery 165(5) 958–964 https://doi.org/10.1016/j.surg.2018.11.008
17. Vazquez BJ, Richards ML, and Lohse CM, et al (2012) Adrenalectomy improves outcomes of selected patients with metastatic carcinoma World J Surg 36(6) 1400–1405 https://doi.org/10.1007/s00268-012-1506-3 PMID: 22411083
18. Strong VE, D’Angelica M, and Tang L, et al (2007) Laparoscopic adrenalectomy for isolated adrenal metastasis Ann Surg Oncol 14(12) 3392–3400 https://doi.org/10.1245/s10434-007-9520-7 PMID: 17665267
19. Sarela AI, Murphy I, and Coit DG, et al (2003) Metastasis to the adrenal gland: the emerging role of laparoscopic surgery Ann Surg Oncol 10(10) 1191–1196 https://doi.org/10.1245/ASO.2003.04.020 PMID: 14654476
20. Mayo-Smith WW and Dupuy DE (2004) Adrenal neoplasms: CT-guided radiofrequency ablation – preliminary results Radiology 231 225–230 https://doi.org/10.1148/radiol.2311031007 PMID: 14990812






