Expression of cyclooxygenase-2 (COX-2) in colorectal carcinoma in an indigenous African population of Kano, Nigeria
Jimoh Ajanaku Abdulrazaq1, Mohammed Aminu Zakari2, Yusuf Ibrahim2 and Hamza Ahmad2
1Department of Pathology, Federal University of Health Sciences Azare, Azare 751101, Bauchi, Nigeria
2Department of Pathology, Aminu Kano Teaching Hospital, Kano 700101, Nigeria
ahttps://orcid.org/0000-0003-4052-3300
bhttps://orcid.org/0000-0002-4545-866x
chttps://orcid.org/0009-0004-4000-9285
dhttps://orcid.org/0009-0000-4078-7328
Abstract
Background: cyclooxygenases-2 (COX-2) over-expression has been noticed in colorectal cancers (CRCs) with adverse outcomes, serving as a potential marker for prognosis, targeted therapy and as a window in CRC prevention. Unfortunately, there are scarce data regarding COX-2 expression in CRC in Africa where CRC incidence is on the increase with younger age affectation and unfavourable outcomes.
Aims: This retrospective study aims to determine the proportion of CRCs that over-express COX-2 and document any relationship between COX-2 over-expression with clinicopathological features such as histologic subtype, tumour grade, age and sex.
Methods: All the 139 CRCs that were histologically diagnosed at Aminu Kano Teaching Hospital over a 5-year period were included, but only 124 Formalin-fixed paraffin-embedded tissue blocks were sectioned and stained with COX-2 antibody. COX-2 expression was scored for distribution (no cells = 0, 1%–10% = 1, 11%–50% = 2, 51%–80% = 3, 81%–100% = 4) and intensity (no stain = 0; weak = 1; moderate = 2, strong = 3). The immunoreactive score (IRS) is a product of intensity (I) and distribution (D) as: 9–12 strongly +, 5–8 moderately +, 1–4 weakly + and 0 negative. Over-expression of COX-2 is an IRS of 5–12. Outcomes were statistically evaluated with clinicopathological data.
Results: The CRCs occurred more commonly in males (M: F, 2:1), in the middle age group (mostly between 30 and 59 years), and 51.1% of cases occurred before 50 years and peaked in the 6th decade. Over-expression of COX-2 was observed in 46.8% (58/124) and was strongly associated with adenocarcinoma (ADC) not otherwise specified (NOS) (moderately and poorly differentiated tumours) but not with age or sex.
Conclusion: The over-expression of COX-2 was significantly associated with ADC NOS (moderately and poorly differentiated tumours), indicating that it may influence the outcome of CRCs with possible variation in tumour subtype.
Keyword: COX-2 expression, colorectal cancer, indigenous Africa population, Kano, Nigeria
Correspondence to: Jimoh Ajanaku Abdulrazaq
Email: jimmeeq@gmail.com and jimmson52@yahoo.com
Published: 06/12/2024
Received: 01/06/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Colorectal carcinoma (CRC) is a malignant epithelial tumour of the colon and rectum, and demonstrates a worldwide variation in incidence and outcomes [1, 2]. Though there are more cases in the Western world, the incidence is rising in Africa and Asia, and the majority of deaths occur in less-developed regions reflecting poorer survival in these regions [3, 4]. The available data from Africa has highlighted major challenges and these include rising incidence, attraction to the Western lifestyle, late presentation, younger age affectation, aggressive tumour sub-type, lack of tailored therapy, inadequate research, high cost of treatment, dearth of radiotherapy centres, lack of government support and poorer outcomes [5–7].
Expression of cyclooxygenases-2 (COX-2) in CRC is associated with adverse outcomes. For the pathological roles it plays and experimental observations, over-expression of COX-2 is associated with inflammation, initiation of cancer, cancer progression, activation of vascular endothelial growth factor, angiogenesis, proliferation of surviving cancer stem cells, invasion, metastasis and tumour relapse after therapy [8–10]. Genetically, the carriers of the COX-2 A-1195G AG genotype have 2 and 2.9 times increased risk of developing colorectal adenoma and cancer, respectively, while the respective risk of developing adenoma and CRC for COX-2 A-1195G AA genotype is 1.8 and 3.1 times lower than the general populace [11]. From epidemiological and clinical point of view, inhibitors of cyclooxygenases (COX1 and COX-2) such as nonsteroidal anti-inflammatory drugs (NSAIDs) are useful in the chemo-prevention and management of CRC [8–10]. Based on the sufficient data available, the use of NSAIDs has been recommended in USA and Australia for the dual benefits of cardiovascular events and cancer chemo-prevention [12, 13]. The burdens of CRC in Africa create the need for CRC prevention and treatment. One important way of preventing CRC is through the use of NSAIDs. Studies on the COX-2 expression in CRCs are limited in Africa as the majority of studies are mainly from Asian and Western Countries. This retrospective study aims to determine the proportion of CRCs that over-express COX-2 and document any relationship between COX-2 over-expression with clinicopathological features such as histologic subtype, tumour grade, age and sex.
Materials and methods
This retrospective hospital-based descriptive study was carried out on CRC cases histologically diagnosed in the Histopathology department of Aminu Kano Teaching Hospital, Kano (AKTH) in north-western Nigeria from 1st January 2015 to 31st December 2019. The hospital (AKTH) is a tertiary hospital with over 700-bed capacity. The histopathology department of AKTH receives an average of 5,500 histological samples per annum and renders services including cytology, histology, autopsy and immunohistochemistry (IHC). The CRCs cases were classified and graded according to World Health Organisation, WHO (2019, 5th edition) classification of tumours of the colon and rectum (Figure 1) [14]. The CRCs were grouped into adenocarcinoma (ADC) not otherwise specified (NOS) and other specific histological subtypes. The two-tiered system of grading was used on the basis of prognosis: Low-grade ADC which comprise of well-differentiated adenocarcinoma (WDA) and moderately differentiated adenocarcinoma (MDA) and high-grade ADC that is poorly differentiated adenocarcinoma (PDA) plus other specific sub-types [14]. Cases with insufficient clinical information, particularly biodata, missing or damaged blocks and tissue blocks with insufficient tissue were excluded.
Data, such as age, site of tumour, histologic diagnosis and grade of the disease was obtained from the Kano cancer registry, pathology request forms, patients’ case notes, duplicate copies of histopathological reports and slide reviews of cases. Ethical approval for this study was obtained from the Health and Research Ethics Committee of AKTH, Kano (ethical review reference number: AKTH/MAC/SUB/12A/P-3/V1/2904). The patient’s identity was concealed at all times and the additional consent for individuals whose samples were used in this study was waived by the institutional ethical committee.
IHC for COX-2 expression
Immunohistochemical staining was performed using anti-COX-2 rabbit polyclonal antibody (Elabscience, USA, catalog No. E-AB-70031), used at a 1:500 dilution according to standard immunohistochemical staining protocols. The antibody was stored at −20 degrees Celsius and it was centrifuged before opening to ensure complete recovery of contents. A kidney sample with intact renal tubules was used as positive control while a negative control was obtained by replacing primary antibody with distilled water.
Tissue preparations (FFPE tissue blocks)
Formalin-fixed paraffin-embedded tissues retrieved were cut or sectioned at 3 µm. The tissue slides were left to dry in an oven for 2 hours at 60º after microtomy. Then, the slides were dewaxed in xylene for 1 minute and then passed through decreasing concentrations of alcohol at 100%, 95% and 70% for 1 minute each for rehydration of sections.
Antigen retrieval or unmasking of antigen sites
Antigen retrieval was done by placing the slides in 100 ml of citrate buffer (pH 6) and then heating using the microwave heating retrieval method at a hundred degrees centigrade (100oC) for 15–20 minutes. Slides were then washed in distilled water for another 2 minutes and then washed with phosphate buffer for 3 minutes twice. They were rinsed in distilled water for 2 minutes, and then 3% of hydrogen peroxide was then added and left for 10 minutes. Slides were then washed in running distilled water for 2 minutes and incubated with a blocking reagent for 10 minutes.
Blockage of endogenous peroxidase
To block non-specific antigen sites, tissue sections were incubated for 1 hour in 1.5% bovine serum albumin at room temperature.
Immunostaining procedure
Each slide was wiped with cotton wool to remove excess blocking solution. Incubation with the primary antibodies was carried out at room temperature for 30 minutes with 200 μl of anti-COX-2. Following the primary antibody incubation step, a secondary antibody from a streptavidin-biotin complex peroxidase kit (LSAB + kit, Dako, Copenhagen, Denmark) was then incubated according to the manufacturer’s instructions. Peroxidase activity was also developed with the substrate 3,3-diaminobenzidine tetrahydrochloride (DAB; Dako) by incubating the tissue sections in DAB for a period of 3 minutes. The tissue slides were washed in running water for a period of 3 minutes and then counterstained with haematoxylin. The tissue sections were then dehydrated in increasing concentrations of alcohol. They were cleared in xylene and the application of cover slip was done using Distyrene Plasticizer Xylene and they were allowed to dry.
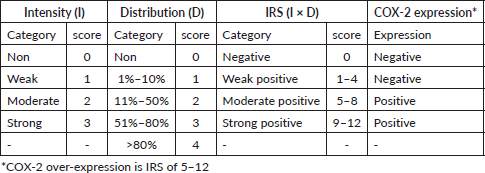
The slides were viewed under the light microscope, brown cytoplasmic and membranous staining was interpreted as positive staining and was scored semi-quantitatively using the immunoreactive score (IRS) system, a final score that is a product of the intensity and distribution of COX-2 immunoreactivity (Figure 2) [15]. The intensity of staining was scored as 0 for no staining, 1 for weak staining, 2 for moderate and 3 for strong staining. The percentage of positive tumour cells was scored: 0 indicating no cell with a positive reaction, 1 indicating 1%–10% of cells with a positive reaction, 2 indicating 11%–50% of cells with a positive reaction, 3 indicating 51%–80% of cells with a positive reaction and 4 indicating greater than 80% of cells with a positive reaction. The final IRS score obtained by multiplying the distribution and intensity for each tumour was graded as follows: 9–12 strongly positive, 5–8 moderately positive, 1–4 weakly positive and 0 negatives. COX-2 was considered over-expressed if the IRS score is moderate to strong (that is a score of 5 to 12), Table 1.
Table 1. Shows COX-2 immunoreactive scoring system (IRS).
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS Inc, Chicago, IL, USA version 25). Chi-square was used with statistical significance set at p < 0.05 to test the association between COX-2 expression and clinico-pathological parameters such as age, sex, histological subtype and grade. Proportions and central tendencies of nominal and continuous variables were obtained by descriptive statistics of frequency, mean and median.
Results
139 CRCs within the study period in the department were analysed and 124 cases had COX-2 IHC. Sixty-four CRC cases were endoscopic biopsies. Each of the 139 cases belongs to only one patient and there were no duplicate cases or combined open biopsies and endoscopic biopsies belonging to a single patient.
Age distribution of CRCs
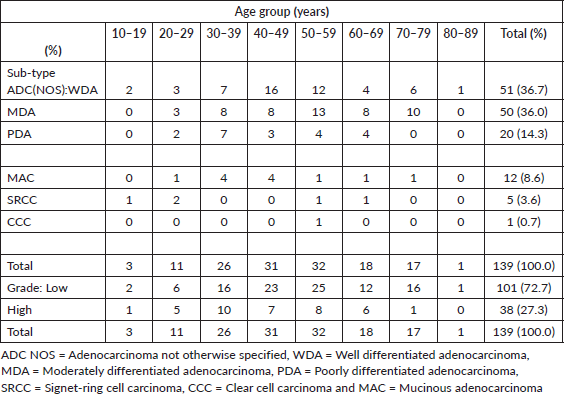
The age distribution of CRC cases ranged from 11 to 85 years with a mean age of 48.2 (S. D-15.5) years (Table 2). The largest proportion of cases (64.0%) clustered within the 30 to 59 age range and peaked in the 6th decade. Seventy-one cases of CRC (51.1%) occurred before the age of 50 years while only one case (0.7%) occurred at the age of 80 years or more. Out of 81 cases that occurred before or at 50 years, 30 cases (37.0%) occurred in females.
Table 2. Shows age distribution of CRCs with sex, histological subtypes and grade.
Gender distribution of CRCs
There were 92 males and 47 females giving a male-to-female ratio of 2:1. Female cases tend to be associated with a younger age group than their male counterpart (mean of 45.7 years for females versus 49.5 years for males, and 63.8% of female cases occurring before or at 50 years versus 55.4%).
Histological types of the CRCs
ADC NOS constitute 87.1% of cases (Table 2). Twelve (60.0%) of the ADC NOS with high-grade features were observed at ≤50 years and 40% of this occurred in females. Overall, 10 (83.3%) cases of mucinous carcinomas occurred at 50 years or below. Unfavourable subtypes such as poorly differentiated ADC NOS, mucinous carcinoma and signet ring cell carcinoma tend to affect younger age groups before or at 50 years (Table 2).
Grades of the CRCs
Low-grade ADC made up 72.7% of cases (Tables 2 and 3). The respective proportions of low-grade and high-grade ADC that occurred at ≤50 years are 71.3% (72 of 101 cases) and 81.6% (31 of 38 cases).
COX-2 IHC for CRCs
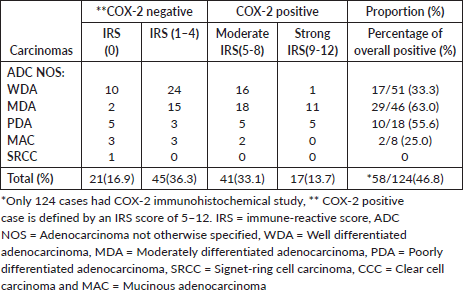
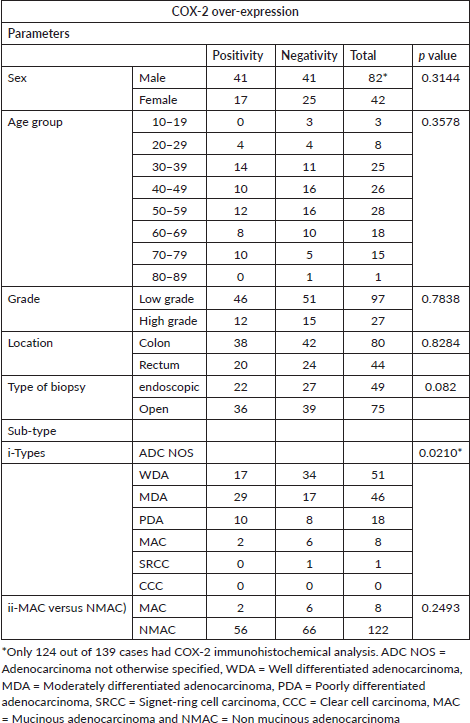
Over-expression of COX-2 was observed in 46.8% (58 out of 124) of cases. Of this number, 70.7% (41 of 58 positive cases) of this was moderately positive while 29.3% (17 of 58 positive cases) were strongly positive (Table 4).
Table 3. Shows sex distribution of 139 CRCs by histological subtype and grade.
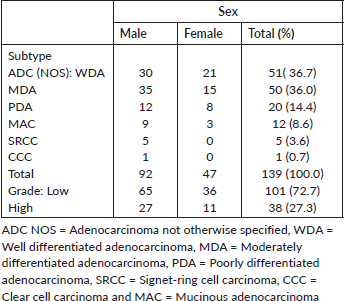
Table 4. Shows COX-2 IRS score distribution of 124 CRCs.
COX-2 expression and age of patients with CRCs
Out of 58 cases that over-expressed COX-2, 75.9% (44 of 58) occurred in the 30–69 age range, but it is interesting to note that no positivity was recorded in the age 10–19 and 80–89 categories which have 3 and 1 case, respectively (Table 5).
COX-2 expression and sex of patients with CRCs
Forty-two out of 124 cases that had COX-2 IHC were females while the remaining 82 cases were males. Seventeen of 42 (40.5%) female cases were COX-2 positive which constitutes 29.3% of all COX-2 positive cases. Half of the male cases expressed COX-2 which accounted for the majority of COX-2 positive cases, 70.7% (41 of 58). The ratio of female to male cases that over-express COX-2 is 1: 2.4, but 40.5% of female and 50% of male cases expressed COX-2.
COX-2 expression and histological sub-type of CRCs
For the category of ADC (NOS), 48.7% of cases (56 of 115) were positive and these include 33.3% of ADC with well-formed glands (17 of 51), 63.0% of ADC with moderate differentiation (29 of 46) and 55.6% of ADC with poor differentiation (10 of 18). Only 2 cases out of 8 (25%) cases of mucinous carcinomas over-expressed COX-2. COX-2 expression was strongly associated with histological sub-type (p-value-0.02101).
COX-2 expression and grade of CRCs
Despite the fact that a large number of MDA tend to be positive compared to other sub-types (29 out of 46 cases, 63.0%), only 46 out of 97 (47.4%) cases of low-grade ADC (both well and MDA) over-expressed COX-2 (Table 5). Thirty-eight (27.3%) CRCs were high-grade ADC but only 12 out of 27 cases (44.44%) over-expressed COX-2.
Table 5. Shows COX-2 over-expression of 124 CRCs with age group, sex, tumour grade and histological sub-type.
Discussion
Age distribution of CRC
Early onset CRC: ‘Early-onset’ CRC refers generally to CRC occurring in patients ≤50 years old. This current study demonstrates that more than half (51.08%) of CRCs are early-onset. Similar findings have also been documented across Africa, India and Filipinos [5 ,16–24]. In Ibadan, Nigeria, 41% of cases occurred before 50 years; in Mali, 60% of cases were before 50 years; in Kenya, 17% of cases were observed before 40 years and in Ghana, 34% of cases manifested before 50 years [5, 16, 17, 19]. In Mumbai, India, Prachi et al showed the mean age of CRC at 47.2 years and 33% of cases were in those below 40 years [20]. Kaw et al [23] observed a mean age of 55.3 years and 17% of cases occurred in patients 40 years of age or younger in the Philippines [21]. In the United States of America, as a result of the rise in CRC among young individuals, the median age of CRC cases has declined from age 72 years in the early 2000s to age 66 years in 2018 [25]. The early onset CRC incidence is a global phenomenon, affecting USA, UK, Australia, Canada and Germany even though at a lower magnitude [26–28].
Certain birth cohorts have been identified as a possible cause of early onset CRC [29–31]. In this regard, a birth cohort effect results because age-specific CRC incidence rates vary due to changes in the ways by which generations are exposed to CRC risk factors. Those who were born in the 1950s have the lowest CRC incidence in the USA, but the risk of developing CRC has since increased with each successive generation [30]. Desk-bound work hours and passive media consumption independently increase the risk for young-onset CRC. In the USA, early onset CRC risk has increased by 69% for men and 20% for women for more than 14 hours per week spent watching television [32, 33]. A family history of CRC, [34–37] and certain key molecular alterations [38, 39] may also give an insight to the cause of early onset CRC.
Sex distribution of CRC
Globally, incidence rates of CRC are higher in males than females. The current study demonstrated a male preponderance and sex (M: F) ratio of 2:1 which echoes the findings in other studies [5, 16, 20]. Contrastingly, a study from Filipinos showed no gender discrimination [23] while two studies from Ghana demonstrated higher female occurrence [19, 40]. The female preponderance in Raskin et al [19] (Ghana) and Kaw et al [23] (Philippines) may stem from the location of tumours as they observed more right-sided colonic tumours (with the exclusion of rectal tumours) which are more common in females compared to the left tumour. Differences in risk factor exposure and access to screening, hormonal factors and socioeconomic factors appear to be important in CRC sex disparity [41–44].
Histological sub-types and grade of CRC
ADC NOS accounted for the majority (87.1%) including WDA and MDA, both of which accounted for 72.7% of all CRC and this finding is in consonance with other studies [5, 18, 19, 23]. However, the proportion (27.3%) of CRC with unfavourable histology put together is quite significant: 14.4% (PDA), 8.6% (mucinous carcinoma), 3.6% (signet-ring cell carcinoma) at 3.6% and 0.7% (clear cell carcinoma) which is similar to findings in Kenya, Ghana and Philippines [18, 19, 23].
COX-2 expression in CRCs
Over-expression of COX-2 was observed in 46.8% of the CRCs in this study, which is in agreement with studies from Japan by Bamba et al [46], Konno et al [47], Tomozawa et al [48] and Yamauchi et al [49], and a study from the UK by Elder et al [45] where the rate of COX-2 over-expression ranged from 25% to 92%. The available studies demonstrated wide variation (25% to 92%) in COX-2 expression (Table 6). Konno et al [47] observed a rate of 25% of COX-2 positivity in Japan while Elder et al [45] demonstrated a 92% rate in the UK [45, 47]. Elder et al [45] examined the over-expression of COX-2 in 35 adenomas and 38 sporadic invasive colorectal ADC at the University of Bristol, UK, and showed that COX-2 expression in CRC was 92% compared to 66% in adenomas [45]. The reason for this wide gap in COX-2 expression in the available studies is difficult to explain, more studies are needed to exclude the possibility of racial factors because most of the available studies are from Asia with few from another continent like Africa. Other possible reasons may include the histological sub-types, tumour grades, tumour stage, study designs, sample size or genetic makeup.
In terms of the histology of CRC, the COX-2 over-expressions in MDA (63.0%) and PDA (55.6%) are higher than WDA (33.3%) and mucinous carcinomas (25%). Similar to this pattern of over-expression, Qi-Bing et al [50] in their large-sized study of 1,026 CRC cases in China, showed that the proportions of well, moderate and poorly differentiated CRC cases that over-expressed COX-2 are 73.5%, 80.8% and 74.5%, respectively, while the overall expression is 77.97% with no significant correlation with sex, age or tumour location but demonstrate a significant correlation with tumour size ≥5 cm, serosa invasion, late stage and distant metastasis. Likewise, these findings are similar to observations by Yamauchi et al [49] in their immunohistochemical analysis of 232 cases of CRC in Japan. Venkatachala and Rajendra [26] on the other hand, observed COX-2 expression in WDA (56.6%), MDA (66.6%) and PDA (100%). The present study demonstrated a strong statistical association between COX-2 expression and histological sub-type of tumour (p values of 0.02101) but not with age or sex similar to the findings of large sample size studies of Wu et al [50] and Yamauchi et al [49]. However, studies with relatively lower sample sizes tend to demonstrate contrasting findings. For instance, findings from the analysis of 30 advanced CRC cases by COX-2 mRNA quantitative PCR, showed that COX-2 mRNA is higher in low-grade CRC (WDA and MDA; 93%) compared to PDA (7%) [51]. Also, two separate studies that analysed 38 and 63 advanced CRC cases by COX-2 IHC showed no significant association between COX-2 expression and histology type and other parameters such as age, sex, tumour size, Duke’s stage, grade, location and depth of invasion [45, 48].
The stage of CRC cases in the studies also affects the rate of COX-2 overexpression. For instance, Venkatachala and Rajendra [26] from India observed overall COX-2 expression in 86.2% of cases. They also observed highly positive COX-2 cases to constitute 33.3% of stage I, 58.8% of stage II, 80% of stage III and 100% of stage IV tumours with association with lymphatic metastasis and invasive depth [26]. Furthermore, the result of COX-2 heterogeneity (varied COX-2 expression among available studies) may also be affected by racial and genetic makeup. Polymorphism in the COX-2 gene with other genes may be key in this regard. Carriers of COX-2 A-1195G AG increase adenomas and CRCs risk, and mutation in the APC gene has been shown to correlate with COX-2 expression in both adenomas and CRCs [11, 52, 53]. More studies are needed to establish the role of race in COX-2 expression in CRCs.
It is also important to decipher why COX-2 expression is relatively low in many studies. For instance, this current study observed only 46.8% expression despite evidence suggesting COX-2 involvement in every stage of carcinogenesis (initiation and progression) and chemo-preventive effects of NSAIDs [8–10, 50, 54–59]. The supporting evidence includes first, some of the CRCs lacking COX-2 expression over-expressed COX-1 and thus, dysregulation in COX-1 and COX-2 expression is found in a higher percentage of CRCs [60]. Second, any alteration in up- or downstream of COX-2 (arachidonic acid metabolic) pathways may mimic the cellular effect of COX-2. Third, evidence indicates the elevation of COX-2 expression in CRC stromal cells such as fibroblasts and macrophages [61]. Expression of COX-2 is present in both adenomas and CRC because it is believed to be involved in the initiation of tumourigenesis as it can be upregulated by the carcinogen. However, the expression is higher in CRC than in adenoma, indicating that it may also serve as a marker for tumour progression. Expression of COX-2 influences all stages of tumour progression [8–10, 50, 54–59].
Limitation of this study
The data utilised in this study is limited to northern Nigeria, so multicenter studies in Nigeria and across Africa will give a better reflection of COX-2 expression in CRC in Africa. This will also give a better understanding between COX-2 expression and the uncommon histological subtypes in Africa. The entire tumour had not been stained with COX-2 IHC. Therefore, sections stained with COX-2 antibody may not entirely represent COX-2 status in each CRC case. The inability of this study to correlate COX-2 expression with survival largely due to the loss of patients to follow-up, and the lack of information on the use of COX-2 inhibitors by the patients equally underlies the weakness in this study.
Table 6. Shows global studies of COX-2 expression on CRC.
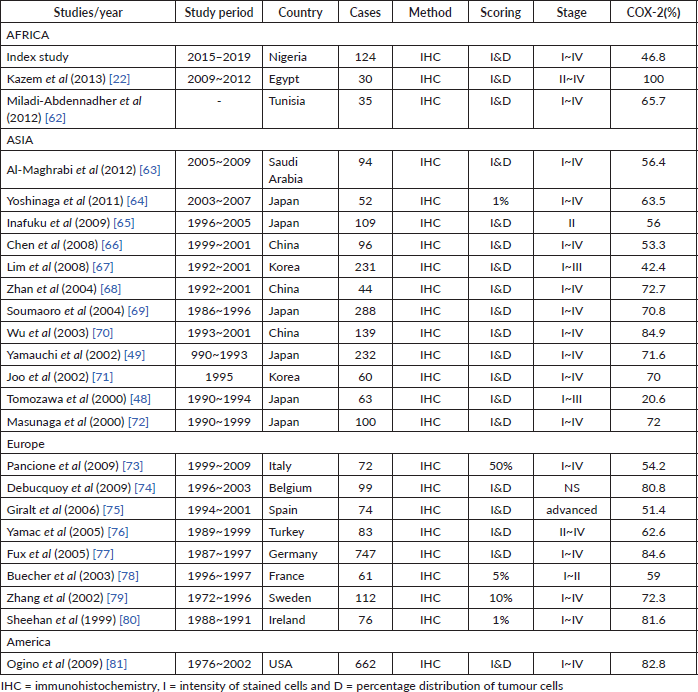
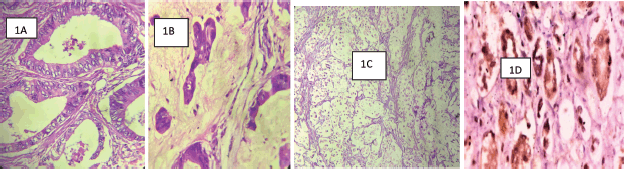
Figure 1. (Photomicrographs): (a): WDA of the colon showing well-formed malignant glands infiltrating muscularis propria. Haematoxylene and eosin (H&E) ×200. (b): A mucinous carcinoma of the rectum showing nests and cords of malignant epithelial cells surrounded by pools of mucin. H&E ×200. (c): A signet-ring cell carcinoma of the rectum showing signet-ring cell. H&E ×200. (d): COX-2 positive control. Typical cytoplasmic and membranous staining of COX-2 protein on the renal tubular epithelial cells ×200.
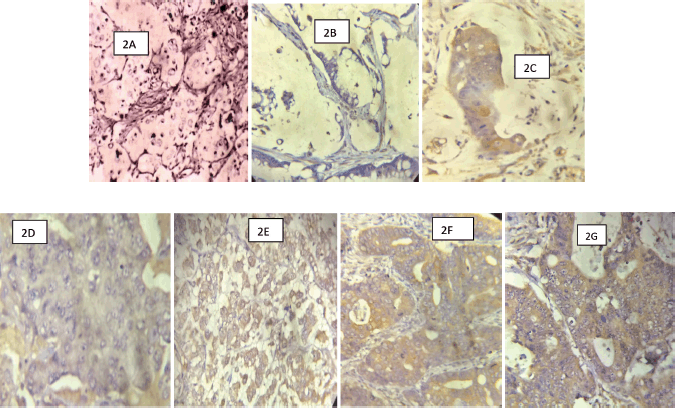
Figure 2. (Photomicrographs of CRCs with COX-2 IHC): (a): A signet-ring cell carcinoma of the colon showing no membranous/cytoplasmic brown staining (COX-2 negative) ×200. (b): A mucinous carcinoma of the rectum showing no membranous/cytoplasmic brown staining (COX-2 negative) ×200. (c): A mucinous carcinoma of the rectum showing strong membranous/cytoplasmic brown staining (COX-2 positive) ×200. (d): A PDA of the colon showing weak COX-2 stain ×200. (e): A PDA of the colon showing strong membranous/cytoplasmic brown staining (COX-2 positive) ×200. (f): A WDA of the colon showing strong membranous/cytoplasmic brown staining (COX-2 positive) ×200. (g): A MDA of the rectum showing strong membranous/cytoplasmic brown staining (COX-2 positive) ×200.
Conclusion
Occurrence of CRC is more frequent in middle age, and more than half of all cases are early onset CRCs. This trend has clinical, social and financial implications. The strong statistical association between COX-2 over-expression and histological sub-type may influence the outcomes and prevention of CRCs with possible variation in tumour sub-type.
Conflicts of interest
The authors have declared that no competing interests exist.
Funding
The authors received no specific funding for this work.
References
1. Bray F and Soerjomataram I (2015) The changing global burden of cancer: transitions in human development and implications for cancer prevention and control Chapter 2 Cancer: Disease Control Priorities 3rd edn, eds H Gelband, P Jha, and R Sankaranarayanan, et al (Washington: International Bank for Reconstruction and Development/The World Bank) Vol 2
2. Bray F, Jemal A, and Grey N, et al (2012) Global cancer transitions according to the human development index (2008-2030): a population-based study Lancet Oncol 13 790–801 https://doi.org/10.1016/S1470-2045(12)70211-5 PMID: 22658655
3. World Health Organization (2018) Global Health Observatory (Geneva: World Health Organization) [who.int/gho/database/en/]
4. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660
5. Rotimi O and Abdulkareem F (2014) Fifty-three years of reporting colorectal cancer in Nigerians – a systematic review of the published literature Niger Postgrad Med J 21(1) 68–73 https://doi.org/10.4103/1117-1936.163708 PMID: 24956622
6. Morhason-Bello IO, Odedina F, and Rebbeck TR, et al (2013) Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer Lancet Oncol 14(4) e142–e151 https://doi.org/10.1016/S1470-2045(12)70482-5 PMID: 23561745
7. Abdel-Wahab M, Bourque JM, and Pynda Y, et al (2013) Status of radiotherapy resources in Africa: an International atomic energy agency analysis Lancet Oncol 14(4) pe168–pe175 https://doi.org/10.1016/S1470-2045(12)70532-6
8. Claria J (2003) Cyclooxygenase-2 biology Curr Pharm Des 9(27) 2177–2190 https://doi.org/10.2174/1381612033454054 PMID: 14529398
9. Wang D, Fu L, and Sun H, et al (2015) Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice Gastroenterology 149(7) 1884–1895 https://doi.org/10.1053/j.gastro.2015.07.064 PMID: 26261008 PMCID: 4762503
10. Xu L, Stevens J, and Hilton MB, et al (2014) COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models Sci Transl Med 6(242) 242 https://doi.org/10.1126/scitranslmed.3008455
11. Shomaf M, Yousef A, and Ababna N, et al (2015) Cyclooxygenase-2 (COX2) gene polymorphisms and the risk of sporadic colorectal cancer and polyps among Jordanian population Turk J Gastroenterol 26 154–158 https://doi.org/10.5152/tjg.2015.6174 PMID: 25835114
12. Bibbins-Domingo K and U.S. Preventive Services Task Force (2016) Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement Ann Intern Med 164(12) 836–845 https://doi.org/10.7326/M16-0577 PMID: 27064677
13. Cancer Council Australia Colorectal Cancer Guidelines Working Party (2017) Clinical Practice Guidelines for the Prevention, Early Detection and Management of Colorectal Cancer. Short Form Summary of NHMRC Approved Recommendations (Sydney) pp 5–44 [https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer]
14. Nagtegaal ID, Arends MJ, and Odze RD, et al (2019) Tumour of colon and rectum World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System eds D Lokuhetty, V White, R Watanabe, et al (Lyon: IARC Press) pp 156–213
15. Soslow RA, Dannenberg AJ, and Rush D, et al (2000) COX-2 is expressed in human pulmonary, colonic and mammary tumors Cancer 89(12) 2637–2645 https://doi.org/10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B
16. Gaudre N, Ly M, and Badiaga Y, et al (2013) Epidemiological and clinical features of colorectal cancer at the hematology and oncology ward of Point G in Bamako, Mali from 2005-2011: 113 cases Mali Med 28(3) 39–44 PMID: 30049166
17. Irabor DO, Afuwape OO, and Ayandipo OO (2014) The present status of the management of colorectal cancer in Nigeria J Cancer Res 1 267190 [
18. Saidi H, Abdihakin M, and Njihia B, et al (2011) Clinical outcomes of colorectal cancer in Kenya Ann Afri Surg 7(1) 42–49
19. Raskin L, Dakubo JC, and Palaski N, et al (2013) Distinct molecular features of colorectal cancer in Ghana Cancer Epidemiol 37(5) 556–561 https://doi.org/10.1016/j.canep.2013.07.007 PMID: 23962701 PMCID: 4267222
20. Patil P S, Saklani A, and Gambhire P, et al (2017) Colorectal cancer in India: an audit from a Tertiary Center in a low prevalence area Indian J Surg Oncol 8(4) 484–490 https://doi.org/10.1007/s13193-017-0655-0 PMID: 29203978 PMCID: 5705504
21. Stigliano V, Sanchez-Mete L, and Martayan A, et al (2014) Early-onset colorectal cancer: a sporadic or inherited World J Gastroenterol 20(35) 12420–12430 https://doi.org/10.3748/wjg.v20.i35.12420 PMID: 25253942 PMCID: 4168075
22. Kazem A, El Sayed K, and El Kerm Y (2014) Prognostic significance of COX-2 and b-catenin in colorectal carcinoma AJM 50(3) 211–220
23. Kaw LL, Punzalan CK, and Crisostomo AC, et al (2002) Surgical pathology of colorectal cancer in Filipinos: implications for clinical practice J Am Coll Surg 195(2) 188–195 https://doi.org/10.1016/S1072-7515(02)01186-9 PMID: 12168965
24. Asombang AW, Madsen R, and Simuyandi M, et al (2018) Descriptive analysis of colorectal cancer in Zambia, Southern Africa using the National Cancer Disease Hospital Database PAMJ 30 248 https://doi.org/10.11604/pamj.2018.30.248.12464
25. SEER*Stat Database November 2018 Submissions: Rate Sessions – Incidence SEER 9 Regs Research Data with Delay Adjustment, Malignant Only, Nov 2018 Sub (1975-2016) <Katrina/Rita Population Adjustment> [https://seer.cancer.gov/data-software/documentation/seerstat/nov2018/]
26. Venkatachala S and Rajendra M (2016) Correlation of COX- 2 expression in colorectal carcinoma with clinicopathological features Turk Patoloji Derg 33(3) 228–234
27. Dimberg J, Samuelsson A, and Hugander A, et al (1999) Differential expression of cyclooxygenase 2 in human colorectal cancer Gut 45(5) 730–732 https://doi.org/10.1136/gut.45.5.730 PMID: 10517910 PMCID: 1727716
28. Keith C, Nigel S, and Pierre G, et al (2002) Analysis of cyclooxygenase expression in human colorectal adenomas Dis Colon Rectum 45 1316–1324 https://doi.org/10.1007/s10350-004-6418-3
29. Araghi M, Soerjomataram I, and Bardot A, et al (2019) Changes in colorectal cancer incidence in seven high-income countries: a population-based study Lancet Gastroenterol Hepatol 4(7) 511–518
30. Siegel RL, Fedewa SA, and Anderson WF, et al (2017) Colorectal cancer incidence patterns in the United States, 1974–2013 J Natl Cancer Inst 109(8) djw322 https://doi.org/10.1016/S2468-1253(19)30147-5 PMID: 31105047
31. Brenner DR, Ruan Y, and Shaw E, et al (2017) Increasing colorectal cancer incidence trends among younger adults in Canada Prev Med 105 345–349 https://doi.org/10.1016/j.ypmed.2017.10.007 PMID: 28987338
32. Liu PH, Wu K, and Ng K, et al (2019) Association of obesity with risk of early-onset colorectal cancer among women JAMA Oncol 5(1) 37–44 https://doi.org/10.1001/jamaoncol.2018.4280 PMCID: 6382547
33. Nguyen LH, Liu PH, and Zheng X, et al (2018) Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer JNCI Cancer Spectr 2(4) pky073 https://doi.org/10.1093/jncics/pky073
34. O'Connell JB, Maggard MA, and Livingston EH, et al (2004) Colorectal cancer in the young Am J Surg 187(3) 343–348 https://doi.org/10.1016/j.amjsurg.2003.12.020 PMID: 15006562
35. Chen FW, Sundaram V, and Chew TA, et al (2017) Low prevalence of criteria for early screening in young-onset colorectal cancer Am J Prev Med 53(6) 933–934 https://doi.org/10.1016/j.amepre.2017.07.016 PMID: 29051017 PMCID: 5873286
36. Chen FW, Sundaram V, and Chew TA, et al (2017) Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis Clin Gastroenterol Hepatol 15(5) 728–737.e3 https://doi.org/10.1016/j.cgh.2016.10.038
37. Pearlman R, Frankel WL, and Swanson B, et al (2017) Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer JAMA Oncol 3(4) 464–471 https://doi.org/10.1001/jamaoncol.2016.5194 PMCID: 5564179
38. Willauer AN, Liu Y, and Pereira AAL, et al (2019) Clinical and molecular characterization of early-onset colorectal cancer Cancer 125(12) 2002–2010 https://doi.org/10.1002/cncr.31994 PMID: 30854646 PMCID: 6583775
39. Khan SA, Morris M, and Idrees K, et al (2016) Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients J Pediatr Surg 51(11) 1812–1817 https://doi.org/10.1016/j.jpedsurg.2016.07.015 PMID: 27558481 PMCID: 5312708
40. Agyemang-Yeboah FA, Yorke J, and Obirikorang C, et al (2017) Patterns and presentations of colorectal cancer at Komfo-Anokye teaching hospital Kumasi, Ghana Pan Afr Med J 28 121
41. CRUK (2016) Cancer Awareness Measure (CAM) Key Findings Report; 2014 & Trends Aanalysis (2008–2014) (London: Cancer Research)
42. Tchernof A and Després JP (2013) Pathophysiology of human visceral obesity: an update Physiol Rev 93(1) 359–404 https://doi.org/10.1152/physrev.00033.2011 PMID: 23303913
43. Bassett JK, Severi G, and English DR, et al (2010) Body size, weight change, and risk of colon cancer Cancer Epidemiol Biomark Prev 19(11) 2978–2986 https://doi.org/10.1158/1055-9965.EPI-10-0543
44. Slattery ML, Potter JD, and Curtin K, et al (2001) Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer Cancer Res 61(1) 126–130 PMID: 11196149
45. Elder DJ, Baker JA, and Banu NA, et al (2002) Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression J Pathol 198(4) 428–434 https://doi.org/10.1002/path.1232 PMID: 12434411
46. Bamba H, Ota S, and Kato A, et al (1999) High expression of cyclooxygenase-2 in macrophages of human colonic adenoma Japan Int J Cancer 83(4) 470–475 https://doi.org/10.1002/(SICI)1097-0215(19991112)83:4<470::AID-IJC6>3.0.CO;2-F PMID: 10508481
47. Konno H, Baba M, and Shoji T, et al (2002) Cyclooxygenase-2 expression correlates with uPAR levels and is responsible for poor prognosis of colorectal cancer Clin Exp Metastasis 19(6) 527–534 https://doi.org/10.1023/A:1020392309715 PMID: 12405290
48. Tomozawa S, Tsuno NH, and Sunami E, et al (2000) Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer Br J Cancer 83(3) 324–328 https://doi.org/10.1054/bjoc.2000.1270 PMID: 10917546 PMCID: 2374554
49. Yamauchi T, Watanabe M, and Kubota T, et al (2002) Cyclooxygenase-2 expression as a new marker for patients with colorectal cancer Dis Colon Rectum 45(1) 98–103 https://doi.org/10.1007/s10350-004-6120-5 PMID: 11786771
50. Wu QB and Sun GP (2015) Expression of COX-2 and HER-2 in colorectal cancer and their correlation World J Gastroenterol 21(20) 6206–6214 https://doi.org/10.3748/wjg.v21.i20.6206 PMID: 26034355 PMCID: 4445097
51. Negi RR, Rana SV, and Gupta V, et al (2019) Over-expression of cyclooxygenase-2 in colorectal cancer patients Asian Pac J Cancer Prev 20(6) 1675–1681 https://doi.org/10.31557/APJCP.2019.20.6.1675 PMID: 31244287 PMCID: 7021602
52. Dimberg J, Hugander A, and Sirsjo A, et al (2001) Enhanced expression of cyclooxygenase-2 and nuclear beta-catenin are related to mutations in the APC gene in human colorectal cancer Anticancer Res 21(2A) 911–915 PMID: 11396184
53. Fujita M, Fukui H, and Kusaka T, et al (2000) Relationship between cyclooxygenase-2 expression and K-ras gene mutation in colorectal adenomas J Gastroenterol Hepatol 15(11) 1277–1281 PMID: 11129221
54. Rigas B, Goldman IS, and Levine L (1993) Altered eicosanoid levels in human colon cancer J Lab Clin Med 122(5) 518–523 PMID: 8228569
55. Loomans-Kropp HA, Pinsky P, and Cao Y, et al (2019) Association of aspirin use with mortality risk among older adult participants in the prostate, lung, colorectal, and ovarian cancer screening trial JAMA Netw Open 2(12) e1916729 https://doi.org/10.1001/jamanetworkopen.2019.16729 PMID: 31800071 PMCID: 6902761
56. Liao X, Lochhead P, and Nishihara R, et al (2012) Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival N Engl J Med 367(17) 1596–1606 https://doi.org/10.1056/NEJMoa1207756 PMID: 23094721 PMCID: 3532946
57. Nan H, Morikawa T, and Suuriniemi M, et al (2013) Aspirin use, 8q24 single nucleotide polymorphism rs6983267, and colorectal cancer according to CTNNB1 alterations J Natl Cancer Inst 105(24) 1852–1861 https://doi.org/10.1093/jnci/djt331 PMID: 24317174 PMCID: 3866156
58. Fink SP, Yamauchi M, and Nishihara R, et al (2014) Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD) Sci Transl Med 6(233) 233re2 https://doi.org/10.1126/scitranslmed.3008481 PMID: 24760190 PMCID: 4030641
59. Burn J, Gerdes AM, and Macrae F, et al (2011) Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial Lancet 378(9809) 2081–2087 https://doi.org/10.1016/S0140-6736(11)61049-0 PMID: 22036019 PMCID: 3243929
60. Watanabe K, Kawamori T, and Nakatsugi S, et al (2000) Inhibitory effect of a prostaglandin E receptor subtype EP1 selective antagonist, ONO-8713, on development of azoxymethane-induced aberrant crypt foci in mice Cancer Lett 156 57–61 https://doi.org/10.1016/S0304-3835(00)00440-7 PMID: 10840160
61. Chapple KS, Cartwright EJ, and Hawcroft G, et al (2000) Localization of cyclooxygenase-2 in human sporadic colorectal adenomas AJP 156(2) 545–553 PMID: 10666384 PMCID: 1850032
62. Miladi-Abdennadher I, Abdelmaksoud-Dammak R, and Ayed-Guerfali DB, et al (2012) Expression of COX-2 and E-cadherin in Tunisian patients with colorectal adenocarcinoma Acta Histochem 114(6) 577–581 https://doi.org/10.1016/j.acthis.2011.11.002
63. Al-Maghrabi J, Buhmeida A, and Emam E, et al (2012) Cyclooxygenase-2 expression as a predictor of outcome in colorectal carcinoma World J Gastroenterol 18(15) 1793–1799 https://doi.org/10.3748/wjg.v18.i15.1793 PMID: 22553404 PMCID: 3332293
64. Yoshinaga M, Taki K, and Somada S, et al (2011) The expression of both peroxisome proliferator-activated receptor delta and cyclooxygenase-2 in tissues is associated with poor prognosis in colorectal cancer patients Dig Dis Sci 56(4) 1194–1200 https://doi.org/10.1007/s10620-010-1389-9
65. Inafuku Y, Furuhata T, and Tayama M, et al (2009) Matrix metalloproteinase-2 expression in stromal tissues is a consistent prognostic factor in stage II colon cancer Cancer Sci 100(5) 852–858 https://doi.org/10.1111/j.1349-7006.2009.01116.x PMID: 19445018 PMCID: 11158796
66. Chen S, Liu J, and Li G, et al (2008) Altered distribution of beta-catenin and prognostic roles in colorectal carcinogenesis Scand J Gastroenterol 43(4) 456–464 https://doi.org/10.1080/00365520701785194 PMID: 18365911
67. Lim SC, Lee TB, and Choi CH, et al (2008) Prognostic significance of cyclooxygenase-2 expression and nuclear p53 accumulation in patients with colorectal cancer J Surg Oncol 97(1) 51–56 https://doi.org/10.1002/jso.20907
68. Zhan J, Liu JP, and Zhu ZH, et al (2004) Relationship between COX-2 expression and clinicopathological features of colorectal cancers Chin Med J (Engl) 117(8) 1151–1154 PMID: 15361286
69. Soumaoro LT, Uetake H, and Higuchi T, et al (2004) Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer Clin Cancer Res 10(24) 8465–8471 https://doi.org/10.1158/1078-0432.CCR-04-0653 PMID: 15623626
70. Wu AW, Gu J, and Ji JF, et al (2003) Role of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients' prognosis World J Gastroenterol 9(9) 1990–1994 https://doi.org/10.3748/wjg.v9.i9.1990 PMID: 12970891 PMCID: 4656659
71. Joo YE, Kim HS, and Min SW, et al Expression of cyclooxygenase-2 protein in colorectal carcinomas Int J Gastrointest Cancer 31(1-3) 147–154 PMID: 12622426
72. Masunaga R, Kohno H, and Dhar DK, et al (2000) Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients Clin Cancer Res 6(10) 4064–4068 PMID: 11051257
73. Pancione M, Forte N, and Sabatino L, et al Reduced beta-catenin and peroxisome proliferator-activated receptor-gamma expression levels are associated with colorectal cancer metastatic progression: correlation with tumor-associated macrophages, cyclooxygenase 2, and patient outcome Hum Pathol 40(5) 714–725 PMID: 19121846
74. Debucquoy A, Goethals L, and Libbrecht L, et al (2009) Molecular and clinico-pathological markers in rectal cancer: a tissue micro-array study Int J Colorectal Dis 24(2) 129–138 https://doi.org/10.1007/s00384-008-0608-8 PMCID: 2745734
75. Giralt J, Navalpotro B, and Hermosilla E, et al (2006) Prognostic significance of vascular endothelial growth factor and cyclooxygenase-2 in patients with rectal cancer treated with preoperative radiotherapy Oncology 71(5-6) 312–319 https://doi.org/10.1159/000107105
76. Yamac D, Celenkoglu G, and Coskun U, et al (2005) Prognostic importance of COX-2 expression in patients with colorectal cancer Pathol Res Pract 201(7) 497–502 https://doi.org/10.1016/j.prp.2005.04.006 PMID: 16164044
77. Fux R, Schwab M, and Thon KP, et al (2005) Cyclooxygenase-2 expression in human colorectal cancer is unrelated to overall patient survival Clin Cancer Res 11(13) 4754–4760 https://doi.org/10.1158/1078-0432.CCR-04-2586 PMID: 16000571
78. Buecher B, Heymann MF, and Lievre A, et al (2003) Cyclo-oxygenase-2 over-expression in sporadic colorectal carcinoma without lymph node involvement Aliment Pharmacol Ther 18(7) 731–740 https://doi.org/10.1046/j.1365-2036.2003.01758.x PMID: 14510747
79. Zhang H and Sun XF (2002) Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer Am J Gastroenterol 97(4) 1037–1041 https://doi.org/10.1111/j.1572-0241.2002.05625.x PMID: 12003384
80. Sheehan KM, Sheahan K, and O'Donoghue DP, et al (1999) The relationship between cyclooxygenase-2 expression and colorectal cancer JAMA 282(13) 1254–1257 https://doi.org/10.1001/jama.282.13.1254 PMID: 10517428
81. Ogino S, Nosho K, and Baba Y, et al (2009) A cohort study of STMN1 expression in colorectal cancer: body mass index and prognosis Am J Gastroenterol 104(8) 2047–2056 https://doi.org/10.1038/ajg.2009.281 PMID: 19513025 PMCID: 2866652






