A hospital-based study of survival in oral cancer patients of Tata Memorial Hospital, Mumbai
Monika Lokhande1,2,a, Sivaranjini Kannusamy1,2, Amey Oak1,2,b, Sandhya Cheulkar1,2, Shalmali Chavan1,2, Varsha Mishra1,2, Pragati Gode1,2, Aijimol S Thakadiyil1,2, Saket Mendhe1,2, Supriya Kadam1,2, Ganesh Balasubramaniam1, Pankaj Chaturvedi1 and Rajesh Dikshit1
1Homi Bhabha National Institute, Mumbai 400094, India
2Division of Cancer Care, Hospital Cancer Registries and Survival Studies, Centre for Cancer Epidemiology, Tata Memorial Centre, Mumbai 400094, India
ahttps://orcid.org/0009-0005-2587-7188
bhttps://orcid.org/0009-0004-1893-4191
Abstract
Introduction: Oral cancer represents a significant global public health concern, with the death rate for lip and oral cavity malignancies experiencing a 1.40-fold increase worldwide in the past three decades. This retrospective study aimed to comprehensively understand overall survival (OS) and the influence of sociodemographic and clinical factors on patients diagnosed with oral cavity cancer.
Materials and methods: The study focused on oral cancer patients enrolled in 2016 and treated at Tata Memorial Hospital, Mumbai, with a follow-up period extending to 5 years until 2021. Utilising the Kaplan–Meier technique and log-rank test, we examined OS and variations based on sociodemographic factors, while the Cox proportional hazard model allowed us to investigate the simultaneous impact of multiple factors on OS.
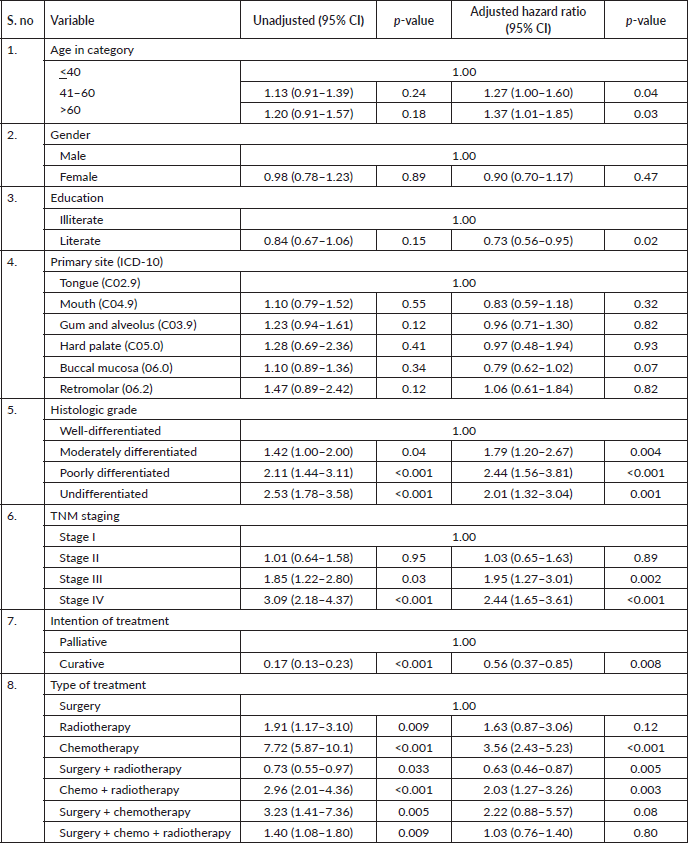
Results: A total of 1,895 eligible participants were included. The overall 5-year survival rate was 65%. After adjusting for age, gender, education, primary site, tumour grade, TNM staging, treatment intention, status and modality, we found in our study oral cancer patients aged more than 60 years (HR = 1.37, 95% CI: 1.01–1.85, p-value 0.03), patients who had poorly differentiated carcinoma (HR = 2.44, 95% CI: 1.56–3.81, p-value < 0.001), belonged to stage IV as per TNM staging (HR = 2.44, 95% CI: 1.65–3.61, p-value < 0.001), patient who have received partial treatment (HR = 2.44, 95% CI: 1.65–3.61, p-value < 0.001) and only chemotherapy (HR = 3.56, 95% CI: 2.43–5.23, p-value < 0.001) found to have a higher hazard of dying while literate (HR = 0.73, 95% CI: 0.56–0.95, p-value 0.02) are protective.
Limitations: The retrospective nature of the study posed constraints in exploring additional variable associations.
Implication: Overall early detection, appropriate treatment, and regular follow-up are critical for improving the survival rate of patients with oral cavity cancer.
Conclusion: This research proposes that improving the socioeconomic status and promoting proactive treatment-seeking behaviour is crucial for enhancing the survival of oral cancer patients. Cancer hospitals, in collaboration with the wider public healthcare system in India, which includes clinicians and policymakers, should consider these suggestions to enhance cancer treatment and control in low–middle-income countries.
Keywords: oral cancer, sociodemographic factors, clinical factors, registry, survival, India
Correspondence to: Amey Oak
Email: ameyoak55@gmail.com
Published: 15/02/2024
Received: 30/11/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oral cancer is a serious public health issue globally. The death rate for malignancies of the lip and oral cavity has increased 1.40 times globally over the past three decades. Although smoking is still contributing to up to one-third (30.5%) of worldwide fatalities from this cancer [1]. In 2018, there were 354,864 new instances of oral cancer and 177,384 oral cancer-related deaths worldwide [2]. As per the GLOBOCAN 2020, India has the highest age-standardised incidence rate for oral cancer, with a rate of 9.6 in the South East Asia region [1]. In India, oral cancer ranks in the top three of all cancers and accounts for more than 30% of all cancer cases [2]. Every year, India reports a quarter of all cases worldwide (approximately 77,000 new cases and 52,000 deaths) [3]. South and Southeast Asian countries including India, have the highest rate of oral cancer, as a result, oral cancer prevention is quickly becoming a major global health concern. Survival rates for patients with oral cancer are significantly influenced by sociodemographic and clinical factors [4, 5]. A comprehensive understanding of sociodemographic and clinical characteristics is required to provide targeted treatments, customised treatment programmes and effective public health initiatives. By addressing these traits, healthcare professionals can increase the survival rate of patients with oral cancer by promoting earlier detection, more treatment compliance and easier access to care.
Despite advancements in cancer detection and treatment over the past few decades, 5-year survival rates for oral cancer are still in the 50%–60% range [6, 7]. This study focuses on sociodemographic and clinical parameters to assess their effects on oral cancer patients’ survival rates. This study was conducted at Tata Memorial Hospital (TMH), a top cancer centre in India that offers cancer diagnosis, treatment, education and research. It gives you access to numerous cancer-related investigations, therapies and patient follow-ups. TMH has a homegrown electronic medical record system (EMR), the contents of which include various modules concerning patients’ information entered by clinicians, the modules are shared with patients and providers via the hospital’s intranet and globally via our website. TMH has been carrying out paperless and filmless operations since 2013, enabling the real-time exchange of information and ensuring a continuum of care. This information will be useful for the various research investigations.
TMH has upgraded treatment facilities for patients so that research can determine which treatment is more effective for the patient’s survival. It was useful to comprehend the cancer survival rates by geographic region because at TMH patients often visit from all around India. This study helped us to understand the relationship between sociodemographic and clinical variables on the prognosis of patients with oral cancer. Current data from 2016 was used in this study, and there was a 5-year follow-up from 2016 to 2021.
Materials and methods
The study was done after the approval from Institute Ethics Committee (IEC). The study included a retrospective review of hospital data from the TMH Cancer Registry, which included all diagnosed oral cancer patients who were recruited at TMH. Oral cancer patients who were recruited at TMH between 1st January 2016 and 31st December 2016, and received treatment at the hospital (TMH). The information was extracted from the patient’s file and the hospital’s EMR. Data collection has been done by using a comprehensive questionnaire that has three sections namely sociodemographic, clinical and follow-up details. Demographic and clinical characteristics such as age, gender, region, occupation, education (a person who cannot read or write is considered as illiterate), marital status, income, initial tumour site, histology of the tumour, grade, location, extent, type of case (new – patients who newly enrolled and received treatment at TMH and old – patients had received treatment outside before coming to TMH) and treatment were noted. Oral cavity subsites were divided into buccal mucosa, tongue, mouth, alveolus, retromolar trigone and hard palate. Surgery, radiation and chemotherapy have all been considered in the treatment analysis. Every 6 months, follow-up was conducted on a regular basis. The follow-up period was commenced from January 2016 till December 2021 for each patient. Follow-up information was collected through the EMR as well as by phone. The follow-up period ended on 31 December 2021. The patients’ overall survival (OS) duration was the period from the date of diagnosis and the date of death or the date of the final follow-up, whichever came first. Overall, 5-year survival was calculated for patients with oral cancer, from January 2016 to December 2021. In addition, the study investigated the influence of sociodemographic and clinical factors on the OS of individuals with oral cancer. The confidentiality of clinical and treatment data was protected. Data entry has been done in-house software. Data analysis was done by using STATA software version 15.0. The mean, standard deviation, median and range, proportion and 95% confidence intervals (CIs) were used to summarise the analytical findings. Kaplan–Meier test and the log-rank test to evaluate OS and variations in survival rates across diverse sociodemographic and clinical factors. Cox proportional hazard models were used to examine the simultaneous impact of various factors on OS in a multifactorial scenario. p-value < 0.05 was taken to be significant.
Results
In 2016, Tata Memorial Centre in Mumbai treated a total of 1,895 cases of oral cancer, with a mean age of 48.36 (11.50). A significant majority of patients (81.74%) were male. Buccal mucosa (C06.0) and tongue (C02) constituted over 70% of cases, while lip (C00) and oral cavity (06.9) were the least common sites. Squamous cell carcinoma was the predominant histology (96.09%). Alarmingly, half of these patients (50.18%) were already at an advanced stage (stage IV). Treatment completion showed that the vast majority (87.49%) completed their treatment, with only 12.51% discontinuing treatment. See Tables 1 and 2 and Figures 1–5.
Table 1. Distribution of oral cancer patients by sociodemographic and clinical characteristics.
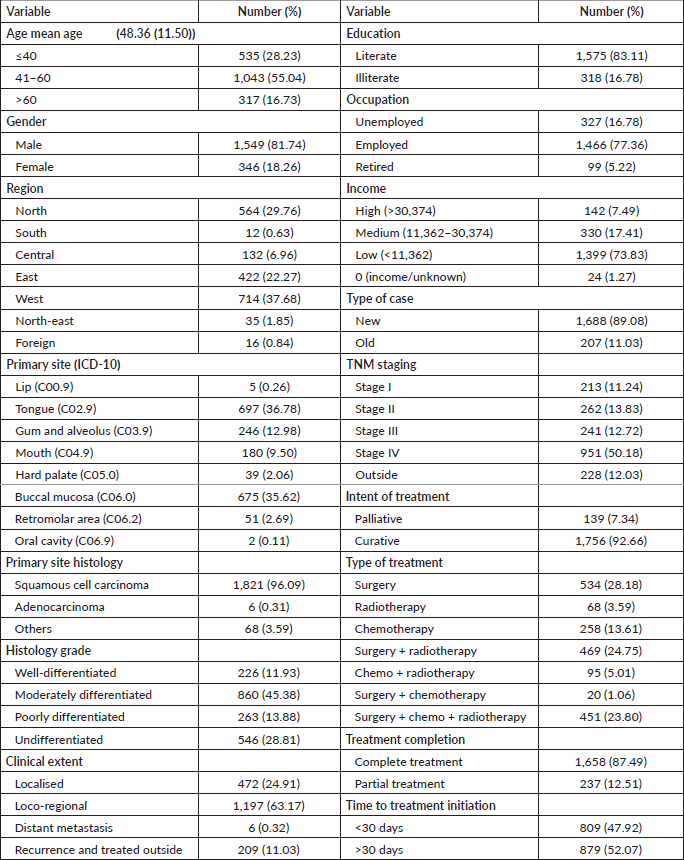
Table 2. OS based on sociodemographic and clinical characteristics.
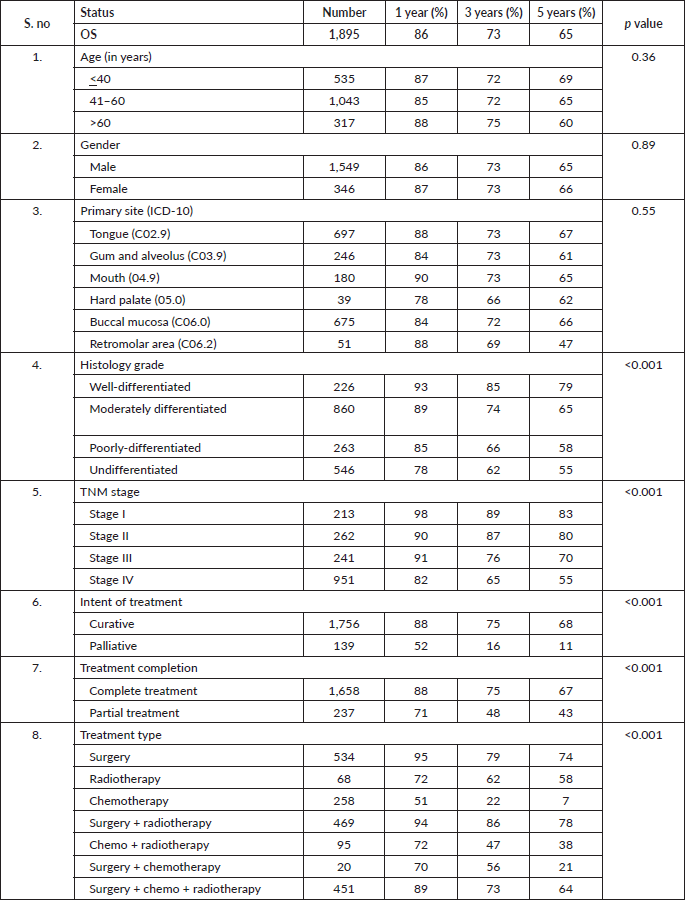
At the end of the follow-up on 31 December 2021, 24% of the 1,895 patients had passed away, 41% were censored and the overall 5-year survival rate was 65%. Notably, patients aged 40 or younger had a 5-year survival rate (69%), which was unrelated to the stage of illness. Patients in younger age groups (stage I, 64; stage II, 65; stage III, 61 and stage IV, 272) outnumbered those aged 41–60 (65%), and above 60 (60%). The 5-year survival rate for males and females was 65% and 66%, respectively. Site-specific 5-year survival rates varied, with the tongue at 67%, floor of the mouth at 65%, gum and alveolus at 61%, hard palate at 62%, buccal mucosa at 66% and retromolar at 47%. Well-differentiated tumours had a 5-year survival rate of 79%, moderately differentiated at 65%, and poorly and undifferentiated at 58% and 55%, respectively. Stage-wise 5-year survival rates showed a decreasing trend from stage I (83%) to stage IV (55%). Curative patients had a higher 5-year survival rate (68%) compared to palliative patients (11%). Patients who received partial treatment had a significantly lower 5-year survival rate (43%) compared to those who completed treatment which was 67%. The 5-year survival rate for surgery, the most common initial treatment, was 74%, radiotherapy was 58%, and chemotherapy was only 7%. Combination therapies varied, with surgery + radiotherapy having the highest (78%) and surgery + chemotherapy having the lowest (21%) 5-year survival rates, showing significant differences. New patients had a 5-year survival rate of 67%, while old patients had a rate of 50%, and this difference was highly significant (p-value < 0.001) (Table 3).
After adjusting for age, gender, education, primary site, tumour grade, TNM staging, treatment–intention, status and modality, we found in our study oral cancer patients aged more than 60 years (HR = 1.37, 95% CI: 1.01–1.85, p-value 0.03), patients who had poorly differentiated carcinoma (HR = 2.44, 95% CI: 1.56–3.81, p-value < 0.001), belonged to stage IV as per TNM staging (HR = 2.44, 95% CI: 1.65–3.61, p-value
< 0.001), patient who have received partial treatment (HR = 2.44, 95% CI: 1.65–3.61, p-value < 0.001) and only chemotherapy (HR = 3.56, 95% CI: 2.43–5.23, p-value < 0.001) found to have a higher hazard of dying while literate (HR = 0.73, 95% CI: 0.56–0.95, p-value 0.02), cancer involving buccal mucosa sites (HR = 0.79, 95% CI: 0.62–1.02, p-value 0.07) and patient who had the curative intention of treatment 0.56 (HR = 0.56, 95% CI: 0.37–0.85, p-value 0.008) had a better prognosis.
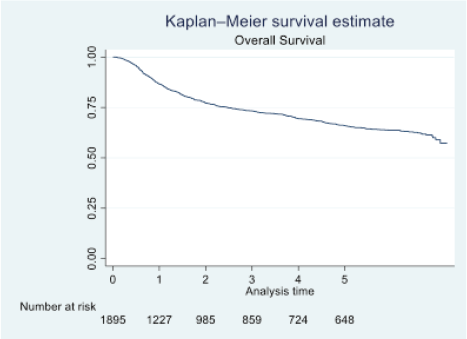
Figure 1. OS of oral cavity cancer patients of TMH, Mumbai, in 2016 (N = 1,895).
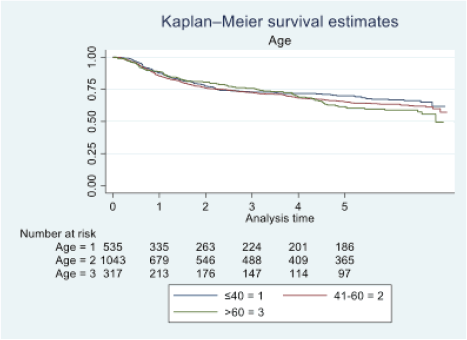
Figure 2. Five-year survival based on the age of oral cancer patients of TMH, Mumbai, in 2016 (N = 1,895).
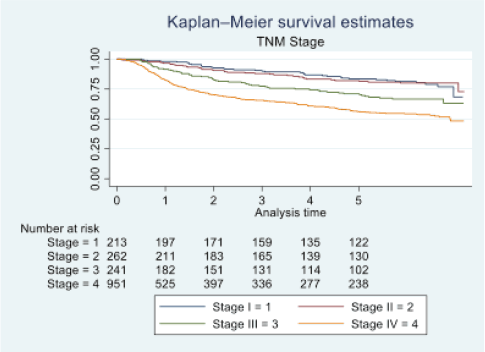
Figure 3. Five-year survival based on TNM stage of oral cancer patients of TMH, Mumbai, in 2016 (N = 1,895).
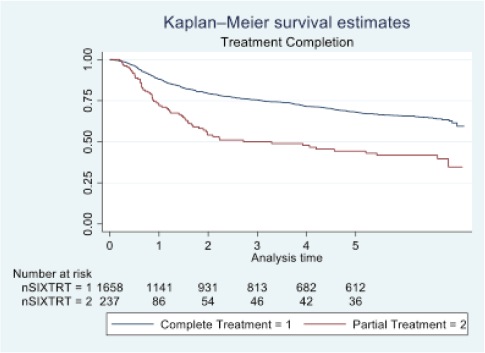
Figure 4. Five-year survival based on treatment completion of oral cancer patients of TMH, Mumbai, in 2016 (N = 1,895).
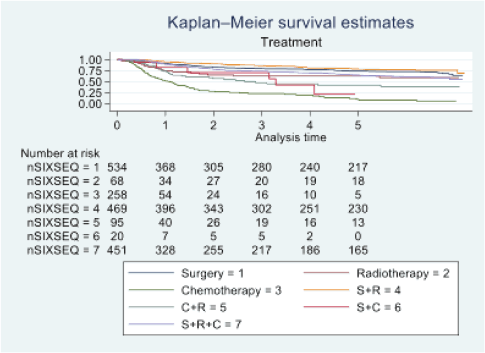
Figure 5. Five-year survival based on the type of treatment of oral cancer patients of TMH, Mumbai, in 2016 (N = 1,895).
Discussion
This study, conducted at TMH, Mumbai in 2016, aims to determine the 5-year survival rates among individuals diagnosed with oral cancer. The research also investigates sociodemographic and clinical prognostic factors influencing oral cancer survival. Among the 1,895 registered oral cancer patients, 24% succumbed within 5 years and 41% were lost to follow-up. The OS rates in our study for the first, third and fifth years were 86%, 73% and 65%, respectively. In comparison to Yeole et al’s [8] findings, our study shows higher survival rates, potentially attributed to differences in data sources. Yeole et al’s [8] study included cases from the Mumbai cancer registry, incorporating data from death certificates with broader criteria. In contrast, our analysis focuses exclusively on patients registered and treated at TMH. The OS rate in Zanoni et al’s [9] study, which considered various factors, closely aligns with our findings. Listl et al’s [10] study reports a lower 5-year relative survival rate of 54.6%, and Kowalski et al [11] found a 51.7% OS rate in their research on oral squamous cell carcinoma patients from 2001 to 2012, both lower than our study’s rates.
According to our findings, individuals under 40 exhibited a higher 5-year survival rate (69%) compared to those over 60 (60%). In a study by Ansarin et al [12] at the European Institute of Oncology, they investigated 577 consecutive patients. Notably, advanced tongue cancer demonstrated lower fatality rates among younger individuals than their older counterparts, aligning with our study’s outcomes. In a study by Lin et al [13], 85 patients over the age of 70 who underwent surgery for early-stage oral cavity squamous cell carcinoma (OCSCC) were retrospectively examined. Disease-free survival and OS were estimated, revealing that elderly individuals with early-stage OCSCC may experience disease progression after surgery. In our study, the hazard ratio indicated that females (0.98) had a slightly more protective effect than males. In terms of education, the majority of patients in our study were literate. Those with some form of education had a 5-year survival rate of 66%, while illiterate individuals had a rate of 61%. Mathew et al [14] found varying survival rates based on literacy levels, with illiterate/primary, middle school and secondary school and above groups having rates of 42%, 35% and 48%, respectively. Income status was divided into high, medium and low categories, with corresponding 5-year survival rates of 66%, 62% and 66%. Goswami et al [15] noted a correlation between oral cancer patients and income, with a high incidence of catastrophic health expenses reported due to the cost of treatment.
Table 3. Multivariate analysis of risk factors for OS of patients with oral cavity cancer using Cox regression (N = 1,895).
The 5-year survival rate for tongue cancer was 67%, while buccal mucosa cancer had a 5-year survival rate of 66%. Comparative studies indicate varying 5-year survival rates for tongue cancer, ranging from 50% to 68%, with our study aligning closely with the higher end of this spectrum [16, 17]. Bobdey et al [18] found a significantly lower 5-year survival rate of 54.01% for buccal mucosa patients in their retrospective analysis of 409 cases. Examining primary site histology, 96% of our study’s patients had squamous cell carcinoma, with a 65% 5-year survival rate. In contrast, Bakshi et al [19] reported a lower 5-year survival rate of 58.3% for OCSCC. Fan et al’s [20] study on young Chinese patients with OCSCC showed a higher 5-year survival rate of 75%. Poorly differentiated tumours in our study exhibited lower 5-year survival rates (58%) compared to well-differentiated (79%) and moderately differentiated (65%) tumours. Low survival rates for poorly differentiated tumours were also discovered in other research studies [21–24]. Regarding the extent of the disease, our study revealed a 5-year survival rate of 56% for localised cases and 59% for loco-regional cases. However, the study had limited data on distant metastasis cases, making it challenging to determine OS for this group. Yeole et al [8] reported lower 5-year survival rates for distant metastases (1.6%) compared to our findings. Analysing survival based on TNM staging, our study demonstrated decreasing survival rates from stage I (83%) to stage IV (55%). This trend aligns with international findings, including a multicentre analysis reporting similar stage-wise survival percentages [25]. The combination of surgery and radiotherapy in our study was associated with the highest 5-year survival rate (78%), while chemotherapy alone had the lowest rate (7%). This aligns with existing research, emphasising the positive impact of surgery and radiotherapy on survival rates [26]. Clinical characteristics in oral cancer, such as primary site, histology, stage and treatment methods, have important implications for public health measures as well as individual patient care. TNM and tumour stage classification were also more significant prognostic variables for these patients’ survival, helping to identify high-risk groupings and guiding therapy. A comprehensive strategy that incorporates prevention, early identification, personalised therapy and supportive care is essential for increasing OS rates and improving the quality of life among individuals impacted by oral cancer.
Strengths of our research include a diverse patient population from across India, enhancing the generalisability of our findings. However, limitations include the exclusion of comorbid conditions that could impact survival and a lack of comprehensive data on patients with distant metastasis.
Recommendations from our study, the study revealed that patients who received their complete course of treatment had a considerably higher rate of survival than those who abandoned their treatment in the middle or discontinued it altogether. Healthcare providers can develop educational materials and counselling programmes that help patients understand the importance of completing their oral cancer treatment to improve their survival rates and quality of life.
After a telephone follow-up, we discovered that the patient’s mental and physical health had deteriorated, prompting the need for support services including pain management and mental health services to assist patients in coping with the mental and physical difficulties brought on by cancer therapy. Overall early detection, appropriate treatment and regular follow-up are critical for improving the survival rate of patients with oral cavity cancer.
Conclusion
We have deduced from this study that age is the predictive factor that really affects the survival of patients with oral cavity cancer. Our study’s findings showed that education significantly influences survival. Factors contributing to this discrepancy may include improved health literacy, access to healthcare information and enhanced ability to navigate healthcare systems effectively among literate individuals. with buccal mucosa primary site patients showing a significant protective effect. Poorly differentiated, TNM stage IV is considered to be hazardous. Treatment also plays a crucial effect in patient survival. Patients who received complete treatment had significantly greater survival rates compared to those who received partial treatment.
Acknowledgment
The authors would like to acknowledge the staff in the Division of HBCR and POCSS for their immense contribution and continuous technical support in completing the study.
Conflicts of interest
There is no conflict of interest.
Funding
There is no funding received for this study.
Informed consent
The database of the hospital-based cancer registry is under the custody of authorised study personnel. The authors have ensured that the user ID and password for accessing the data are protected. The authors ensure that the rights of the patients are not violated in any manner.
Author contributions
Ms Monika Lokhande has contributed to the design, statistical analysis, data interpretation and writeups of the manuscript. Dr Sivaranjini has been involved in concept, design, statistical analysis and data interpretation. Dr Amey Oak gave knowledgeable direction and oversight to ensure the study’s methodological rigor and write-ups. Sandhya Cheulkar has contributed to data validation and interpretation. To obtain ethical approval, Dr Shalmali Chavan assisted as well as communicated with the Institutional Review Board (IRB). Dr Varsha, Dr Pragati and Dr Aijimol carried out the data collection and coding and Dr Ganesh Balasubramanium and Dr Pankaj Chaturvedi made substantial contributions to the study’s administration and conceptualisation. Dr Rajesh Dikshit has contributed to the concept, design and management, and assisted in drafting the final report.
References
1. Nocini R, Lippi G, and Mattiuzzi C (2019) The worldwide burden of smoking‐related oral cancer deaths Clin Exp Dent Res 6(2) 161–164 https://doi.org/10.1002/cre2.265
2. Zhang SZ, Xie L, and Shang ZJ (2022) Burden of oral cancer on the 10 most populous countries from 1990 to 2019: estimates from the global burden of disease study 2019 Int J Environ Res Public Health 19(2) 875 https://doi.org/10.3390/ijerph19020875 PMID: 35055693 PMCID: 8775770
3. Borse V, Konwar AN, and Buragohain P (2020) Oral cancer diagnosis and perspectives in India Sens Int 1 100046 https://doi.org/10.1016/j.sintl.2020.100046 PMID: 34766046 PMCID: 7515567
4. Ogden GR (2020) Factors affecting survival for oral cancer Textbook of Oral Cancer: Prevention, Diagnosis and Management [Internet] eds S Warnakulasuriya and JS Greenspan (Cham: Springer International Publishing) Date accessed: 03/05/23 https://doi.org/10.1007/978-3-030-32316-5_25
5. Wong YK, Tsai WC, and Lin JC, et al (2006) Socio-demographic factors in the prognosis of oral cancer patients Oral Oncol 42(9) 893–906 https://doi.org/10.1016/j.oraloncology.2005.12.007 PMID: 16730220
6. Saka-Herrán C, Jané-Salas E, and Mari-Roig A, et al (2021) Time-to-treatment in oral cancer: causes and implications for survival Cancers [Internet] 13(6) 1321 Date accessed: 03/10/23 https://doi.org/10.3390/cancers13061321 PMID: 33809427 PMCID: 8000007
7. Jitender S, Sarika G, and Varada HR, et al (2016) Screening for oral cancer J Exp Ther Oncol [Internet] 11(4) 303–307 PMID: 27849341
8. Yeole BB, Ramanakumar AV, and Sankaranarayanan R (2003) Survival from oral cancer in Mumbai (Bombay), India Cancer Causes Control CCC 14(10) 945–952 https://doi.org/10.1023/B:CACO.0000007965.61579.b2
9. Zanoni DK, Montero PH, and Migliacci JC, et al (2019) Survival outcomes after treatment of cancer of the oral cavity (1985-2015) Oral Oncol 90 115–121 https://doi.org/10.1016/j.oraloncology.2019.02.001 PMID: 30846169 PMCID: 6417804
10. Listl S, Jansen L, and Stenzinger A, et al (2013) Survival of patients with oral cavity cancer in Germany PLoS One 8(1) e53415 https://doi.org/10.1371/journal.pone.0053415 PMID: 23349710 PMCID: 3548847
11. Kowalski LP, de Oliveira MM, and Lopez RVM, et al (2020) Survival trends of patients with oral and oropharyngeal cancer treated at a cancer center in São Paulo, Brazil Clinics 75 e1507 https://doi.org/10.6061/clinics/2020/e1507
12. Ansarin M, De Berardinis R, and Corso F, et al (2021) Survival outcomes in oral tongue cancer: a mono-institutional experience focusing on age Front Oncol [Internet] 11 616653 [https://www.frontiersin.org/articles/10.3389/fonc.2021.616653] PMID: 33912446 PMCID: 8075362
13. Lin FY, Huang CC, and Hung LC, et al (2020) Older patients with early-stage oral cavity squamous cell carcinoma: outcomes for elderly patients aged 70 years or older Ther Radiol Oncol [Internet] 4(0) [https://tro.amegroups.com/article/view/6321]
14. Mathew A, George PS, and Kunnambath R, et al (2020) Educational status, cancer stage, and survival in South India: a population-based study JCO Glob Oncol 6 GO.20.00259 PMID: 33156718 PMCID: 7713566
15. Goswami S, Gupta S, and Gangane N, et al (2022) Financial impact of oral cancer treatment on the households in rural India Indian J Cancer
16. Cancer Treatment Centers of America [Internet] (2022) Tongue Cancer: Symptoms, Causes, Treatment & Survival Rate (Cancer Treatment Centers of America) [https://www.cancercenter.com/cancer-types/oral-cancer/types/tongue-cancer] Date accessed: 01/05/23
17. A brief description about the survival rates of tongue cancer – RGCIRC [Internet] [https://www.rgcirc.org/blog/what-are-the-survival-rates-of-tongue-cancer/] Date accessed: 01/05/23
18. Bobdey S, Sathwara J, and Jain A, et al (2018) Squamous cell carcinoma of buccal mucosa: an analysis of prognostic factors South Asian J Cancer 7(1) 49–54 Date accessed: 01/05/23 https://doi.org/10.4103/sajc.sajc_317_16 PMID: 29600236 PMCID: 5865098
19. Bakshi J, Kaur N, and Tiwana H, et al (2023) Survival analysis of oral squamous cell carcinoma patients attending tertiary care centre of North India Indian J Surg Oncol 14(1) 234–242 https://doi.org/10.1007/s13193-020-01187-3 PMID: 36891418 PMCID: 9986144
20. Fan Y, Zheng L, and Mao MH, et al (2014) Survival analysis of oral squamous cell carcinoma in a subgroup of young patients Asian Pac J Cancer Prev APJCP 15(20) 8887–8891 https://doi.org/10.7314/APJCP.2014.15.20.8887 PMID: 25374224
21. Gleber-Netto FO, Yakob M, and Li F, et al (2016) Salivary biomarkers for detection of oral squamous cell carcinoma in a Taiwanese population Clin Cancer Res 22(13) 3340–3347 https://doi.org/10.1158/1078-0432.CCR-15-1761 PMID: 26847061 PMCID: 4930722
22. Nguyen TV and Yueh B (2002) Weight loss predicts mortality after recurrent oral cavity and oropharyngeal carcinomas Cancer 95(3) 553–562 https://doi.org/10.1002/cncr.10711 PMID: 12209747
23. Kosunen A, Pirinen R, and Ropponen K, et al (2007) CD44 expression and its relationship with MMP-9, clinicopathological factors and survival in oral squamous cell carcinoma Oral Oncol 43(1) 51–59 https://doi.org/10.1016/j.oraloncology.2006.01.003
24. Noguchi M, Kido Y, and Kubota H, et al (1999) Prognostic factors and relative risk for survival in N1-3 oral squamous cell carcinoma: a multivariate analysis using Cox’s hazard model Br J Oral Maxillofac Surg 37(6) 433–437 https://doi.org/10.1054/bjom.1999.0146
25. Macquarie University [Internet] Improvement in Survival of Patients With Oral Cavity Squamous Cell Carcinoma: an International Collaborative Study (Macquarie University) [https://researchers.mq.edu.au/en/publications/improvement-in-survival-of-patients-with-oral-cavity-squamous-cel/fingerprints/] Date accessed: 01/05/23
26. Leite IC and Koifman S (1998) Survival analysis in a sample of oral cancer patients at a reference hospital in Rio de Janeiro, Brazil Oral Oncol 34(5) 347–352 https://doi.org/10.1016/S1368-8375(98)00019-0 PMID: 9861339






