Cervical cancer: a meta-analysis, therapy and future of nanomedicine
Jeaneen Venkatasa and Moganavelli Singhb
Nano-Gene and Drug Delivery Group, Discipline of Biochemistry, School of Life Sciences, University of KwaZulu-Natal, Private Bag X54001, Durban 4000, South Africa
ahttps://orcid.org/0000-0001-5061-0788
bhttps://orcid.org/0000-0002-9985-6567
Abstract
Cervical cancer is one of the leading causes of female death, with an annual mortality rate exceeding 200,000 in developing communities. Despite the past decade bearing witness to a reduction in cervical cancer cases throughout developed countries, the prevalence in developing countries continues to rapidly rise. The increase in cervical cancer cases is attributed to the lack of financial resources and the unavoidable risk factors of the disease. Traditional means of anticancer therapy are compromised by reduced drug potency, non-specificity, negative side effects and the development of multiple drug resistance (MDR), which leads to a decrease in the long-term anticancer therapeutic efficacy. Recent advances in nanomedicine have elucidated the potential of nanoparticles to reduce the side effects and improve the survival rate of patients, by enhancing selective delivery and uptake of photosensitive, therapeutic and genetic material to cervical cancer cells, thereby enhancing antitumour efficiency. This review paper analyses the risk factors and epidemiology of cervical cancer globally, especially in developing communities, whilst demonstrating the enhanced anticancer treatment using selected nanoparticles.
Keywords: cervical cancer, nanotechnology, nanomedicine, therapeutics, risk factors
Correspondence to: Moganavelli Singh
Email: singhm1@ukzn.ac.za
Published: 24/09/2020
Received: 08/05/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer produces one of the highest mortalities worldwide, with cervical cancer being the second most common malignancy amongst women. Cervical cancer is a growing health concern, with a global estimate of 570,000 novel cases and 311,000 deaths annually [1–3]. Despite the prevention of the disease by screening and treatment of pre-cancerous lesions, cervical cancer is the most common cause of cancer mortality amongst women [4]. The identification of key risk factors plays a fundamental role in cervical cancer prevention. Numerous studies have demonstrated the association between several risk factors and cancer [5–8].
An association between cervical cancer survival rate and the socioeconomic status of women has been reported [9]. In addition, venereal diseases, reproductive factors, long-term oral contraceptives and behavioural issues such as smoking and obesity have also been identified as risk factors for the disease [10]. High-risk human papillomavirus (HPV) infections have been established as the primary risk factor for the development of cervical cancer with HPV 16 and 18 being declared to be the cause of 71% of cervical cancer cases within the African continent [5].
Over the years, various treatment strategies have been developed for cervical cancer, including radiotherapy, chemotherapy and, in extreme cases, surgery [1]. However, these therapies are limited by a lack of anticancer drug potency, non-specificity, negative side effects and the development of MDR, which leads to a decrease in the long-term efficacy of anticancer therapy [11]. Nanotechnology has the potential to overcome these limitations, by increasing the selectivity and potency of chemical, physical and biological approaches for eliciting cancer cell death whilst minimising collateral toxicity to non-malignant cells [12, 13]. The favourable and unique properties of nanoparticles can improve the delivery of therapeutics, thereby enhancing their activity in cervical cancer cells whilst reducing harmful side effects in healthy cells [14, 15]. Hence, this meta-analysis aims to review the potential of nanomedicine in the improvement of cervical cancer therapeutics, especially in developing countries.
Literature search criteria
To assess the potential of nanomedicine in cervical cancer therapeutics, several databases from the National Centre for Biotechnology Information, WorldCat.org and National Library of Medicine were searched from 2013 to March 2020 for studies throughout the world which were published in English. The literature was limited to case-controlled studies, randomised controlled trials and observational studies, with comparison groups addressing the incidence and mortality rate of cervical cancer in women aged 15–70, and risk factors including reproductive and behavioural factors and the economic status of a country. These databases were further searched to identify and compare both novel and traditional means of cervical cancer therapies. A total of 111 research articles that followed a consistent, precise and direct design, with minimised bias according to the PRISMA guidelines, were selected. The search terms were developed using a combination of the following keywords, cervical cancer, nanomedicine, nanoparticles, cervical cancer therapy, incidence and mortality rates, apoptotic pathways and risk factors.
The burden of cervical cancer
Cancer cells modify the natural pathways of healthy cells enabling them to survive in harmful environments. These modified cells allow for metastasis and uncontrollable invasion of surrounding cells, inhibiting the normal function of fundamental organs. Cervical cancer results from a malignancy in the interior cervical lining and junction between the vagina and uterus [16]. Cervical cancer is primarily classified by histologic abnormalities, namely squamous cell carcinoma (SCC) and adenocarcinoma. The former accounts for 70%, whereas the latter accounts for 18% of cervical cancer cases. The remaining cases of cervical carcinomas consist of adenosquamous (4%) and additional carcinomas or malignancies (6.5%) [17, 18].
SCC develops within the transformation zone, located between cervical columnar and squamous cells, and migrates to the distal endocervical canal from the exocervix with an age-related progression [19]. Adenocarcinoma develops within the endocervix mucus glands [18]. Cervical cancer within the neuroendocrine is aggressive and often misdiagnosed and rare [20]. Cervical melanomas result from the migration of metastasised lesions from any other part of the body of all susceptible women, whereas cervical adenoid cystic carcinomas are mostly present in elderly women in the early stages of diagnosis [21]. Cervical lymphoma is another rare type of cervical cancer that occurs within the lymph nodes in the cervical area [22].
The development of cervical cancer is preceded by precancerous changes within the cervix. Precancerous lesions are referred to as squamous intraepithelial lesions (SIL) or cervical intraepithelial neoplasm (CIN). CIN is categorised into three broad categories, namely CIN1 (mild dysplasia), CIN2 (moderate dysplasia) and CIN3 (severe dysplasia) [23, 24]. It was reported that CIN1 and CIN2 were more likely to regress than progress, with a less than 1% progression rate from mild to severe dysplasia [25]. These results were confirmed by other researchers who also indicated that higher-grade lesions of CIN2 and CIN3 are more likely to progress to invasive carcinomas, with CIN3 displaying a progression rate of 31.1% [26].
The severity and site of infection denote the various stages of cervical cancer [27]. The initial stage, stage 0 or cervical carcinoma in situ, occurs within the upper cervical cell layer. Although stage 0 cervical cancer may develop malignancies if left untreated, it is not regarded by many as cancer [28]. Stage 1 is limited to the cervix only and is divided into two groups, stage 1A (5–7 mm) and 1B (>7 mm) with regards to the size of cancerous tissue. Cancer progresses from stage 1 to 2 when it begins to spread from the cervix into the upper vagina.
Similar to stage 1, stage 2 is divided into two categories, namely, stage 2A cervical cancer which spreads to about two-thirds of the vagina and stage 2B which progresses within the vagina to the surrounding tissues of the uterus [29]. Stage 3A cervical cancer spreads to the lower vagina and surrounding lymph nodes and is followed by stage 3B, whereby cervical cancer spreads to the pelvic wall. At this stage, the large size of tumour blocks the ureters causing the kidney to enlarge or even cease to function. Stage 4 is the most crucial stage, whereby cancer has the potential to spread to the rectum, bladder, intestinal tract and lungs [30, 31]. Cervical cancer prevention and control are targeted at the epidemiological studies describing the distribution and incidence of cervical cancer whilst accessing risk factors.
Epidemiology of cervical cancer
Global distribution and incidence rates of cervical cancer
Cervical cancer is the second most common malignancy worldwide, with the incidence and mortality rate varying with geographical location (Figure 1) [2, 3]. Cervical cancer incidence and mortality rate in high-income countries have halved over the past 30 years due to the introduction of formalised screening programmes [30]. Sriplung et al [32] reported the incidence of cervical cancer across five continents. Although the authors postulated a decrease in the incidence rate in high-income countries, an increase in newly diagnosed cervical cancer cases was observed in low-income, developing countries.
Bray et al [2] depicted the inverse correlation between a country’s economic status and incidence of cervical cancer, whereas prevalence increased as economic status decreased. Ferlay et al [33] estimated that for every 50% of women screened in developed countries, only 5% are screened in developing countries. Arbyn et al [16] reported the lowest cervical cancer incidence and mortality rate to occur in high-income, developed countries. The authors stipulated the lowest incidence rates of cervical cancer (5,092 per 100,000) to occur in western Asia, whereas the lowest mortality rate was observed in Australia–New Zealand (403 per 100,000).
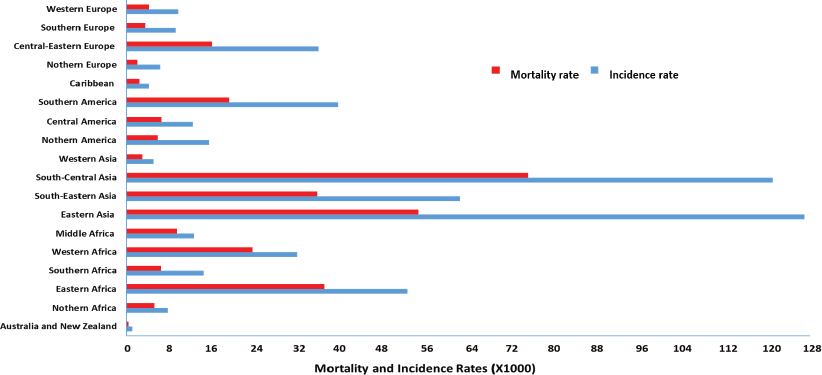
Figure 1. Global mortality and incidence rate of cervical cancer (per 100,000 individuals) [2, 34–38].
Eighty percent of cervical cancer cases occur in the developing world, in countries such as Switzerland (52/100,000) and Haiti (93/100,000) [36, 37]. The cervical cancer mortality rate in high-to-low human development index countries was reported to be 6.8 per 100,000 individuals [36] and a leading cause of incidence and mortality in Indian women with 122,844 novel cases and 67,477 deaths annually [38]. In 2015, approximately 98,900 new cases were reported in China, which accounted for 18.7% of the global incidence [39]. It was postulated that in rural China, which contains more than 70% of its population, 90% of women diagnosed with cervical cancer die within a 5-year period [40], making cervical cancer a major health concern in China.
Southern African countries bear a highly disproportionate incidence (92,000) and mortality (60,098) of cervical cancer [1, 2]. Similar results were reported revealing the incidence of cervical cancer to vary within Sub-Saharan Africa, with Malawi having the highest incidence of >72.9 per 100000 and mortality rates of 49.8 per 100,000 [41]. Conversely, in Nigeria, the cervical cancer incidence rate is 27.1/100,000 as a result of the National Cancer Control Policy implemented by the government to improve and control cancer, including cervical cancer [42].
The variation in socioeconomic status amongst different regions of the world plays a fundamental role in the development and implementation of screening programmes. An inadequate screening of cervical cancer in low-resource or developing countries is attributed to reduced health care due to financial constraints, lack of resources, inadequate knowledge of cervical cancer, healthcare infrastructure, laboratory supplies, trained practitioners and patient management guidelines and cultural and socioreligious barriers preventing routine pelvic screening [9, 43]. Like many cancers, the incidence and mortality rate discrepancies of cervical cancer in different regions are attributed to these risk factors.
Cervical cancer risk factors
Epidemiological studies have identified several risk factors for cancer. Lifestyle factors such as reproduction (parity, pregnancy and contraceptives), obesity, diet, smoking and alcohol consumption have been included as major contributors to the development of cancer. Accessing the mechanism of each risk factor allows researchers to modify their intrinsic and extrinsic effects and effectively reduce the incidence of various cancers [10]. Cervical cancer prevalence begins to increase in women aged 18–29 years, peaks in women aged 48–64 and decreases in women older than 65 years [44].
Venereal diseases
Genetic factors play an essential role in the incidence of cervical cancer [35]. An important etiological factor for cancerous cervical lesions is a highly prevalent, sexually transmitted infection, HPV. HPV detection and vaccination measures need to be implemented as a means of cervical cancer prevention. Whilst 90% of HPV-induced lesions disappear within 6–12 months, others progress into cancer [35], with HPV types 16, 18, 45, 33, 35, 52, 51 and 31 associated with cervical cancer [45].
High-risk HPVs 16 and 18 account for 70% of novel cases globally [46], with a reported 71% of cervical cancer cases in Africa. The higher HPV prevalence in females compared to males has been reported to be due to a larger surface area within the epithelium of the cervix enduring squamous metaplasia and lack of certain adaptive immune responses observed in males. HPVs 16 and 18 induce carcinogenesis by oncoprotein E6 and E7 activities, which inhibit the function of p53 and retinoblastoma tumour suppressor genes [5]. These oncoproteins are associated with changes in the methylation of both the host and viral DNA by altering cellular pathways that regulate cell adhesion, genetic integrity, cellular control, immune response and, most importantly, apoptosis [35, 47].
Cervical cancer forms a large portion of opportunistic diseases with an increased susceptibility of human immunodeficiency virus (HIV)-infected women to high-risk HPV-related cancers [36, 48]. HIV-1 increases the severity of lesions and the proportion of cervical cancer cases [49, 50], by attacking the CD4 or T-helper cells of the immune system and hijacking the cell’s replication machinery to replicate its own DNA. As HIV destroys more CD4 cells, the individual’s immune system becomes weak and unable to fight secondary diseases [51]. HIV may be directly involved in the oncogenesis or indirectly, due to immunosuppression [52]. HIV-positive females are at a higher risk of developing SIL which rapidly progresses to cervical cancer, which is aggravated in immunosuppressed individuals (Figure 2) [48]. HIV-1 increases the expression of HPV oncoproteins E6 and E7, which bind to and degrade the tumour suppressor proteins [49].
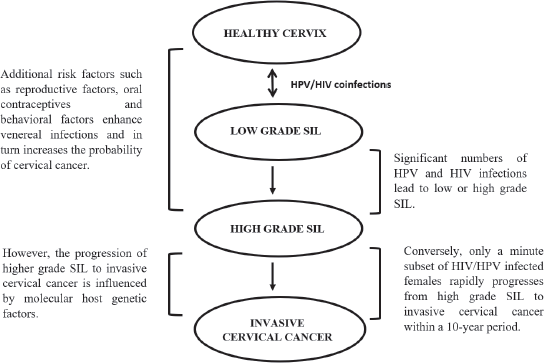
Figure 2. The influence of venereal diseases, HPV and HIV infections on cervical cancer development [5, 35, 47, 48, 52].
Reproductive factors
Cervical cancer is also linked to an increased susceptibility of HPV infections in individuals with multiple sexual partners [53]. A multivariate analysis model demonstrated that an earlier age of sexual debut and a higher parity is associated with a high-risk HPV infection [5]. Numerous studies have illustrated a direct correlation between parity and the prevalence of cervical cancer, whereby the age of a female’s first pregnancy is inversely associated with their susceptibility of cervical cancer [7, 8, 54]. Parity is regarded as the number of times an individual gives birth to a foetus within a 24-week gestational period [55]. It was illustrated that women who had a higher parity (greater than 3 pregnancies) were more likely to obtain invasive cervical cancer (odds ratio 2.1–2.5), compared to those with fewer pregnancies [8, 55]. It was further reported that delivery was a major predictor of CIN 3 and was significantly higher in women with HPV-infections and that parity influenced the quantity of oestrogen within a fertile woman, whereas oestradiol enhanced the immortalisation of HPV-infected cells [56].
Pregnancy may also produce some dysplastic lesions of the cervix, which may regress, persist or progress to produce a carcinoma [55, 57]. Despite the alarming evidence that parity is a predictor of cervical cancer, no positive relationship between parity and HPV has been established. Contradictory, the authors observed a higher parity in cervical cancer patients who were infected with HPV compared to cervical cancer patients who were not infected with HPV. However, it was suggested that the relationship between parity and cervical cancer is the result of sexual activity as a cofounding factor and may be eliminated with the use of contraceptives [58].
Oral contraceptives
From the late 1950s to the present day, billions of women have been utilising steroid hormones as a means of contraception. Oral contraceptives are synthetic hormone-containing drugs that are ingested to prevent pregnancy by inhibiting ovulation [54]. Several studies have depicted the strong correlation between cervical cancer and long-term oral contraceptive use [7, 54, 59, 60]. Naturally occurring oestrogen and progesterone have been observed to stimulate the growth and development of cancer cells. Hence, the introduction of additional synthetic hormones will accelerate cell growth and increase the risk of cancer [59]. It has been reported that the incidence of cervical cancer doubled in individuals who had consumed steroid hormones for longer than 5 years, with an increase in invasive cervical cancer prevalence in individuals taking injectable progesterone [35, 57]. The additional hormones increase the incidence of cervical cancer by increasing the susceptibility of high-risk HPV infections [59]. However, despite the increased incidence of unusual histological types in women who are long-term contraceptives users, the role of oral contraceptives in cervical cancer is still controversial [7, 60]. Hence, further studies on this association need to be undertaken.
Behavioural factors
The link between smoking and cervical cancer has been well elucidated, with smoking influencing the incidence of CIN 3 and invasive cervical cancer [6, 54, 61]. This positive correlation is due to the direct mutagenic effect of nicotine (decomposed product of cigarettes) on the DNA in squamous cells. Smoking also causes epigenetic changes within the epithelial cells contributing to the pathogenesis of cervical neoplasia [62]. Hence, smoker females are 2–4 times more susceptible to developing cervical cancer than non-smokers [57, 60]. It was also reported that the incidence of cervical cancer decreased dramatically (50%) in individuals who quit smoking for a 10-year period [54]. Smoking reduces the efficiency of an individual’s immune system, enhancing their susceptibility to HPV infections. Benzo[α]pyrene, a constitute of tobacco, upregulates the amplification of the HPV genome, thus enhancing the integration of the viral genomes into the host’s genome [62]. The long-term effects of nicotine in vivo include the stimulation of the vascular endothelial growth factor, persistent cell proliferation, apoptosis inhibition, suppression of T lymphocyte activity and immunoglobulin levels and increased microvessel density [61].
The hormonal changes observed in an obese individual is positively correlated with the incidence of cervical cancer, whereby females with a body mass index (BMI) over 30 displayed a 2-fold higher vulnerability to cervical adenocarcinoma than individuals with a normal or slightly overweight BMI (≤25) [63]. This results from the conversion of androgen to oestrogen within the peripheral adipose tissue. The additional oestrogen will accelerate cell growth and increase the risk of cancer as observed with oral contraceptives [64].
Traditional treatment limitations
Cervical cancer treatment is traditionally determined by the stage and extent of the disease. There are essentially three types of standard cervical cancer treatments, including surgery, radiation and chemotherapy. Surgical treatment consists of a radical hysterectomy with pelvic lymphadenectomy (RHL) and conisation for stage 1 and certain stage 2 cervical cancer cases to abrogate the side-effect on fertility [65, 66]. Surgery involves the physical removal of the metastatic tissue from the cervix or cervical canal [66]. Although cervical cancer surgery is minimally invasive, a complete pelvic lymphadenectomy is associated with lymphocele, pelvic nerve impairment and lymphoedema. This anticancer method can cause damage to the sympathetic and parasympathetic branches of the autonomous innervation and blood supply of pelvic organs, leading to colorectal dysfunction [65].
Radiotherapy attempts to destroy cancer cells utilising various forms of radiation including high energy X-rays. Radiotherapy is administered in the advanced phases of stage 1 cervical cancer as a primary, radical curative. This form of therapy consists of brachytherapy, concomitant cisplatin and external beams of radiotherapy [67]. Postoperative, adjuvant radiotherapy aimed to reduce the reoccurrence of cervical cancer is administered to tumours larger than 4 cm with lymph node metastases [68]. Post-radical therapy individuals with adverse histopathologic influences are required to undergo adjuvant pelvic radiation or chemoradiation [69]. Deep cervical stromal invasion, metastatic disease in regional nodes, parametrial extension and positive surgical margins serve as risk factors for the reoccurrence of cervical tumours. Radiotherapy is limited by the induction of vaginal discomfort and dryness, rectal bleeding and stenosis, radiation cystitis, menstrual changes and lymphoedema [70].
Chemoradiation is the preferred method of treatment as it enhances the anticancer properties of radiation. Chemotherapy inhibits the growth of cervical cancer cells by preventing their active division and growth with the aid of chemotherapeutic compounds, such as carboplatin, cisplatin, methotrexate, paclitaxel and topotecan [69, 71]. Although it is able to treat advanced cervical cancer which has spread to subsequent organs, this form of therapy is limited by its detrimental side effects [72]. Several studies have illustrated a higher survival rate in women treated with chemotherapy followed by radiation, as a result of the selection of cross-resistant tumour cells which delay the initiation of the curative therapy [69, 70]. The sensitivity of chemotherapeutic drugs is limited by their inability to distinguish cancer cells from their non-metastatic counterparts; hence, the drugs also inhibit the functioning of healthy cells [71]. Although most side effects are short-term, such as fatigue, hair loss, nausea and loss of appetite, the long-term effects negatively impact the quality of life of the patients [72]. These include anaemia, neutropenia, thrombocytopenia due to blood marrow cell damage, neuropathy or peripheral neuropathy due to nerve damage (cisplatin and paclitaxel), nephrotoxicity or kidney damage, premature menopause and, in severe cases, infertility [73].
These cervical cancer treatment approaches lack satisfactory outcomes despite the rapid elucidation of biological and the etiological understanding of HPV and cervical cancer. The main limitation lies within their side effects, which, in turn, limits their optimal dosages and efficiency. Hence, it is fundamental to develop novel strategies to treat pre-invasive and invasive cervical cancer [11]. Advances in nanomedicine have the potential to overcome these limitations by improving the management and treatment of cervical cancer. Nanomedicine aims to improve the survival rate of cancer patients by reducing side effects and enhancing the selective delivery of drugs to tumour tissues and uptake of therapeutic compounds, thus increasing antitumour activity [14].
Nanomedicine
The past decade has elucidated the potential and application of nanotechnology in various aspects of day-to-day life. The use of nanotechnology in pharmaceutical research and development of novel medical approaches has been precluded as a key enabling technology, which enhances the development of innovative medical solutions to address unfulfilled medical needs [74, 75]. The application of nanotechnology for medical purposes has been termed as nanomedicine and is defined as the use of nanomaterials to diagnose, treat, monitor and control diseases [12].
Nanoparticles are classified according to their particle size, surface area and particle size distribution (PSD). Size is the most fundamental characteristic in nanotechnology. Nanoparticles usually range between 1 and 100 nm; however, the size varies with respect to the function, physicochemical and biological characteristics of the nanomaterial utilised [76, 77]. Size is controlled by the ‘bottom-up’ and ‘top-down’ techniques, by which the particle increases to the desired size from an atomic state or is produced by the breakdown of a bulk material into smaller pieces using chemical or mechanical energy [78]. Boverhof et al. [78] further reported the importance of surface area (approximately 60 m2/cm3) and PSD due to the polydispersion of nanomaterials.
Nanomedicine has the potential to overcome the limitations of conventional therapeutic approaches by improving the targeted delivery of therapeutics to tumour sites and enhancing efficiency [13]. Numerous studies have elucidated the potential of nanomedicine in cervical cancer therapy, showing that nanoparticles increased the therapeutic index with reduced side effects by using the passive targeting approach which causes nanocomplex accumulation in tumours [14, 77].
Medical properties of nanoparticles
Nanomedicine is categorised into three fundamental branches, namely regenerative medicine, nanodiagnosis (diagnosis) and nanotherapy (controlled drug delivery). A fourth emerging category, which combines therapy and diagnostics, termed theranostics displays a promising approach for future cancer therapy [79, 80]. Nanomedicine has revolutionised clinical practices by exploiting multiple mechanisms of effective molecules which could not be utilised to date due to their toxicity, maximising efficacy and reducing harmful side effects, toxicity and required dose [12–14]. Nanoparticles provide a preferential distribution throughout the body by facilitating targeted, controlled and site-specific drug delivery, in addition to improving movement across biological barriers [80].
The compact, small size of nanoparticles offers a high specific surface area with relation to volume, thus resulting in an increase in the surface energy of the particle and making the nanomaterial more reactive [76]. Dynamic changes in the plasma biomolecule adsorption layer surrounding the surface of the nanoparticle, referred to as the ‘corona’, facilitate the transition of the nanomaterial across biological barriers, such as the gastrointestinal and lung fluid, and blood [81, 82]. Nanoparticles also possess variable sizes, shapes, chemical compositions and magnetic, optical and electric properties [83].
The physiopathological nature of the diseases in question and the interaction between the biological fluid of the environment and surface of the nanomaterial must be taken into consideration when designing biologically effective nanoparticles [76, 82]. Understanding the biological processes behind specific diseases enables researchers to design nanomaterials which perform site-specific action on misfolded proteins, mutated genes and infections caused by microorganisms [83]. The interaction between the nanoparticle and the cell is determined by the characterisation of the corona’s biomolecules. This interaction is influenced by the size, surface area, shape, chemistry, energy, porosity, hydrophobicity, presence of ligands and valence and conductance states of the nanomaterials [81, 84]. The intrinsic properties of nanomaterials are highly advantageous in the development of pharmaceutical therapies, including cancer treatment [79].
Induction of apoptotic pathways for cervical cancer
Apoptosis is defined as programmed cell death through biochemical mechanisms [85]. Apoptosis is a vital instrument in the functioning and development of the embryo and the immune system, hormone-dependent atrophy and normal cell turnover [86]. In cancer therapy, the apoptosis of metastatic cells can be induced by inhibiting essential cellular pathways. These include intrinsic pathways that target the mitochondria or extrinsic pathways that involve tumour necrosis factor (TNF) receptors and extrinsic signals (Figure 3) [87].
In intrinsic pathways, the p53 protein increases the production of the apoptosis regulator Bcl-2 homologous antagonist (BAK) and bcl-2-like protein 4 (BAX), which, in turn, increases the mitochondrial membrane permeability and the leakage of cytochrome c [85]. Cytochrome c then forms an apoptosome protein complex by binding with apoptotic protease activating factor-1 (Apaf-1), procaspase 9 and adenosine triphosphate. Cleavage of the pro-caspase from the apoptosome produces active caspase 9 proteins, which activate caspase 3 leading to cell death [11]. In extrinsic pathways, apoptosis is induced by a TNF model or first apoptosis signal (Fas) ligand model. The receptors of both these models bind to the TNF receptor-associated death domain and the Fas-associated death domain protein, respectively, to activate caspases 8, which induces the caspase 3 production and cell death [87].
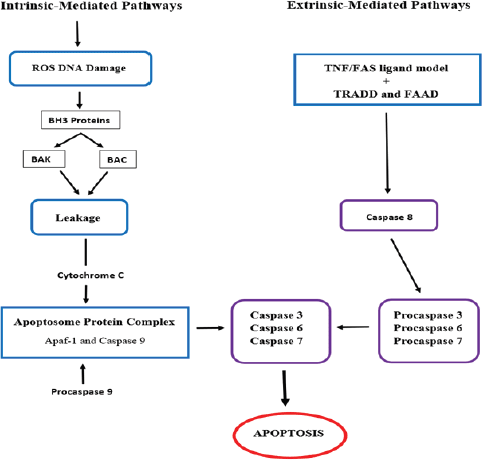
Figure 3: Targeted intrinsic and extrinsic pathways for cervical cancer treatment [11, 85, 87].
Nanoparticles have been used to deliver anticancer therapeutics which initiate the apoptotic pathway in cancer cells. Nanoparticles coupled with apoptotic pathway stimulators reduce the stimulator’s toxic side effects whilst enhancing cytotoxicity [86]. Silver nanoparticle-bound carboplatin was observed to stabilize the DNA-topoisomerase I, resulting in the accumulation of breaks in the DNA, inhibiting replication and transcription and leading to HeLa cell death at significantly higher toxicity and lower concentrations compared to carboplatin [88].
Targeting mitochondrial apoptotic pathways has also been studied for cervical cancer therapy [11, 87]. The ability of mesoporous silica nanoparticles carrying cytochrome c was shown to effectively induce apoptosis in cervical cancer cells by targeting mitochondrial apoptotic pathways [89]. The cytotoxic and apoptotic effect of silver nanoparticles (AgNPs) in HeLa cells was demonstrated [90]. AgNPs upregulated the mRNA expression of BAX, P53 and caspase 3 in AgNP-treated HeLa cells by targeting the mitochondrial apoptotic pathways. Nanomedicine has revolutionised gene therapy, chemotherapy and photodynamic therapy by improving the delivery of insoluble drugs, circulation, direct targeting or delivery of drugs, delivery of multiple drugs and transcytosis of the drugs across endothelial and epithelial barriers and imaging [12, 91].
Gene therapy
Gene therapy modifies the human genome by introducing exogenous genetic material into a cell to treat the underlying cause of a disease [92]. Cancer gene therapy aims to enhance the sensitivity of tumours towards chemotherapeutic drugs and radiation therapies or induces apoptosis by the introduction of suicide or wild-type tumour suppressor genes [93]. Although gene modification has successfully improved cervical cancer therapy through alterations in the cellular signalling pathways, the uptake of genetic cargo by cells is limited [94]. Genetic cargo such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) is unable to effectively cross epithelial membranes due to their negative charge, susceptibility to degradation and large size [95], hence the need for suitable nanocarriers of these nucleic acids.
Nanoparticles serve as vehicles by facilitating the binding, condensation and delivery of small interfering RNA (siRNA), messenger RNA (mRNA), microRNA (miRNA) and plasmid DNA (pDNA), to the target sites [96-98]. A targeting ligand is generally attached to a nanocarrier to improve the sensitivity and selectivity of uptake of the genetic cargo by the cancer cells. An increase in transfection efficacy of DNA containing nanoparticles with folic acid moieties over naked DNA was observed in HeLa (cervical cancer) cells [99], whereas efficient luciferase gene silencing using siRNA-selenium nanocomplexes was observed in HeLa-tat-Luc cells [98].
Chemotherapy
Chemotherapy remains a standard approach to treat cervical cancer. However, the efficiency of conventional chemotherapeutic drugs is limited by their undesirable and severe side effects, lack of specificity, rapid in vivo degradation, poor biodistribution, suboptimal concentrations and intervals between treatments [15]. Common cervical cancer chemotherapeutic drugs include cisplatin, paclitaxel and methotrexate. Cisplatin disrupts biological functions and apoptosis of the cancer cells by binding and crosslinking to the DNA of cells. However, this drug has been observed to induce renal failure and neurotoxicity [100]. Other drugs, such as methotrexate, decrease the conversion of dihydrofolates to tetrahydrofolates required for RNA and DNA replication, by inhibiting dihydrofolate reductase and stimulating neutropenia and stomatitis [101].
Nanoparticles can improve the efficacy, bioavailability and distribution of the chemotherapeutic drugs to cancerous tissues whilst reducing side effects and cytotoxicity in healthy tissue, by creating a targeted drug delivery system [88]. Nanoparticles are able to bind and transport drugs across physiological barriers that limit traditional drug delivery systems [16]. The coating of nanoparticles with a polymer layer (e.g., polyethylene glycol) inhibits the binding of proteins in vivo and allows the nanoparticles to evade immune responses induced by the reticuloendothelial system and macrophages, which eliminate foreign bodies from the blood system [101].
Silver nanoparticles carrying carboplatin (a platinum-based drug) was shown to increase cytotoxicity in HeLa cells compared to using carboplatin alone, with a further reduction of undesired side effects, improvement in pharmacokinetics and synergism and suppression of drug resistance through distinct mechanisms of action at a low dose [88]. Improved efficiency in cervical cancer treatment using several proteins, polymers and lysosome nanocomplexes containing cisplatin, paclitaxel and methotrexate was also reported [11]. The ability of chitosan and methoxypolyethylene glycol-coated nanoparticles were seen to effectively deliver methotrexate both in vivo and in vitro and to reduce HeLa cell proliferation [103]. Although polyphenols are promising anti-cervical cancer agents, their clinical application is hindered by their poor solubility and low oral bioavailability [15].
Photodynamic therapy
Photodynamic therapy (PDT) induces apoptosis of cancerous cells using light-sensitive compounds that react with biological compounds under selective lighting [14]. As a minimally invasive anticancer therapy method, photodynamics is considered as a promising modality for the ablation of tumour cells in a variety of cancers, including cervical cancer [14]. PDT involves the use of a non-toxic near-infrared light (620 nm and 850 nm) which has maximum tissue permeability and a photosensitizer to improve anticancer effects [99]. Exposure to light stimulates the activation of the photosensitizers which are reduced to a photosensitizers’ triplet, which further reacts with oxygen to produce reactive oxygen species. These cytotoxic molecules induce a series of biological reactions which eventually lead to the death of the cancer cells [102].
Most photosensitizers occur naturally in plant extracts such as hypericin (H. perforatum, 514–593 nm), thiophenes (E. latifolius Tausch, 225 and 400 nm) and curcumin (Curcuma longa, 350 to 450 nm). Although these extracts are capable of treating cancer cells, the compounds have poor solubility properties and are ineffective in clinical applications [103]. Lipid nanocapsules embedded with hypericin were found to improve the solubility and efficiency of the compound in HeLa cells [104].
Gold nanoparticles have shown potential in the transport of protoporphyrin IX, a heterocyclic organic photosensitive compound, to HeLa cells [14]. These gold nanoparticles increased cytotoxicity and produced higher reactive oxygen species by absorbing radiation at 630 nm. Furthermore, gold nanoparticle-mediated 2-mercapto-5-nitro benzimidazole (MNBI) was also reported to increase cytotoxicity in cervical cancer cells. MNBI produces nitric oxide (NO), which is toxic to healthy cells; however, the MNBI nanocomplex mediated delivery directly to the HeLa cells minimising harmful side effects on healthy cells [105].
Dual-role nanoparticles
Nanoparticles often enhance cancer therapy by serving a dual role, whereby the particle can carry as well as co-deliver multiple anticancer drugs, including genetic material, photosensitising molecules, proteins and chemotherapeutic drugs. Polymeric nanoparticles which co-delivered siRNA and cisplatin were shown to effectively decrease the viability of cervical cancer cell lines in vitro [106]. PLGA magnetic nanoparticles were also used to co-deliver curcumin and 5-fluorouracil to cancer cells [107], whereas methotrexate and 5-fluorouracil-loaded layered double hydroxide produced enhanced cytotoxicity in cervical cancer cells compared to the drugs alone [108]. The potential of a siRNA, anti-MCL 1 and anti-E7 cocktail against oncoproteins E6 and E7 and anti-apoptotic protein MCL-1 against HPV-related cervical cancer was also noted [109]. Besides carrying and delivering therapeutics, some nanoparticles themselves exhibit anticancer properties.
The concept of gold nanoparticles was envisaged over 100 years ago by Faraday and are popular in nanomedicine [110]. They have been utilised as delivery vehicles for anticancer therapeutics due to their favourable and unique characteristics which include biocompatibility, small size and dispersive shapes, strong surface plasmon resonance and high dispersity. They also display antibacterial properties [90], as well as antiproliferative properties towards certain cancer cell lines [107]. The anticancer properties of gold nanoparticles in HeLa cells have been reported [110]. Silver nanoparticles have also illustrated antibacterial and anticancer properties by targeting and inhibiting the nuclear DNA-dependent serine/threonine-protein kinase (DNA-PKcs) and c-Jun N-terminal kinases causing extensive damage to the telomeres in cancer cells [21]. Selenium nanoparticles possess antioxidant and cytotoxic activity in cancer cells, high bioavailability and low toxicity, in addition to serving as delivery vehicles for anticancer compounds [111]. Nanoparticles have also shown their potential for combination therapy further enhancing their anticancer potential in the treatment of cervical cancer.
Conclusion
Cervical cancer remains a global health crisis with several common and unavoidable risk factors. Despite the rapid elucidation of the biological and etiological understanding of HPV and cervical cancer, traditional treatments, such as surgery, radiation and chemotherapy, lack satisfactory outcomes due to numerous limitations. Nanomedicine has revolutionised and can extensively improve cervical cancer therapy by enhancing the targeting of apoptotic pathways. The low toxicity, biocompatibility, photosensory and anticancer properties of nanoparticles, together with their potential for dual treatment by acting synergistically with its payload, make them ideal platforms for cervical cancer treatment. This review serves to enhance the understanding and need for these non-toxic, safe, stable delivery systems, which are capable of protecting their therapeutic cargo against degradation and offering high therapeutic indices. The reduced cost implications in particular will be attractive to low-income and developing countries. However, nanomedicine in cervical cancer therapy is still in its infancy, and further clinical studies need to be undertaken to fully appreciate its benefits.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank the NRF South Africa-BRICS funding for financial support (Grant No: 120455).
References
1. Small Jr W, Bacon MA, and Bajaj A, et al (2017) Cervical cancer: a global health crisis Cancer 123 2404–2412 https://doi.org/10.1002/cncr.30667
2. Bray F, Ferlay J, and Soerjomataram I, et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68 394–424 https://doi.org/10.3322/caac.21492 PMID: 30207593
3. Koh WJ, Abu-Rustum NR, and Bean S, et al (2019) Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology J Natl Compr Canc Netw 17 64–84 https://doi.org/10.6004/jnccn.2019.0001 PMID: 30659131
4. Aggarwal P (2014) Cervical cancer: can it be prevented? World J Clin Oncol 5 775–780 https://doi.org/10.5306/wjco.v5.i4.775 PMID: 25302177 PMCID: 4129540
5. Ginindza TG, Dlamini X, and Almonte M, et al (2017) Prevalence of and Associated Risk Factors for High Risk Human Papillomavirus among Sexually Active Women, Swaziland PLoS One 12 1371–1389 https://doi.org/10.1371/journal.pone.0170189
6. Sugawara Y, Tsuji I, and Mizoue T, et al (2019) Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women Jpn J Clin Oncol 49 77–86 https://doi.org/10.1093/jjco/hyy158
7. Asthana S, Busa V, and Labani S (2020) Oral contraceptives use and risk of cervical cancer – a systematic review & meta-analysis Eur J Obstet Gyn R B 247 163–175 https://doi.org/10.1016/j.ejogrb.2020.02.014
8. Weng Q, Jiang J, and Haji FM, et al (2020) Women’s knowledge of and attitudes toward cervical cancer and cervical cancer screening in Zanzibar, Tanzania: a cross-sectional study BMC Cancer 20 63–75 https://doi.org/10.1186/s12885-020-6528-x
9. Akinyemiju T, Ogunsina K, and Sakhuja S, et al (2016) Lifecourse socioeconomic status and breast and cervical cancer screening: analysis of the WHO Study on Global Ageing and Adult Health (SAGE) BMJ Open 11 1275–1281
10. Momenimovahed Z, Tiznobaik A, and Taheri S, et al (2019) Ovarian cancer in the world: epidemiology and risk factors Int J Women’s Health 11 287–294 https://doi.org/10.2147/IJWH.S197604
11. Chen J, Gu W, and Yang L, et al (2015) Nanotechnology in the management of cervical cancer Rev Med Virol 25 72–83 https://doi.org/10.1002/rmv.1825 PMID: 25752817
12. Hira I, Saini RV, and Kumari R, et al (2018) Inspection and remedy of cervical cancer using nanoparticles Nanosci Nanotech Asia 8 186–192 https://doi.org/10.2174/2210681207666170323155831
13. Chaturvedi VK, Singh A, and Singh VK, et al (2019) Cancer nanotechnology: a new revolution for cancer diagnosis and therapy Curr Drug Metab 20 416–429 https://doi.org/10.2174/1389200219666180918111528
14. Juárez AAS, Alvarado EM, and Gallegos ER (2019) Cell death induced by photodynamic therapy with the conjugate of gold nanoparticles-PpIX in HeLa cell line AIP Conf Proc 209 4008–4012
15. Yadav N, Parveen S, and Banerjee M (2020) Potential of nano-phytochemicals in cervical cancer therapy. Clin Chim Acta 505 60–72 https://doi.org/10.1016/j.cca.2020.01.035 PMID: 32017926
16. Arbyn M, Weiderpass E, and Bruni L, et al (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis Lancet Glob Health 8 191–203 https://doi.org/10.1016/S2214-109X(19)30482-6
17. Bjurberg M, Holmberg E, and Borgfeldt C, et al (2019) Primary treatment patterns and survival of cervical cancer in Sweden: a population-based Swedish Gynecologic Cancer Group Study Gyn Oncol 155 229–236 https://doi.org/10.1016/j.ygyno.2019.08.022
18. Rivera-Colon G, Chen H, and Niu S, et al (2020) Cervical adenocarcinoma: histopathologic features from biopsies to predict tumor behavior Am J Surg Pathol 44 247–254 https://doi.org/10.1097/PAS.0000000000001379
19. Yang EJ, Quick MC, and Hanamornroongruang S, et al (2015) Microanatomy of the cervical and anorectal squamocolumnar junctions: a proposed model for anatomical differences in HPV-related cancer risk. Modern Pathol 28 994–1000 https://doi.org/10.1038/modpathol.2015.54
20. Gil Ibanez B, Regueiro P, and Llurba E, et al (2018) Challenges in the management of neuroendocrine cervical cancer during pregnancy: a case report Mol Clin Oncol 9 519–522 PMID: 30402233 PMCID: 6200965
21. Lim HK, Gurung RL, and Hande MP (2017) DNA-dependent protein kinase modulates the anti-cancer properties of silver nanoparticles in human cancer cells Mutat Res Genet Toxicol Environ Mutagen 824 32–41 https://doi.org/10.1016/j.mrgentox.2017.10.001
22. Vranic S, Cyprian FS, and Akhtar S, et al (2018) The role of epstein–barr virus in cervical cancer: a brief update Front Oncol 8 113 https://doi.org/10.3389/fonc.2018.00113
23. Saglam A, Usubütün A, and Dolgun A, et al (2017) Diagnostic and treatment reproducibility of cervical intraepithelial neoplasia/squamous intraepithelial lesion and factors affecting the diagnosis Turkish J Pathol 33 177–191
24. Lingappanoor S, Meesala V, and Manupati GR, et al (2019) Cervical cancer, an emerging health burden for womenhood Int J Med Sci Curr Res 92 151–160
25. von Knebel Doeberitz M, Meijer CJ, and Lorincz A, et al (2020) Infection to cancer—finding useful biomarkers for predicting risk of progression to cancer Human Papillomavirus 2020 269–282 https://doi.org/10.1016/B978-0-12-814457-2.00017-9
26. Siegler E, Reichman Y, and Kugelman N, et al (2019) Low-risk human papillomavirus types in cervical intraepithelial neoplasia 2–3 and in invasive cervical cancer patients J Low Genit Tract Dis 23 248–252 https://doi.org/10.1097/LGT.0000000000000486 PMID: 31592971
27. Longo D, Fauci A, and Kasper D, et al (2011) Harrison’s Principles of Internal Medicine 18th edn (McGraw-Hill Professional) p 322 [www.acessmedicine.com] Date accessed: 14/04/20
28. Zhang Q, Li W, and Kanis MJ, et al (2017) Oncologic and obstetrical outcomes with fertility-sparing treatment of cervical cancer: a systematic review and meta-analysis Oncotarget 8 46580 https://doi.org/10.18632/oncotarget.16233 PMID: 28418849 PMCID: 5542294
29. Cohen PA, Jhingran A, and Oaknin A, et al (2019) Cervical cancer Lancet 393 169–182 https://doi.org/10.1016/S0140-6736(18)32470-X PMID: 30638582
30. Landy R, Pesola F, and Castañón A, et al (2016) Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case–control study Br J Cancer 115 1140–1146 https://doi.org/10.1038/bjc.2016.290 PMID: 27632376 PMCID: 5117785
31. Fokdal L, Tanderup K, and Pötter R, et al (2019) Risk factors for ureteral stricture after radiochemotherapy including image guided adaptive brachytherapy in cervical cancer: results from the EMBRACE studies Intl J Radiat Oncol Biol Phys 103 887–894 https://doi.org/10.1016/j.ijrobp.2018.11.006
32. Sriplung H, Singkham P, and Iamsirithaworn S, et al (2014) Success of a cervical cancer screening program: trends in incidence in Songkhla, Southern Thailand, 1989–2010, and prediction of future incidences to 2030 Asian Pac J Cancer Prev 15 10003–10008 https://doi.org/10.7314/APJCP.2014.15.22.10003
33. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136 359–386 https://doi.org/10.1002/ijc.29210
34. Momenimovahed Z, Ghoncheh M, and Pakzad R, et al (2017) Incidence and mortality of uterine cancer and relationship with human development index in the world Cukurova Med J 42 233–240 https://doi.org/10.17826/cutf.322865
35. Momenimovahed Z and Salehiniya H (2017) Incidence, mortality and risk factors of cervical cancer in the world Biomed Res Ther 4 1795–1811 https://doi.org/10.15419/bmrat.v4i12.386
36. Ochs K, Meili G, and Diebold J, et al (2018) Incidence trends of cervical cancer and its precancerous lesions in women of Central Switzerland from 2000 until 2014 Front Med 5 580–589 https://doi.org/10.3389/fmed.2018.00058
37. DeGennaro Jr V, Shafer M, and Kelly M, et al (2019) Cervical cancer treatment in Haiti: a vertically-integrated model for low-resource settings Gynecol Oncol Rep 28 71 https://doi.org/10.1016/j.gore.2019.03.009
38. Husain RA, Rajakeerthana R, and Sreevalsan A, et al (2018) Prevalence of human papilloma virus with risk of cervical cancer among south Indian women: a genotypic study with meta-analysis and molecular dynamics of HPV E6 oncoprotein Infect Genet Evol 62 130–140 https://doi.org/10.1016/j.meegid.2018.04.029
39. Xu HH, Wang K, and Feng XJ, et al (2018) Prevalence of human papillomavirus genotypes and relative risk of cervical cancer in China: a systematic review and meta-analysis Oncotarget 9 15386–15397 https://doi.org/10.18632/oncotarget.24169 PMID: 29632652 PMCID: 5880612
40. Chen G, Zheng P, and Gao L, et al (2020) Prevalence and genotype distribution of human papillomavirus in women with cervical cancer or cervical intraepithelial neoplasia in Henan Province, Central China J Med Virol 2020 1–7
41. Beddoe AM (2019) Elimination of cervical cancer: challenges for developing countries ecancermedicalscience 13 128–131 https://doi.org/10.3332/ecancer.2019.975
42. Adebamowo SN, Dareng EO, and Famooto AO, et al (2017) Cohort profile: African Collaborative Center for Microbiome and Genomics Research’s (ACCME’s) Human Papillomavirus (HPV) and Cervical Cancer Study Int J Epidemiol 46 1745–1745 https://doi.org/10.1093/ije/dyx050
43. Kuhn L, Denny L, and Pollack A, et al (2020) Human papillomavirus DNA testing for cervical cancer screening in low-resource settings J Natl Cancer Inst 92 818–825 https://doi.org/10.1093/jnci/92.10.818
44. Kashyap N, Krishnan N, and Kaur S, et al (2019) Risk factors of cervical cancer: a case-control study Asia Pac J Oncol Nurs 6 308 https://doi.org/10.4103/apjon.apjon_73_18 PMID: 31259228 PMCID: 6518992
45. Mbatha JN, Taylor M, and Kleppa E, et al (2017) High-risk human papillomavirus types in HIV-infected and HIV-uninfected young women in KwaZulu-Natal, South Africa: implications for vaccination Infect Dis 49 601–608 https://doi.org/10.1080/23744235.2017.1312513
46. Wardak S (2016) Human papillomavirus (HPV) and cervical cancer Med Dosw Mikrobiol 68 73–78
47. Crosbie EJ, Einstein MH, and Franceschi S, et al (2013) Human papillomavirus and cervical cancer Lancet 382 889–899 https://doi.org/10.1016/S0140-6736(13)60022-7 PMID: 23618600
48. Cameron JE, D’Antoni CD, and Herrel NR, et al (2020) Risk of abnormal cervical cytology in HIV-infected women testing positive for both human papillomavirus and Epstein-Barr virus in genital tract specimens Cancer Causes Control 31 365–375 https://doi.org/10.1007/s10552-020-01287-z PMID: 32112173
49. Clifford GM, Tully S, and Franceschi S (2017) Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer Clin Infect Dis 69 1228–1235 https://doi.org/10.1093/cid/cix135
50. Liu G, Sharma M, and Tan N, et al (2018) HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer Aids 32 795–808 https://doi.org/10.1097/QAD.0000000000001765 PMID: 29369827 PMCID: 5854529
51. Rohner E, Bütikofer L, and Schmidlin K, et al (2020) Cervical cancer risk in women living with HIV across four continents: a multicohort study Int Journal Cancer 146 601–609 https://doi.org/10.1002/ijc.32260
52. Chibwesha CJ and Stringer JS (2019) Cervical cancer as a global concern: contributions of the dual epidemics of HPV and HIV JAMA 322 1558–1560 https://doi.org/10.1001/jama.2019.16176 PMID: 31638658
53. Liu ZC, Liu WD, and Liu YH, et al (2015) Multiple sexual partners as a potential independent risk factor for cervical cancer: a meta-analysis of epidemiological studies Asian Pac J Cancer Prev 16 3893–3900 https://doi.org/10.7314/APJCP.2015.16.9.3893 PMID: 25987056
54. Roura E, Travier N, and Waterboer T, et al (2016) The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: results from the EPIC cohort PLoS One 11 147029–147032
55. Rani R, Kumar R, and Trivedi V, et al (2016) Age, parity and stages of cervix cancer: a hospital based study Br J Med Health Res 3 73–82
56. Jensen K, Schmiedel S, Norrild B, Frederiksen K, Iftner T and Kjaer S (2013) Parity as a cofactor for high-grade cervical disease among women with persistent human papillomavirus infection: a 13-year follow-up Br J Cancer 108 234–239 https://doi.org/10.1038/bjc.2012.513 PMCID: 3553518
57. Kusmiyati Y, Prasistyami A, and Wahyuningsih HP, et al (2019) Duration of hormonal contraception and risk of cervical cancer Kesmas: Natl Public Health J 14 9–13
58. Schluterman NH, Sow SO, and Traore CB, et al (2013) Differences in patterns of high-risk human papillomavirus infection between urban and rural low-resource settings: cross-sectional findings from Mali BMC Womens Health 13 68–74 https://doi.org/10.1186/1472-6874-13-4
59. Peng Y, Wang X, and Feng H, et al (2017) Is oral contraceptive use associated with an increased risk of cervical cancer? An evidence-based meta-analysis J Obstet Gynaecol Res 43 913–922 https://doi.org/10.1111/jog.13291 PMID: 28759170
60. Lukac A, Sulovic N, and Smiljic S, et al (2018) The prevalence of the most important risk factors associated with cervical cancer Materia Socio-medica 30 131–139 https://doi.org/10.5455/msm.2018.30.131-135 PMID: 30061804 PMCID: 6029895
61. Fang JH, Yu XM, and Zhang SH, et al (2018) Effect of smoking on high-grade cervical cancer in women on the basis of human papillomavirus infection studies J Cancer Res Ther 14 184–189 https://doi.org/10.4103/0973-1482.179190
62. Ono A, Nakagawa M, and Ikuta E, et al (2019) Relationship between tobacco smoking and cervical cancer Women Health Open J 5 19–21 https://doi.org/10.17140/WHOJ-5-133
63. Poorolajal J and Jenabi E (2016) The association between BMI and cervical cancer risk Eur J Cancer Prev 25 232–238 https://doi.org/10.1097/CEJ.0000000000000164
64. Arfailasufandi R, Mudigdo A, and Sudiyanto A (2019) The effect of obesity, oral contraceptive and passive smoking on the risk of cervical cancer J Epidemiol Public Health 4 189–197 https://doi.org/10.26911/jepublichealth.2019.04.03.06
65. Derks M, van Lonkhuijzen LR, and Bakker RM, et al (2017) Long-term morbidity and quality of life in cervical cancer survivors: a multicenter comparison between surgery and radiotherapy as primary treatment Int J Gynecol Cancer 27 350–356 https://doi.org/10.1097/IGC.0000000000000880
66. Fader AN (2018) Surgery in cervical cancer N Engl J Med 379 1955–1957 https://doi.org/10.1056/NEJMe1814034 PMID: 30379600 PMCID: 6989030
67. Vordermark D (2016) Radiotherapy of cervical cancer Oncol Res Treat 39 516–520 https://doi.org/10.1159/000448902 PMID: 27614991
68. Takekuma M, Kasamatsu Y, and Kado N, et al (2017) The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I–II cervical cancer: a review J Obstet Gynaecol Res 43 617–626 https://doi.org/10.1111/jog.13282 PMID: 28190285
69. Liontos M, Kyriazoglo A, and Dimitriadis I, et al (2019) Systemic therapy in cervical cancer: 30 years in review Crit Rev Oncol Hematol 137 9–17 https://doi.org/10.1016/j.critrevonc.2019.02.009 PMID: 31014518
70. Hofsjo A, Bohm-Starke N, and Blomgren B, et al (2017) Radiotherapy-induced vaginal fibrosis in cervical cancer survivors Acta Oncol 56 661–666 https://doi.org/10.1080/0284186X.2016.1275778 PMID: 28084859
71. Murtono M, Ndii MZ, and Sugiyanto S (2019) Mathematical model of cervical cancer treatment using chemotherapy drug Biol Med Nat Prod Chem 8 11–15 https://doi.org/10.14421/biomedich.2019.81.11-15
72. Ilancheran A (2016). Neoadjuvant chemotherapy in cervical cancer in pregnancy Best Pract Res Clin Obstet Gynaecol 33 102–107 https://doi.org/10.1016/j.bpobgyn.2015.10.008
73. Kong SY, Huang K, and Zeng C, et al (2018) The association between short-term response and long-term survival for cervical cancer patients undergoing neoadjuvant chemotherapy: a system review and meta-analysis Sci Rep 8 1–8 https://doi.org/10.1038/s41598-018-19948-0
74. Tinkle S, McNeil SE, and Mühlebach S, et al (2014) Nanomedicines: addressing the scientific and regulatory gap Ann N Y Acad Sci 1313 35–56 https://doi.org/10.1111/nyas.12403 PMID: 24673240
75. Pita R, Ehmann F, and Papaluca M (2016). Nanomedicines in the EU—regulatory overview AAPS J 18 1576–1582 https://doi.org/10.1208/s12248-016-9967-1 PMID: 27527889
76. Soares S, Sousa J, and Pais A, et al (2018) Nanomedicine: principles, properties, and regulatory issues Front Chem 63 60
77. Sun Q, Barz M, and De Geest BG, et al (2019) Nanomedicine and macroscale materials in immuno-oncology Chem Soc Rev 48 351–381 https://doi.org/10.1039/C8CS00473K
78. Boverhof DR, Bramante CM, and Butala JH, et al (2015) Comparative assessment of nanomaterial definitions and safety evaluation considerations Regul Toxicol Pharmacol 73 137–150 https://doi.org/10.1016/j.yrtph.2015.06.001 PMID: 26111608
79. von Roemeling C, Jiang W, and Chan CK, et al (2017) Breaking down the barriers to precision cancer nanomedicine Trends Biotechnol 35 159–171 https://doi.org/10.1016/j.tibtech.2016.07.006
80. Ji X, Kang Y, and Ouyang J, et al (2019) 2D black mica nanosheets: synthesis of ultrathin biotite nanosheets as an intelligent theranostic platform for combination cancer therapy Adv Sci 6 1970118 https://doi.org/10.1002/advs.201970118
81. Pearson RM, Juettner VV, and Hong S (2014) Biomolecular corona on nanoparticles: a survey of recent literature and its implications in targeted drug delivery Front Chem 2 108 https://doi.org/10.3389/fchem.2014.00108 PMID: 25506050 PMCID: 4245918
82. Louro H (2018) Relevance of physicochemical characterization of nanomaterials for understanding nano-cellular interactions. Cellular and molecular toxicology of nanoparticles Adv Exp Med Biol 1048 123–142 https://doi.org/10.1007/978-3-319-72041-8_8
83. Zengin A, Sutthavas P, and van Rijt S (2020) Inorganic nanoparticle-based biomaterials for regenerative medicine Nanostructured Biomaterials for Regenerative Medicine 1st edn, eds V Guarino, M Iafisco, and S Spriano S (Cambridge: Woodhead Publishing) 293–312 https://doi.org/10.1016/B978-0-08-102594-9.00011-5
84. Corbo C, Molinaro R, and Parodi A, et al (2016). The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery Nanomedicine 11 81–100 https://doi.org/10.2217/nnm.15.188 PMCID: 4910943
85. Peña‐Blanco A and García‐Sáez AJ (2018) Bax, Bak and beyond—mitochondrial performance in apoptosis FEBS J 285 416–431 https://doi.org/10.1111/febs.14186
86. Hudayah T, Chaudhry G, and Taib M, et al (2017) Methanol extracts of four selected marine sponges induce apoptosis in human breast cancer cell line, MCF-7 Int J Res Pharm Sci 8 667–675
87. Nikoletopoulou V, Markaki M, and Palikaras K, et al (2013) Crosstalk between apoptosis, necrosis and autophagy Biochim Biophys Acta 1833 3448–3459 https://doi.org/10.1016/j.bbamcr.2013.06.001 PMID: 23770045
88. Wang J (2020) Combination treatment of cervical cancer using folate-decorated, ph-sensitive, carboplatin and paclitaxel co-loaded lipid-polymer hybrid nanoparticles Drug Design Dev Ther 14 823 https://doi.org/10.2147/DDDT.S235098
89. Mendez J, Morales Cruz M, and Delgado Y, et al (2014) Delivery of chemically glycosylated cytochrome c immobilized in mesoporous silica nanoparticles induces apoptosis in HeLa cancer cells Mol Pharm 11 102–111 https://doi.org/10.1021/mp400400j PMCID: 3905321
90. Pandurangan M, Enkhtaivan G, and Venkitasamy B, BY, et al (2016) Time and concentration-dependent therapeutic potential of silver nanoparticles in cervical carcinoma cells Bio Trace Elem Res 170 309–319 https://doi.org/10.1007/s12011-015-0467-4
91. Maney V and Singh M (2019) The synergism of platinum-gold bimetallic nanoconjugates enhance 5-Fluorouracil delivery in vitro Pharmaceutics 11 439 https://doi.org/10.3390/pharmaceutics11090439
92. Xu X, Liu A, and Bai Y, et al (2019) Co-delivery of resveratrol and p53 gene via peptide cationic liposomal nanocarrier for the synergistic treatment of cervical cancer and breast cancer cells J Drug Deliv Sci Technol 51 746–753 https://doi.org/10.1016/j.jddst.2018.05.008
93. Jain R, Frederick JP, and Huang EY, et al (2018) MicroRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct Nucleic Acid Ther 28 285–296 https://doi.org/10.1089/nat.2018.0734 PMID: 30088967 PMCID: 6157376
94. Hill AB, Chen M, and Chen CK, et al (2016) Overcoming gene-delivery hurdles: physiological considerations for nonviral vectors Trends Biotechnol 34 91–105 https://doi.org/10.1016/j.tibtech.2015.11.004 PMID: 26727153 PMCID: 5800990
95. Xiao-Jie L, Hui-Ying X, and Zun-Ping K, et al (2015) CRISPR-Cas9: a new and promising player in gene therapy J Med Genet 52 289–296 https://doi.org/10.1136/jmedgenet-2014-102968 PMID: 25713109
96. Maiyo F and Singh M (2019) Folate-Targeted mRNA delivery using chitosan functionalized selenium nanoparticles: potential in cancer immunotherapy Pharmaceuticals 12 164 https://doi.org/10.3390/ph12040164
97. Mbatha LS and Singh M (2019) Starburst poly(amidoamine) dendrimer grafted gold nanoparticles as a scaffold for folic acid-targeted plasmid DNA delivery in vitro J Nanosci Nanotechnol 19 1959–1970 https://doi.org/10.1166/jnn.2019.15798
98. Maiyo F and Singh M (2020) Polymerized selenium nanoparticles for folate-receptor targeted delivery of anti-Luc-siRNA: potential for gene silencing Biomedicines 8 76 https://doi.org/10.3390/biomedicines8040076
99. Liu B, Han SM, and Tang XY, et al (2014) Cervical cancer gene therapy by gene loaded PEG-PLA nanomedicine Asian Pac J Cancer Prev 15 4915–4921 https://doi.org/10.7314/APJCP.2014.15.12.4915 PMID: 24998563
100. Gupta S and Gupta MK (2017) Possible role of nanocarriers in drug delivery against cervical cancer Nano Rev Exp 8 1335567 https://doi.org/10.1080/20022727.2017.1335567 PMID: 30410707 PMCID: 6167030
101. Chen J, Huang L, and Lai H (2014) Methotrexateloaded PEGylated chitosan nanoparticles: synthesis, characterization, and in vitro and in vivo antitumoral activity Mol Pharm 11 2213–2223 https://doi.org/10.1021/mp400269z
102. Kou J, Dou D, and Yang L (2017) Porphyrin photosensitizers in photodynamic therapy and its applications Oncotarget 8 81591–81603 https://doi.org/10.18632/oncotarget.20189 PMID: 29113417 PMCID: 5655312
103. Mansoori B, Mohammadi A, and Amin Doustvandi M, et al (2019) Photodynamic therapy for cancer: role of natural products Photodiagnosis Photodyn Ther 26 395–404 https://doi.org/10.1016/j.pdpdt.2019.04.033 PMID: 31063860 PMCID: 6579671
104. Chen C, Wang J, and Li X, et al (2017) Recent advances in developing photosensitizers for photodynamic cancer therapy Comb Chem High Throughput Screen 20 414–422 https://doi.org/10.2174/1386207320666170113123132 PMID: 28088891
105. Vannini F, Lenzi C, and Lubrano V (2017) Nitric oxide-based anticancer therapeutics: the new technologies of the nanoparticles Nitric Oxide (Donor/Induced) in Chemosensitizing 1st edn, ed B Bonavida (Massachusetts: Academic Press) pp 143–154 https://doi.org/10.1016/B978-0-12-811020-1.00008-9
106. Xu X, Xie K, and Zhang XQ, et al (2013) Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug Proc Natl Acad Sci 110 18638–18643 https://doi.org/10.1073/pnas.1303958110 PMID: 24167294 PMCID: 3832000
107. Balasubramanian S, Girija AR, and Nagaoka Y, et al (2014) Curcumin and 5-fluorouracil-loaded, folate-and transferrin-decorated polymeric magnetic nanoformulation: a synergistic cancer therapeutic approach, accelerated by magnetic hyperthermia Int J Nanomed 9 437–445
108. Kim TH, Lee GJ, and Kang JH, et al (2014) Anticancer drug-incorporated layered double hydroxide nanohybrids and their enhanced anticancer therapeutic efficacy in combination cancer treatment BioMed Res Int 2014 193401–193411 PMID: 24860812 PMCID: 4016841
109. Lechanteur A, Furst T, and Evrard B, et al (2017) Promoting vaginal distribution of E7 and MCL-1 siRNA-silencing nanoparticles for cervical cancer treatment Mol Pharm 14 1706–1717 https://doi.org/10.1021/acs.molpharmaceut.6b01154 PMID: 28350964
110. Baharara J, Ramezani T, and Divsalar A, et al (2016) Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells Avicenna J Med Biotechnol 8 75–81 PMID: 27141266 PMCID: 4842245
111. Maiyo F and Singh M (2017) Selenium nanoparticles: potential in cancer gene and drug delivery Nanomedicine 12 1075–1089 https://doi.org/10.2217/nnm-2017-0024 PMID: 28440710






