Association between oesophageal cancer and biomass smoke exposure: a case-control study
Violet Kayamba1a, Chola Mulenga1, Malambo Mubbunu1, Lydia Kazhila1, Phoebe Hodges1,2 and Paul Kelly1,2
1Tropical Gastroenterology and Nutrition Group, University of Zambia School of Medicine, PO Box 50398, Lusaka, Zambia
2Blizard Institute, Barts & The London School of Medicine and Dentistry, Queen Mary University of London, 4 Newark Street, London E1 2AT, UK
ahttps://orcid.org/0000-0002-6521-2501
Abstract
Most African populations are regularly exposed to biomass smoke, but knowledge of associated health implications is limited. This study aimed to investigate the association between oesophageal cancer (OC) and exposure to biomass smoke. This case-control study was conducted in Lusaka, Zambia. Cases were patients with endoscopically diagnosed OC, while controls were healthy volunteers. Questionnaires were used to collect lifestyle risk factors. Two sets of data were analysed; one with unmatched cases and controls and the other one with matching by age and sex. We enrolled 366 patients (131 cases and 235 controls). Among the cases, 50 (38%) were female and the median age was 56 years (IQR = 46–65 years). OC was significantly associated with domestic exposure to biomass smoke in univariate analysis (OR: 3.1; 95% CI: 1.7–5.6, p < 0.001) and after adjusting for potential confounders (OR: 2.1; 95% CI: 1.1–3.8, p = 0.017). Matched comparisons showed similar results for this association in univariate analysis (OR: 2.9; 95% CI: 1.5–5.8, p < 0.001) and using conditional logistic regression (OR: 2.8; 95% CI: 1.3–5.9, p = 0.005). Other risk factors found to be associated with OC were rural residence (OR: 2.3; 95% CI: 1.0–5.3, p = 0.004), lack of formal education (OR: 3.9; 95% CI: 1.5–9.9, p = 0.04) and living in poor housing (OR: 2.4; 95% CI: 1.1–5.6, p = 0.034). In conclusion, there is an association between OC and domestic exposure to biomass smoke and other lifestyle factors linked to low socio-economic status.
Keywords: oesophageal cancer, Zambia, biomass, risk factors
Correspondence to: Violet Kayamba
Email: viojole@yahoo.com
Published: 04/07/2022
Received: 06/01/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In 2020, 604,100 oesophageal cancer (OC) cases and 544,076 deaths were recorded worldwide [1]. The two main histological subtypes of OC are oesophageal squamous cell carcinoma (OSCC) and adenocarcinoma [2]. About 80% of the OSCC cases occur in low- and middle-income countries. Definite risk factors of OSCC are tobacco and alcohol with evidence for the others, including poor oral hygiene, caustic injury to the oesophagus and radiation [3], which is still being investigated. Recently, there has been growing evidence implicating the consumption of hot beverages as a risk factor for OSCC [4–6]. On the contrary, obesity and Barrett’s oesophagus are significant risk factors for oesophageal adenocarcinoma [7].
There are clear geographical variations in OSCC incidence across Africa [8, 9]. The highest incidence of OSCC in Africa is along the eastern corridor—extending from Ethiopia to parts of South Africa [10]. The OSCC epidemiological variation across Africa justifies the need for further aetiological research within individual countries. There is growing evidence linking lifestyle and environmental factors [10–12] in OSCC pathology across Africa. In addition, recent studies have explored the role of genetics on OSCC development in Africa, such as that by Moody et al [13].
While biomass smoke has not yet been confirmed on the list of definite OC [14] risk factors, we recently reported a significant association between increased OSCC risk and biomass smoke exposure [15]. Exposure to biomass smoke is very common in Africa with over 70% of its population relying on biomass fuels [16]. Biomass smoke constitutes a complex mixture of compounds, some of which are similar to known tobacco smoke-related carcinogens [17]. It is therefore possible that biomass smoke could be influencing carcinogenesis using similar mechanisms to cigarette smoke.
This study aimed to investigate the influence of biomass smoke exposure (in connection with other risk factors) on OC development using a case-control study approach.
Methods
This hospital-based case-control study was conducted at the University Teaching Hospital (UTH) in Lusaka, Zambia, between October 2018 and May 2021. UTH is the largest referral hospital in Zambia. OC patients above the age of 18 years, presenting to the UTH endoscopy unit for upper gastrointestinal endoscopy were considered for enrolment. Healthy controls were enrolled among caregivers and other asymptomatic volunteers within the UTH. They were above 18 years and without any obvious health condition. All study participants gave written consent. We excluded individuals with prior history of cancer diagnosis or therapy. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the University of Zambia Biomedical Research Ethics Committee (ref. no. 001-11-17).
Study procedure
Enrolment of study participants
All patients with oesophageal lesions suspected to be malignant on endoscopy (during the study period) were requested to participate as cases. During the endoscopy, at least six biopsies were collected from the lesions in accordance with standard of care. These were fixed in 10% neutral buffered formalin for histopathological processing. Slides were stained using routine haematoxylin and eosin to facilitate histological diagnosis of OC subtypes. Enrolled as controls were healthy volunteers who did not have any gastrointestinal symptoms. In addition, we also enrolled controls from among persons who had escorted relatives for endoscopy. We used interviewer-administered questionnaires to collect information on OC risk factors. Included in the questionnaire were demographic, medical history, lifestyle and OC environmental risk questions.
Exposure assessment
To determine exposure to biomass smoke, we asked study participants about the source of fuel used for cooking, heating and lighting in their homes. Those who admitted to being reliant on firewood, charcoal, dung or grass were categorised as having exposure to biomass smoke. We collected details on housing from the participants, including the type of roofing, wall, number of rooms and cooking areas. We also asked about the availability of basic items such as television sets, fridges, computers, microwave ovens, cars, etc. We gathered information on whether or not the water sources to participants’ households were piped. Study participants were asked about a history of eating soil (geophagia). The same questionnaires also sought a history of alcohol consumption and tobacco use.
Data analysis and sample size calculation
To calculate the required sample size, we used previously published work [14]. We found that 68% of the OC patients and 38% of the endoscopic controls were regularly exposed to biomass smoke in their homes. At 90% power and an alpha level of 0.05, at a 1:1 proportion of cases to controls, we needed at least 63 cases and 63 controls.
Data were analysed using Stata version 15. Continuous variables were summarised using median and interquartile ranges as the data were non-parametric when tested using the Shapiro–Wilk test. Percentages were used to summarise categorical variables. Analysis was carried out for both matched and unmatched cases and controls. Matching was done by sex and age. McNemar’s test was used for matched data and Fisher’s exact test for unpaired data. In order to adjust for potential confounding, conditional logistic regression for matched and unconditional logistic regression for unmatched data was used. A two-sided alpha value of 0.05 at a confidence level of 95% was considered a threshold for statistical significance.
Results
Basic demographic characteristics of oesophageal cancer patients enrolled in the study
We enrolled a total of 366 participants, of which 131 were cases and 235 were controls. Among the cases, 50 (38%) were female and the median age was 56 years (IQR = 46–65 years). The majority of OC patients (88%) were from Lusaka, eastern, central and southern provinces (Table 1). Histopathology reports were available for 100 OC patients, and of these, 89 (89%) were squamous cell carcinoma and 11 (11%) were adenocarcinomas. For the remaining 31, histological confirmation was made at private facilities with a similar proportion of histological sub-types.
Risk factors for oesophageal cancer
We analysed the occurrence of risk factors by comparing cases and controls. In univariate analysis, exposure to biomass smoke, poor housing, lack of basic household goods, lack of formal education and cigarette smoking were associated with increased odds of OC (Table 2). Analysis of matched cases and controls revealed that exposure to biomass smoke, rural residence, cigarette smoking, poor housing, lack of basic household goods and formal education were risk factors for OC in Zambian patients, as shown in Table 2. Use of charcoal (OR: 2.4; 95% CI: 1.4–4.5, p = 0.001) or firewood (OR: 3.9; 95% CI: 1.8–8.3, p < 0.001) was each associated with OC.
We ran three different models using stepwise logistic regression of unmatched cases and controls. Model 1 included all the variables analysed in univariate analysis. In model 2, we removed variables less than 300, as that reduced the total numbers and could have introduced some biases. Model 3 was run on variables related to the environment. The first model showed that age more than 45 years (p = 0.009), rural residence (p = 0.002), lack of formal education (p = 0.035) and tobacco smoking were associated with OC, while in the second and third models, biomass smoke exposure showed a statistically significant association (Table 3).
For matched cases and controls, we used conditional logistic regression. The same variables were used for models 1, 2 and 3 as described above. Exposure to biomass smoke was significantly associated with OC in models 2 and 3 with OR: 2.8, 95% CI: 1.3–5.9, p = 0.005 and OR: 2.4, 95% CI: 1.2–4.5, p = 0.016, respectively.
Domestic fuel use and oesophageal cancer
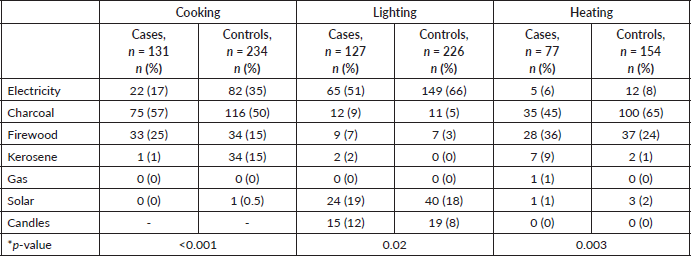
The main fuels used by study participants included electricity, charcoal, firewood, kerosene, gas and solar. Overall, 89 (92%) rural residents relied on biomass fuels compared to 165 (63%) urban dwellers. A comparison of the cases and controls showed significant differences in the fuel used for cooking (p < 0.001), lighting (p = 0.02) and heating (p = 0.003) (Table 4). These effects remained significant after adjusting for cooking and sleeping areas within the household.
Table 1. Basic characteristics of the oesophageal cancer patients included in the study.
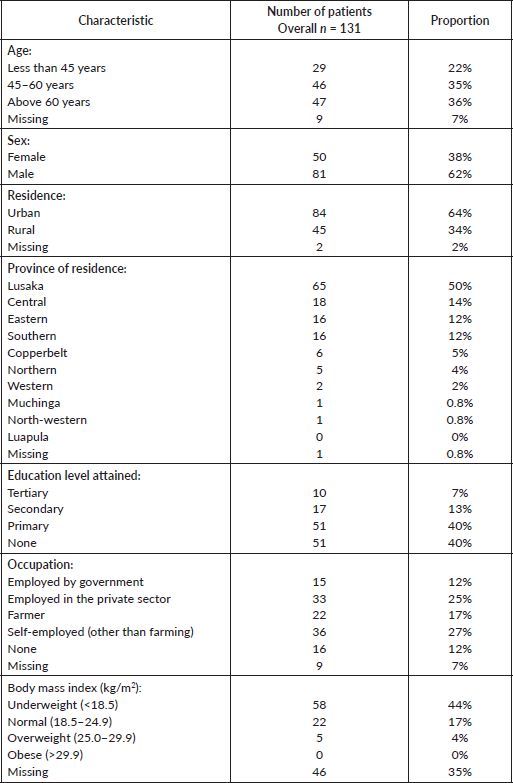
Table 2. Univariable analysis of risk factors for oesophageal cancer.

Discussion
OC is a growing problem in south-eastern Africa, with several aetiological factors implicated. This study has established biomass smoke exposure as a possible risk factor, which is consistent with previous findings from Zambia and the surrounding regions [15, 18, 19]. We further report that residing in a rural area, lack of formal education and poor housing are also risk factors for OC.
The World Health Organisation reports that household air pollution, including biomass smoke exposure, increases the risk of developing numerous lethal and chronic conditions, such as heart diseases [20]. Biomass smoke exposure is very common in many parts of the world, with over 70% of the African population regularly exposed [16]. Previously, we presented preliminary data linking biomass fuel use with increased risk of oesophageal and stomach carcinogenesis [15, 21]. An Iranian prospective cohort study of up to 50,045 participants reported that household burning of biomass fuels increased the risk of digestive tract cancers among individuals [22]. There is an urgent need for experimental studies (involving both human and animal models) to identify internal dose carcinogenic biomarkers. In an area of high OC prevalence in south-west Kenya, significantly higher urinary polycyclic aromatic hydrocarbons (probable carcinogens) were established [23].
Cancer development is a complex process, with several factors acting synergistically. We explored multiple lifestyle factors and found a significant association between proxies of poverty and OC risk. This finding is common elsewhere, where it is shown that individuals from the low socio-economic class than the higher socio-economic class are more prone to developing OC risk than the latter [24]. It is not clear which poverty-driven factors lead to OC, but pollution compounded by poor ventilation might be a contributing factor.
Similar to our previous findings, we failed to demonstrate a link between alcohol intake and OC. This could result from the data collection approach in which we relied on recall and categorised individuals into two: those who drank alcohol and those who did not. This approach did not allow us to establish a threshold amount of alcohol intake responsible for OC increased risk. We, however, found that cigarette smoking was an independent risk factor in unmatched comparisons. Cigarette smoking is a well-established risk factor for OC [25], but with varying molecular evidence. In a similar population to ours, whole-exome sequencing and RNA transcriptomic analysis of 59 Malawian OC patients did not find any signatures of tobacco smoking [26], while mutational signatures for tobacco were reported in a study that included samples from 8 countries, including East Africa [13].
Table 3. Logistic regression models evaluating risk factors for oesophageal cancer.
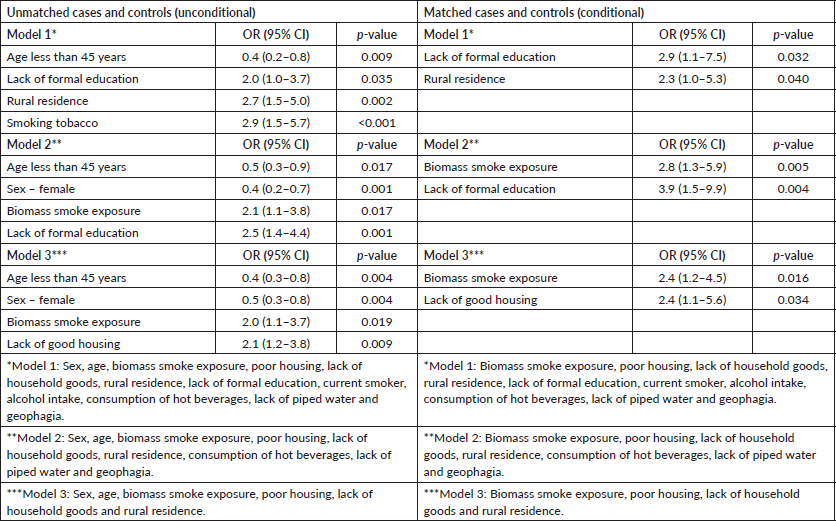
Table 4. Association between oesophageal cancer and type of energy used for cooking, lighting and heating in homes.
Africa has had a relatively low cancer research output, with most of its publications coming out of South Africa and Egypt [27]. With limited outputs from countries most heavily affected by OC, risk factors that could be affecting African populations have not been thoroughly investigated. The need for intensifying cancer research in Africa is an urgent one. In addition to understanding risk factors, there is a need to enhance data collection on treatment outcomes and streamline where the intention is curative or palliative [28]. Early OC detection offers the best chance at good outcomes, but unfortunately this seldom occurs, and in Africa, such information is not well documented or reported.
From our sample size calculations, we enrolled enough study participants to allow us draw evidence-based conclusions. However, a major limitation was the lack of an objective way to measure biomass smoke exposure. Still, we based our assessment purely on household use of biomass fuel. In future, we intend to institute objective measurements using wearable devices to determine exposure more accurately.
Conclusion
There is an association between OC and biomass smoke exposure. Therefore, minimising exposure could potentially impact the growing burden of OC in Zambia. Moreover, there is a need to investigate how other factors (lack of formal education, suitable housing and rural residence) interact with biomass smoke in OC pathogenesis.
Acknowledgments
The authors acknowledge the efforts made by Joyce Sibwani, Rose Soko and Kachinga Masheke in gathering information from the study participants.
Declarations
Not applicable.
Funding
None.
Conflicts of interest
The authors have no conflicts to declare.
Data availability
Data will be available from the corresponding author upon reasonable request
Authors’ contributions
VK and PK designed and conceptualised the study. VK, CM, MM and PH enrolled the study participants and collected the data, VK, LK and PK analysed the data. All the authors were involved in manuscript writing and approval of the final version.
References
1. Ferlay J, Ervik M, and Lam F, et al (2020) Global Cancer Observatory: Cancer Today (France: International Agency for Research on Cancer) [https://gco.iarc.fr/today] Date accessed: 18/10/21
2. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136(5) E359–E386 https://doi.org/10.1002/ijc.29210
3. Hull R, Mbele M, and Makhafola T, et al (2020) A multinational review: esophageal cancer in low to middle-income countries Oncol Lett 20(4) 42 https://doi.org/10.3892/ol.2020.11754 PMID: 32802164 PMCID: 7412736
4. Lin S, Xu G, and Chen Z, et al (2020) Tea drinking and the risk of esophageal cancer: focus on tea type and drinking temperature Eur J Cancer Prev 29(5) 382–387 https://doi.org/10.1097/CEJ.0000000000000568 PMID: 32740163
5. Mwachiro MM, Parker RK, and Pritchett NR, et al (2019) Investigating tea temperature and content as risk factors for esophageal cancer in an endemic region of Western Kenya: validation of a questionnaire and analysis of polycyclic aromatic hydrocarbon content Cancer Epidemiol 60 60–66 https://doi.org/10.1016/j.canep.2019.03.010 PMID: 30925281 PMCID: 6559237
6. Tai WP, Nie GJ, and Chen MJ, et al (2017) Hot food and beverage consumption and the risk of esophageal squamous cell carcinoma: a case-control study in a northwest area in China Medicine (Baltimore) 96(50) e9325 https://doi.org/10.1097/MD.0000000000009325
7. Marabotto E, Pellegatta G, and Sheijani AD, et al (2021) Prevention strategies for esophageal cancer-an expert review Cancers (Basel) 13(9) 2183 https://doi.org/10.3390/cancers13092183
8. African Esophageal Cancer Consortium (2022) Expanding oesophageal cancer research and care in eastern Africa Nat Rev Cancer 22(5) 253–254 https://doi.org/10.1038/s41568-022-00458-1 PMID: 35246668
9. Kayamba V (2019) Esophageal cancer hotspots in Africa Lancet Gastroenterol Hepatol 4(11) 818–820 https://doi.org/10.1016/S2468-1253(19)30253-5 PMID: 31609235
10. Van Loon K, Mwachiro MM, and Abnet CC, et al (2018) The African esophageal cancer consortium: a call to action J Glob Oncol 4 1–9
11. Lipenga T, Matumba L, and Vidal A, et al (2021) A concise review towards defining the exposome of oesophageal cancer in sub-Saharan Africa Environ Int 157 106880 Date accessed: 17/09/21 https://doi.org/10.1016/j.envint.2021.106880 PMID: 34543937
12. Chetwood JD, Finch PJ, and Kankwatira A, et al (2018) Five-year single-centre experience of carcinoma of the oesophagus from Blantyre, Malawi BMJ Open Gastroenterol 5(1) e000232 https://doi.org/10.1136/bmjgast-2018-000232 PMID: 30397505 PMCID: 6203015
13. Moody S, Senkin S, and Islam SMA, et al (2021) Mutational signatures in esophageal squamous cell carcinoma from eight countries with varying incidence Nat Genet 53 1553–1563 https://doi.org/10.1038/s41588-021-00928-6 PMID: 34663923
14. International Agency for Research on Cancer [https://monographs.iarc.who.int/agents-classified-by-the-iarc/] Date accessed:20/08/21
15. Kayamba V, Bateman AC, and Asombang AW, et al (2015) HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: a case-control study Cancer Med 4(4) 588–595 https://doi.org/10.1002/cam4.434 PMID: 25641622 PMCID: 4402073
16. Bonjour S, Adair-Rohani H, and Wolf J, et al (2013) Solid fuel use for household cooking: country and regional estimates for 1980-2010 Environ Health Perspect 121(7) 784–790 https://doi.org/10.1289/ehp.1205987 PMID: 23674502 PMCID: 3701999
17. Kayamba V, Heimburger DC, and Morgan DR, et al (2017) Exposure to biomass smoke as a risk factor for esophageal and gastric cancer in low-income populations: a systematic review Malawi Med J 29(2) 212–217 https://doi.org/10.4314/mmj.v29i2.25 PMID: 28955435 PMCID: 5610298
18. Mlombe YB, Rosenberg NE, and Wolf LL, et al (2015) Environmental risk factors for esophageal cancer in Malawi: a case-control study Malawi Med J 27(3) 88–92 https://doi.org/10.4314/mmj.v27i3.3 PMID: 26715952 PMCID: 4688868
19. Okello S, Akello SJ, and Dwomoh E, et al (2019) Biomass fuel as a risk factor for esophageal squamous cell carcinoma: a systematic review and meta-analysis Environ Health 18(1) 60 https://doi.org/10.1186/s12940-019-0496-0 PMID: 31262333 PMCID: 6604279
20. Health and Environment Linkages Initiative Website [https://www.who.int/heli/risks/indoorair/indoorair/en/] Date accessed: 30/11/21
21. Kayamba V, Zyambo K, and Mulenga C, et al (2020) Biomass smoke exposure Is associated with gastric cancer and probably mediated via oxidative stress and DNA samage: a case-control study JCO Glob Oncol 6 532–541 https://doi.org/10.1200/GO.20.00002 PMID: 32228314 PMCID: 7113078
22. Sheikh M, Poustchi H, and Pourshams A, et al (2020) Household fuel use and the risk of gastrointestinal cancers: the golestan cohort study Environ Health Perspect 128(6) 67002 https://doi.org/10.1289/EHP5907 PMID: 32609005 PMCID: 7299082
23. Mwachiro MM, Pritchett N, and Calafat AM, et al (2021) Indoor wood combustion, carcinogenic exposure and esophageal cancer in southwest Kenya Environ Int 152 106485 https://doi.org/10.1016/j.envint.2021.106485 PMID: 33689906 PMCID: 8832867
24. Wu CC, Chang CM, and Hsu TW, et al (2016) The effect of individual and neighborhood socioeconomic status on esophageal cancer survival in working-age patients in Taiwan Medicine (Baltimore) 95(27) e4140 https://doi.org/10.1097/MD.0000000000004140 PMCID: 5058858
25. IARC Monograph on Tobacco Smoking [https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100E-6.pdf] Date accessed: 29/11/21
26. Liu W, Snell JM, and Jeck WR, et al (2016) Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis JCI Insight 1(16) e88755 https://doi.org/10.1172/jci.insight.88755 PMID: 27734031 PMCID: 5053149
27. Kayamba V, Mutale W, and Cassell H, et al (2021) Systematic review of cancer research output from Africa, with Zambia as an example JCO Glob Oncol 7 802–810 https://doi.org/10.1200/GO.21.00079 PMID: 34077269 PMCID: 8459799
28. Buckle GC, Mahapatra R, and Mwachiro M, et al (2021) Optimal management of esophageal cancer in Africa: a systemic review of treatment strategies Int J Cancer 148(5) 1115–1131 https://doi.org/10.1002/ijc.33299






