The relationship between tumour infiltrating lymphocytes, Epstein–Barr virus and Helicobacter pylori infection in gastric cancer
Carlos Castañeda1,2a, Miluska Castillo3b, Luis Bernabe3c, Nancy Suarez3d, Matteo Fassan4,5e, Joselyn Sanchez3f, Katherine Tello3g, Raul Alatrista3, Ivan Chavez6h, Eloy Ruiz6i, Yaqueline Bazan6j, Fernando Barreda7k, Daniel Valdivia7l, Wei Meng8, Arnab Chakravarti8, Juvenal Sanchez9m, Luis Taxa9 and Paola Montenegro2
1Facultad de Ciencias de la Salud, Universidad Cientifica del Sur, Lima 15067, Peru
2Departamento de Oncologia Medica, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
3Departamento de Investigación, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
4Department of Medicine-DIMED, Surgical Pathology Unit, University of Padua, Padua 35122, Italy
5Veneto Institute of Oncology, IOV-IRCCS, Padua 35128, Italy
6Departamento de Cirugía en Abdomen, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
7Departamento de Especialidades Médicas, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
8Department of Radiation Oncology, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
9Departamento de Patologia, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
ahttps://orcid.org/0000-0001-6200-0856
bhttps://orcid.org/0000-0002-0111-3176
chttps://orcid.org/0000-0003-1896-7060
dhttps://orcid.org/0000-0001-5955-3919
ehttps://orcid.org/0000-0001-6515-5482
fhttps://orcid.org/0000-0002-6764-4180
ghttps://orcid.org/0000-0002-4981-3411
hhttps://orcid.org/0000-0002-3431-3262
ihttps://orcid.org/0000-0002-4644-0074
jhttps://orcid.org/0000-0002-7337-2396
khttps://orcid.org/0000-0002-7923-6299
lhttps://orcid.org/0000-0002-5917-6452
mhttps://orcid.org/0000-0002-9825-8573
Abstract
Objective: Epstein–Barr virus (EBV) and Helicobacter pylori (HP) infections have been extensively recognised as gastric cancer (GC) triggers, and recent publications suggest they could behave as predictive markers for immune-modulating therapies. Tumour-infiltrating lymphocytes (TILs) have also been identified as a predictive biomarker for immunotherapy in different malignancies. This study aimed to investigate the association between EBV and HP infection with TIL levels in GC.
Methods: TIL evaluation in haematoxylin-eosin was performed by a pathologist and density of CD3, CD8 and CD163 positive (immunohistochemistry staining) immune cells was calculated with the use of digital pathology software. EBV infection was detected by in situ hybridisation (ISH) and by quantitative polymerase chain reaction (qPCR). Methylation status of EBV-related genes was detected by PCR and a methylome analysis was performed by the Illumina Infinium MethylationEPIC BeadChip. HP status was detected by qPCR.
Results: We included 98 resected GC Peruvian cases in our evaluation. Median TIL percentage was 30. The proportion of EBV+ detected by ISH was 24.1%, of EBV+ detected by qPCR was 41.8%, while 70% showed methylation of EBV-related genes, and 58.21% of cases were HP+. Younger age (p = 0.024), early stages (p = 0.001), HP+ (p = 0.036) and low CD8 density (p = 0.046) were associated with longer overall survival (OS). High TIL level was associated with intestinal subtype (p < 0.001), with grade 2 (p < 0.001), with EBV qPCR+ (p = 0.001), and with methylation of EBV-related genes (p = 0.007). Cases with high TIL level and cases that are EBV positive share eight genes with similarly methylated status in the metabolomic analysis. High CD8 density was associated with EBV PCR+ (p = 0.012) and HP− (0.005).
Conclusion: Lower CD8 density and HP+ predict longer OS. High TIL level is associated with EBV+ and methylation of EBV-related genes, while lower CD8 density is associated with HP+ GC.
Keywords: Epstein–Barr virus, Helicobacter pylori, lymphocytes, in situ hybridisation
Correspondence to: Carlos Castaneda
Email: carloscastanedaaltamirano@yahoo.com
Published: 01/03/2022
Received: 29/09/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastric cancer (GC) represents the fourth most common type of cancer and the second leading cause of cancer death worldwide. The incidence of GC varies up to 10-fold by geographic region, suggesting that genetic or environmental factors influence carcinogenesis and clinicopathological features. In Peru, GC is the second in males and the third most frequent malignancy in women [1]. Epstein–Barr virus (EBV) is uniformly present in gastric adenocarcinoma subtype with extensive lymphocyte infiltration [2, 3] and is associated with cytosine-guanine (CpG) dinucleotide hypermethylated pattern and longer survival [4, 5]. Recent studies indicate that EBV presence could also predict efficacy of checkpoint immune inhibitors in GC [6].
Persistent infection with Helicobacter pylori (HP) induces chronic inflammation and gastric carcinogenesis. HP prevalence in adults living in developing Latin American countries is 70% to 80%, and its presence in GC has been suggested to be associated with a better prognosis and response to checkpoint immune inhibitors [7, 8].
Some reports have shown that a high level of tumour-infiltrating lymphocytes (TILs) is related to both longer survival in GC [9] and a higher response to checkpoint inhibitors. However, methodologies for evaluating TILs have been diverse, and only a few studies have evaluated the relationship between TIL levels and clinicopathological features [10–13]. Recently, the International Immuno-Oncology Biomarkers Working Group (IBWG) has proposed a standardised methodology to evaluate TIL in different malignancies including gastric carcinoma [14]. Furthermore, some recent reports have described that density of immune cell subpopulations belonging to TIL like CD3 positive (T-cell) lymphocytes, CD8 positive (cytotoxic T-cell) lymphocytes and CD163 positive (pro-tumour M2) macrophages could be responsive to the mentioned associations [13, 15].
We developed an exploratory study to evaluate the relation between the TIL level based on IBWG methodology [6, 14] and the infection of HP and EBV in a cohort of EBV enriched GC population. Additionally, density of CD3, CD8-and CD163positive immune cell subpopulations and their relationship with HP and EBV infections were calculated. Evaluation of EBV infection included the analysis of methylation status of EBV-related genes.
Materials and methods
Subjects
The study included histology confirmed gastric adenocarcinomas from patients who underwent surgery at the Instituto Nacional de Enfermedades Neoplasicas (Lima–Peru) from 2015 to 2018. All the patients were selected by convenience sampling. Cases were selected among those with the highest EBV gene counts by polymerase chain reaction (PCR) (previously selected [1] with counts that reached up to 166,210.5
copies/μL) in order to obtain a group with high rates of positive in situ hybridisation (ISH) evaluations for EBV. All included cases had ISH status for EBV and/ or methylation evaluation of EBV-related genes.
This single-centre retro-prospective cohort research was presented and approved by the Research and Ethics committee (Protocol Number #050-2015-CIE/INEN), and the patients were invited to read and sign the informed consent.
Tumour specimens
Every tumour sample was collected and stored at −80°C until use at the Institute Biobanking for quantitative PCR (qPCR) for EBV, as well as paired Formalin-Fixed Paraffin-Embedded (FFPE) samples saved at pathology archive. Tissue microarrays (TMAs) were constructed from tumour cores (6.0 mm diameter) through the invasive areas of each specimen from the selected FFPE blocks. Serial 4-μm sections were prepared and used for immunohistochemistry (IHC) staining [6].
Immunohistochemistry (IHC)
Sections from paraffin samples were rehydrated in phosphate-buffered saline (PBS), and antigen retrieval was performed by immersing in 0.1% trypsin solution in PBS at 37°C for 5–10 minutes or by microwave heating for 5 minutes × 4 (total, 20 minutes) in buffer solution. The sections were treated for 45 minutes with 10% normal goat serum or normal horse serum in PBS. The primary antihuman antibodies used for IHC were CD3 (IS503, Dako, Glostrup, Denmark), CD8 (clone C8/144B, IS623, Dako, Glostrup, Denmark) and CD163 (clone EP324, Master Diagnostica, Granada, España). The sections were incubated further in alkaline phosphatase–streptavidin (Vector Laboratories, Burlingame, CA; 1:1,000 dilution) for 30 minutes at room temperature, reacted with Fast-Red Substrate System (Dakopatts) or with Dako® Fuchsin + Substrate-Chromogen. Background staining was performed with Mayer’s haematoxylin solution, and sections were then dehydrated through ascending alcohols to xylene and mounted on slides.
Measurement of TILs
FFPE tissue samples were retrieved and haematoxylin-eosin stained slides were obtained. The IBWG method for TIL assessment in the stromal compartment was applied by one pathologist (J Sanchez) (Figure 1). The densities of T lymphocytes were assessed following our previous report [6]. Immunostained slides were digitally scanned using a BX63 Olympus scanner (Olympus, Tokyo, Japan) at 20× magnification. Digital images were viewed with Visiopharm Integrator System software version 6.6.1.2572 (Visiopharm, Hørsholm, Denmark). Density of CD3, CD8 and CD163-positive cells were calculated, in cases with enough stained tissue, by VisionPharm software by counting the number of positive cells for staining/total number of cells in five high power fields located in the stromal compartment (M Castillo and LA Bernabe) under supervision by a pathologist (J Sanchez) [6] (Figure 2).
EBV ISH
Chromogenic ISH for EBV-encoded RNA (EBER) was performed in FFPE tissue samples with fluorescein-labelled oligonucleotide probes (EBER probe, Ventana) with enzymatic digestion (ISH protease 3, Ventana) and an iViewBlue detection kit (Ventana) with use of the BenchMark ULTRA staining system (K Tello).
Evaluation of the EBV gene and HP gene expression
The detection of EBV gene expression was performed in a region of BNRF1, and HP genes by the hspA and UreA genes in DNA from frozen samples as targets by qPCR in the LightCycler 96 Instrument Thermal Cycler (Roche, Mannheim, Germany) as described in our previous publication [1]. Values were considered as positive when ≥10 copies/μL was detected (N Suarez).
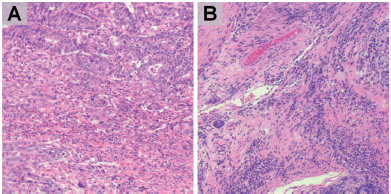
Figure 1. HE staining of GC. Representative slides of stromal compartment with high (a, b) level of tumour infiltrating lymphocytes. (magnification: ×200).
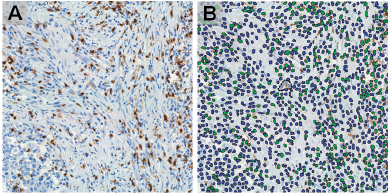
Figure 2. Identification of CD8 in IHC staining images (a) by machine learning-based image processing (b) showing positive (green) and negative (blue) cells, (magnification: ×400).
Evaluation of methylation in genes related to EBV
Genomic DNA was isolated from GC frozen samples using the classical method of phenol/chloroform/isoamylalcohol and proteinase K. The bisulfite treatment was performed as previously described with the EpiTect Bisulfite (Qiagen, Germany) kit and the thermal cycler Mastercycler nexus gradient (Eppendorf, Germany). The methylation status of six gene promoters (RASSF1, CDKN2A, MGMT, GSTP1, HOXA10 and TP73) were analysed in the LightCycler® 96 Instrument (ROCHE®) thermal cycler, and the results were interpreted regarding the threshold cycle presence in the software LightCycler® 96 System Version 2.0 [5]. Primer sequences were forward: 5′ TGGAGTTTTCGGTTGATTGGTT 3′ and reverse: 5′ AACAACGCCCGCACCTCCT 3′ for CDNK2A; forward: 5′ ATCGGAAGTGCGTTATTTCGTG 3′ and reverse: 5′ TTCCGTCTCTCGACTCGAAACT 3′ for HOXA10; forward: 5′ GGGTCGGGTAGTTCGTTTTG 3′ and reverse: 5′ CGATTTCGCTACGTCCCCT 3′ for TP73; forward: 5′ ATTGAGTTGCGGGAGTTGGT 3′ and reverse: 5′ ACACGCTCCAACCGAATACG 3′ for RASSF1A; forward: 5′ GTCGGCGTCGTGATTTAGTATTG 3′ and reverse: 5′ AAACTACGACGACGAAACTCCAA 3′ for GSTP1; forward: 5′ GCGTTTCGACGTTCGTAGGT 3′ and reverse: 5′ CACTCTTCCGAAAACGAAACG 3′ for MGMT.
DNA methylation profiling was performed using the Illumina Infinium MethylationEPIC BeadChip that features over 850,000 CpGs in enhancer regions, gene bodies, promoters, and CpG islands according to the manufacturer’s instructions, in FFPE tumour tissues of a subset of 24 GC cases in the Molecular Genomics Core lab, USC Norris Comprehensive Cancer Center (Los Angeles, CA). The methylation values for the individual CpG sites were obtained as β values. The β-value generated for each CpG locus reflected a measure of the percentage of the methylated (β = 1) and unmethylated probes (β = 0). The β-values of each probe are continuous variables that are calculated by dividing the intensity of the methylated probe by the combined methylated and unmethylated probe intensities, and the resultant values range from 0 to 1.
Statistical analysis
Comparisons of categorical variables were done by a Chi-square test or Fisher’s exact test as appropriate. Correlation between density of CD3, CD8 and CD163-positive cells were calculated by intraclass correlation coefficient (ICC). Overall survival (OS) was defined as the time from the date of surgery until death from any cause or last vital status obtained from patient file or from National Registry of Identification and Marital Status webpage (www.reniec.gob.pe). The follow-up for vital status was completed in September 2021. Survival rates were estimated by the Kaplan–Meier method. All tests were two-sided, and differences were considered to be significant when p < 0.05. Statistical analyses were performed with IBM SPSS Statistics version 21 (IBM, Armonk, NY). Genome-wide DNA methylation was analysed, identifying differentially methylated regions (DMR) with respect to TIL level (with a cutoff of 30%) and ISH EBER status (positive or negative) through Illumina Infinium MethylationEPIC BeadChip. The raw intensity data were imported into R (3.6.0, https://cran.r-projecto.org/) and then analysed with the minfi package for data preprocessing normalisation and comparison between groups. Statistical analyses of the data were performed in RStudio (1.2.1335) (http://www.rstudio.com/) using an R environment (3.6.0) (https://www.R-project.org).
Results
Clinicopathological features
In the whole series of 98 cases, the median age was 68 years and 41.8% were women. Clinicopathological features are described in Table 1. HP was detected in 56.1%. Median TIL percentage was 30 (interquartile range (IQR) = 10%–60%). Median densities of CD3 positive cells, CD8 (T lymphocytes) and CD163 positive cells were 21.07% (IQR = 10%–60%%), 10.48% (IQR = 5.77%–20.04%) and 11.17% (IQR = 5.68%–17.29%), respectively. Density of CD3 positive cells had close correlation with CD8 (ICC = 0.834 (95% CI: 0.725 to 0.899, p < 0.001)). Density of CD3 positive cells (ICC = 0.241 (95% CI: −0.33 to 0.567, p = 0.167)) had poor and CD8 (ICC = 0.551 (95% CI: 0.183 to 0.753, p = 0.005)) had a high direct correlation with CD163.
Median follow up was 21.7 months and 47 patients (48%) were alive at 3 years. Older age (p = 0.024), presence of lymphovascular invasion (p = 0.007), advanced pathological stage (p = 0.001), recurrence (p = 0.002), HP absence (p = 0.036) and high CD8 density (p = 0.046) were associated with shorter OS (Figure 3). The level of TIL (p = 0.594), CD3 density (p = 0.241) and CD163 density (p = 0.152) were not associated with survival (Table 1).
Determination of EBV status through ISH, qPCR and methylation
Status for EBV was positive using EBER-ISH method in 19 of the 79 (24.1%) evaluated cases and was positive using qPCR method in 41 of 98 (41.8%) cases. There was no significant relationship between EBER ISH and qPCR detection (kappa index = 0.012, p = 0.864). There was no significant association between OS and EBER ISH (p = 0.345) or qPCR (p = 0.809).
An upregulated methylation status in at least one gene was found in 55 of the 78 (70%) evaluated cases (Table 1). There was methylation of RASSF1, CDKN2A, MGMT, GSTP1, HOXA10 and TP73 in 37.2%, 48.7%, 34.6%, 11.5%, 15.4% and 10.3%, respectively. There was a significant association between methylation status and presence of EBV infection evaluated through qPCR (kappa index = 0.328, p = 0.003) or ISH (kappa index = 0.158, p = 0.025).
The methylome analysis using Infinium MethylationEPIC BeadChip in 24 tumour samples found that DMR cutoff >30% with <0.05 p-value regarding EBER ISH status identified 88 DMR-related genes (from 116 DMRs).
Table 1. Clinicopathological features.
Association between TILs and clinicopathological features in gastric tissues
High TIL level was associated with intestinal subtype (p < 0.001) and well/ moderately differentiated grade (p < 0.001). CD3 density was not associated with HP (p = 0.531) nor other variables (p > 0.01). High CD8 density was associated with absence of HP infection. High CD163 density was associated with male gender (p = 0.02), diffuse subtype (0.040) and stage III (p = 0.02) (Table 2).
Table 2. Evaluation of relationship among tumour-infiltrating-lymphocytes and clinicopathological features.
Association between TILs and EBER-ISH status, Epstein-Barr gene expression or methylation signature
High TIL level and high CD8 density were associated with positive EBV status through qPCR (p = 0.001 and p = 0.012, respectively) and a trend with EBER ISH status (p = 0.098 and 0.077, respectively) (Table 1). High TIL level was also associated with the methylation of at least one of the 6 EBV-related genes (p = 0.007) and methylation of RASSF1A (p = 0.011). Additionally, high density of CD8-positive cells was associated with the methylation status of RASSF1A (p = 0.048) and CDKN2A (p = 0.025) (Table 2). CD3 density wasnot associated with EBER ISH (p = 0.124) or with other EBV evaluation (p > 0.05).
The methylome analysis using Infinium MethylationEPIC BeadChip found that a DMR cutoff>30% with a <0.05 p-value identified 32 DMRs related genes (from 146 DMR) associated with >30% TIL. Furthermore, eight genes were shared among EBER ISH positive status and TIL>30%: HEATR4 (HEAT repeat containing 4) (high), RGMA (repulsive guidance molecule BMP co-receptor a) (high), MTRNR2L1 (MT-RNR2like 1) (low), SH2D4A (SH2 domain containing 4A) (low), SORCS3 (sortilin related VPS10 domain containing receptor 3) (low), TBC1D14 (TBC1domain family member 14) (low), TMEM260 (transmembrane protein 260) (low) and TNNT3 (troponin T3, fast skeletal type) (low).
Discussion
We found a remarkable association between TIL level and EBV infection. High TIL level and density of CD8 T lymphocytes were significantly associated with positive EBV status and methylation of EBV-related genes.
Methylation patterns have been extensively described in EBV-positive GC [5], and our finding that the TIL level was also associated with methylation in at least one of six evaluated cancer genes suggests that methylation process could mediate the immune activity against EBV-infected tumours. This explanation is also supported by our finding that altered methylation status of eight genes is similar in those tumours with high TIL level and in those with EBER ISH positive status in the genome-wide DNA methylation analyses. SORCS3 is one of the genes with altered methylation and has been previously described to be related to gastrointestinal tumour progression [16, 17]. Hanahan [20] concluded that nonmutational epigenetic reprogramming is a characteristic process that facilitates the acquisition of hallmark capabilities, and recent studies find that methylation of tumour genes can mediate ability of the immune system to detect GC cells [18, 19]. The hypothesis that the association between EBV infection and high TIL level (and high CD8 density) is mediated by the methylated pattern deserves further research because it could lead to the identification of tumour biomarkers that predict activity of immunotherapy in GC.

Figure 3. Kaplan–Meier curve for OS by age (a), stage (b), HP status (c) and CD8 positive T cell density (d).
A significant association between high density of CD8-positive lymphocytes and shorter survival was also found in our series. This effect could be related to the previously described direct correlation between CD8 T lymphocytes and the level of pro-tumour PD-L1 and FOXP3 positive T lymphocytes [10–13, 21]. High density of CD163 positive cells (M2 macrophages) was associated with aggressive features like diffuse subtype and stage III disease and had a trend to shorter survival (39% versus 56% OS at 3 years, p = 0.24). This protumour effect has been previously described [22, 23] and our finding that it was directly correlated with CD8 T cell density could also add to the negative survival impact of CD8 density.
HP infection was also significantly associated with longer survival in our series, a relationship that has previously been described by other groups [7]. Furthermore, our finding that the good prognostic feature of a low density of CD8-positive lymphocytes is associated with the infection, suggests that an effective immune response against GC could explain the better prognosis of HP+ tumours.
Recent publications suggest that the tumour response to immunotherapy could be reduced in patients with HP infection [8]. This fact could be explained by our finding of reduced tumour infiltration by CD8 positive T cells.
A limitation of our exploratory research is the small size of our series that makes our results need to be validated in larger-size studies. The analysed tumour sample volume was small since we used TMAs; however, the evaluated area of invasive tumour was selected by a pathologist, and most samples obtained in real-world are similarly small as they are obtained from gastroscopies.
Conclusion
In conclusion, we found that high level of TIL in GC was associated with EBV infection, and this association could be mediated by the methylation status. HP infection is associated with longer survival, and this association could be mediated by lower CD8 T cell infiltration.
Conflicts of interest
The author(s) declare that they have no conflict of interest.
Funding
Universidad Cientifica del Sur, Consejo Nacional de Ciencia, Tecnologia e Innovacion Tecnologica (under projects #198-2015-FONDECYT and #196-2015-FONDECYT), and INNOVATEPERU (under project #317-PNICP-EC-2014).
References
1. Castaneda CA, Castillo M, and Chavez I, et al (2019) Prevalence of Helicobacter pylori infection, its virulent genotypes, and Epstein-Barr virus in peruvian patients with chronic gastritis and gastric cancer J Glob Oncol 5 1–9 PMID: 31479342 PMCID: 6733198
2. Kuzushima K, Nakamura S, and Nakamura T, et al (1999) Increased frequency of antigen-specific CD8+ cytotoxic T lymphocytes infiltrating an Epstein-Barr virus–associated gastric carcinoma J Clin Invest 104(2) 163–171 https://doi.org/10.1172/JCI6062 PMID: 10411545 PMCID: 408473
3. Strong MJ, Xu G, and Coco J, et al (2013) Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: implications for possible immune adjuvant therapy PLoS Pathog 9(5) e1003341 https://doi.org/10.1371/journal.ppat.1003341 PMID: 23671415 PMCID: 3649992
4. Murphy G, Pfeiffer R, and Camargo MC, et al (2009) Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location Gastroenterology 137(3) 824–833 https://doi.org/10.1053/j.gastro.2009.05.001 PMID: 19445939 PMCID: 3513767
5. Chang MS, Uozaki H, and Chong JM, et al (2006) CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus Clin Cancer Res 12(10) 2995–3002 https://doi.org/10.1158/1078-0432.CCR-05-1601 PMID: 16707594
6. Castaneda CA, Castillo M, and Aliaga K, et al (2019) Level of tumor-infiltrating lymphocytes and density of infiltrating immune cells in different malignancies Biomark Med 13(17) 1481–1491 https://doi.org/10.2217/bmm-2019-0178 PMID: 31621387
7. Tsai KF, Liou JM, and Chen MJ, et al (2017) Distinct clinicopathological features and prognosis of Helicobacter pylori negative gastric cancer PLoS One 12(2) e0170942 https://doi.org/10.1371/journal.pone.0170942 PMID: 28152027 PMCID: 5289528
8. Oster P, Vaillant L, and Riva E, et al (2021) Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies Gut [https://doi.org/10.1136/gutjnl-2020-323392]
9. Zhang D, He W, and Wu C, et al (2019) Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer Front Immunol 10 71 [https://doi.org/10.3389/fimmu.2019.00071] https://doi.org/10.3389/fimmu.2019.00071 PMID: 30761139 PMCID: 6361780
10. Lee HE, Chae SW, and Lee YJ, et al (2008) Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer Br J Cancer 99(10) 1704–1711 https://doi.org/10.1038/sj.bjc.6604738 PMID: 18941457 PMCID: 2584941
11. Kulangara K, Zhang N, and Corigliano E, et al (2019) Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer Arch Pathol Lab Med 143(3) 330–337 https://doi.org/10.5858/arpa.2018-0043-OA
12. Lee K, Hwang H, and Nam KT (2014) Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer Gut Liver 8(2) 131–139 https://doi.org/10.5009/gnl.2014.8.2.131 PMID: 24672653 PMCID: 3964262
13. Fuchs CS, Doi T, and Jang RW, et al (2018) Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial JAMA Oncol 4(5) e180013 https://doi.org/10.1001/jamaoncol.2018.0013 PMID: 29543932 PMCID: 5885175
14. Hendry S, Salgado R, and Gevaert T, et al (2017) Assessing tumor infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinom Adv Anat Pathol 24(6) 311–335 https://doi.org/10.1097/PAP.0000000000000161 PMID: 28777143 PMCID: 5638696
15. Muro K, Bang YJ, and Shankaran V, et al (2015) Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012 Am Soc Clin Oncol [https://ascopubs.org/doi/abs/10.1200/jco.2015.33.3_suppl.3] https://doi.org/10.1200/jco.2015.33.3_suppl.3
16. Oh T, Kim N, and Moon Y, et al (2013) Genome-wide identification and validation of a novel methylation biomarker, SDC2, for blood-based detection of colorectal cancer J Mol Diagnostics 15(4) 498–507 https://doi.org/10.1016/j.jmoldx.2013.03.004
17. Network CGAR (2014) Comprehensive molecular characterization of gastric adenocarcinoma Nature 513(7517) 202–209 https://doi.org/10.1038/nature13480
18. Wang HC, Chen CW, and Yang CL, et al (2017) Tumor-associated macrophages promote epigenetic silencing of gelsolin through DNA methyltransferase 1 in gastric cancer cells Cancer Immunol Res 5(10) 885–897 https://doi.org/10.1158/2326-6066.CIR-16-0295 PMID: 28835422
19. Kim Y, Rhee YY, and Wen X, et al (2019) Combination of L1 methylation and tumor-infiltrating lymphocytes as prognostic marker in advanced gastric cancer Gastric Cancer 23(3) 464–472 https://doi.org/10.1007/s10120-019-01025-8 PMID: 31691036
20. Hanahan D (2022) Hallmarks of Cancer: new dimensions Cancer Discov 12(1) 31–46 https://doi.org/10.1158/2159-8290.CD-21-1059 PMID: 35022204
21. Kawazoe A, Kuwata T, and Kuboki Y, et al (2017) Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein–Barr virus status in a large cohort of gastric cancer patients Gastric Cancer 20(3) 407–415 https://doi.org/10.1007/s10120-016-0631-3
22. Zhou W, Zhang Y, and He F, et al (2020) Abundance of CD163-positive tumor-associated macrophages in the early gastric cancer predicts the recurrence after curative resection Dig Dis 38(6) 458–465 https://doi.org/10.1159/000506122 PMID: 32721976
23. Yan Y, Zhang J, and Li JH, et al (2016) High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial–mesenchymal transition in gastric cancer Onco Targets Ther 9 3975–3983 https://doi.org/10.2147/OTT.S103112 PMCID: 4935103






