Barriers and facilitators of colorectal cancer screening in Asia
Sare Hatamian1, Fatemeh Hadavandsiri2, Zohre Momenimovahed3 and Hamid Salehiniya4
1Department of Epidemiology, School of Public Health and Safety, Iran University of Medical Sciences, Tehran, Iran
2Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Department of Midwifery and Reproductive Health, Qom University of Medical Sciences, Qom, Iran
4Social Determinants of Health Research Center, Birjand University of Medical Sciences, Birjand, Iran
Abstract
Purpose: One of the most common cancers in Asia is colorectal cancer (CRC). Early diagnosis and timely treatment are necessary for preventing complications and advanced stages of the disease. It is important to evaluate barriers and facilitators of screening in different countries. This systematic review aimed to identify the barriers and facilitators of CRC screening in Asia.
Methods: In this systematic review, for identifying barriers and facilitators of CRC screening, a comprehensive search was conducted in PubMed, Web of Science and Scopus in 12 December 2020. Combination keywords such as colorectal cancer, screening, sigmoidoscopy, colonoscopy, faecal occult blood test, barriers, facilitators and the names of each Asian country were used for searching. Full text original studies in English language were accepted in the review.
Results: In total, 36 articles were included in the review. Barriers and facilitators were evaluated. The most common reported barriers were lack of knowledge, fear of result, fear of procedure, fear of pain, lack of awareness, high cost and lack of gastrointestinal symptoms. The most frequent facilitators were having knowledge and awareness of CRC screening, perceived risk and severity, family history of cancer and physician recommendation.
Conclusion: For promoting success in CRC screening programmes, knowing what the barriers and facilitators are is necessary. Awareness and various personal, professional and social factors have been shown to be the major barriers toward CRC screening in most Asian countries.
Keywords: colorectal cancer, screening, facilitators, barriers, Asia
Correspondence to: Hamid Salehiniya
Email: alesaleh70@yahoo.com
Published: 13/09/2021
Received: 27/02/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer is recognised as a global problem nowadays. Colorectal cancer (CRC) is ranked as the third most common cancer in the world by International Agency for Research on Cancer which reported 0.8 million deaths related to CRC in 2018 [1]. It is estimated by the year 2030, the worldwide burden of CRC will rise by 60% to more than 2.2 million new cases and 1.1 million deaths [2, 3]. In Asia, a high prevalence and an increasing number of CRC in both genders have been reported [4, 5].
Due to the high prevalence and incidence of CRC, early diagnosis and timely treatment are necessary for preventing complications and advanced stages of disease. With prevention, 40% of cancers can be prevented and by early detection 90% of cancers can be treated [6–8]. Results of previous studies show that by timely screening in CRC, 100% of genetic cases can be prevented [9, 10]. For early diagnosis of CRC, regular screening is the best control measure and effective method [11–13].
As people become more aware of the risk factors for CRC, their participation in screening programmes increases. Factors leading to CRC are increasing age, life style, family history of CRC, smoking, alcoholism, a low fibre diet, red and processed meat consumption [14–16]. Lack of public knowledge about risk factors of CRC leads to development of disease [14]. The United States Preventive Services Taskforce recommends colorectal screening methods such as: Faecal occult blood test (FOBT), as the simplest way of screening that should be done every year, sigmoidoscopy is done once every 5 years and colonoscopy done at least every 10 years in older than 40-year-old participants [17, 18].
Screening programmes are challenging in developing countries; programmes need huge allocations of financial and logistic resources. Before intending for screening projects, financial and individual factors such as knowledge, attitude, awareness and belief of health promotion should be considered [11, 19–25]. Due to the importance of knowing the causes of participation-status in screening, this study was conducted to determine the barriers and facilitators of colorectal screening programme in Asia.
Materials and methods
Search strategy
For this systematic review which was designed in 2020, comprehensive search was conducted in PubMed/Medline, Web of Science and Scopus in December 2020. Combined keywords such as colorectal cancer, screening, sigmoidoscopy, colonoscopy, faecal occult blood test (FOBT, barriers, facilitators and names of each Asian country were used for searching. We used manual searches in valid journals and followed articles and full text articles for comprehensive search. The articles were entered to EndNote and duplicate articles were deleted automatically by EndNote-X8 software. After removing duplicates, a screening of titles and abstracts was performed and eligible articles were selected. Full-text articles were then reviewed and articles that determined barriers and facilitators of CRC screening were included.
Inclusion criteria
In this study, inclusion criteria were being an original article, observational studies (cross-sectional studies, case–control and longitudinal cohort studies) that investigated CRC screening barriers and facilitators, referring to CRC screening modalities and factors, using keywords in their title or abstracts.
Exclusion criteria
Articles such as letters to editor, case reports, conference abstracts, editorials, review studies, clinical trials and studies not having the full text were the exclusion criteria.
Data selection and synthesis
Searching the article was done by one of the researchers, two researchers’ evaluated articles by prepared checklist for data extraction. After excluding irrelevant articles, the full text of remaining studies was reviewed. Extracting the results was done qualitatively. Information was extracted from each study: the first author’s last name, year of study, study location, type of statistical analysis (descriptive, analytic), type of cancer screening, study population, study objectives and main findings.
Qualitative assessment and analysis
For quality assessment of included studies, we used NewCastle Ottawa Quality Assessment Scale for quantitative studies. The tool uses eight items, categorised into three groups: selection, comparability and ascertainment of either exposure or outcome. Numbers showing awarded for each quality items as visual assessment. No studies were excluded based on their quality score [26].
Results
Specification of included studies
Total search of databases determined 1,150 studies; 482 studies were excluded because of duplication. After checking the title and abstract, 550 studies were excluded that were not related to the purpose of review article and its criteria. Besides, full text screening was done on 118 studies, and finally 36 articles were accepted for this systematic study (Figure 1).
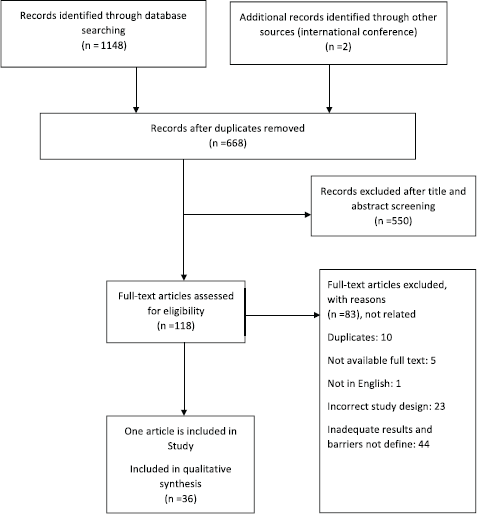
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart illustrating the process for the selection of the included articles for the systematic review.
Study characteristics
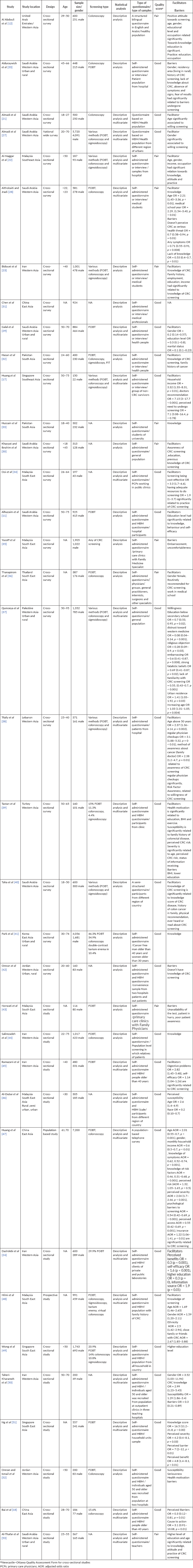
Basic characteristics of the included studies in this review are presented in Tables 1 and 2. Studies selected for literature were cross-sectional studies. The number of samples in studies varied from 116 to 7,200. Most of the participants were 40 years old and the majority of them were men.
Study quality assessment
Scores for cross-sectional studies ranged 5–7 by Ottawa scale. Samples of studies were representative of target population in 30 studies. Data collection procedures were described well by all of studies. Reported studies using self-administered questionnaire and some of them using health belief model (HBM) questionnaire. A total of 36 studies were used sufficient analysis methods and analysis linkage. A total of 26 studies had good quality score, and 10 studies had fair quality score by Ottawa quality scale (Table 2).
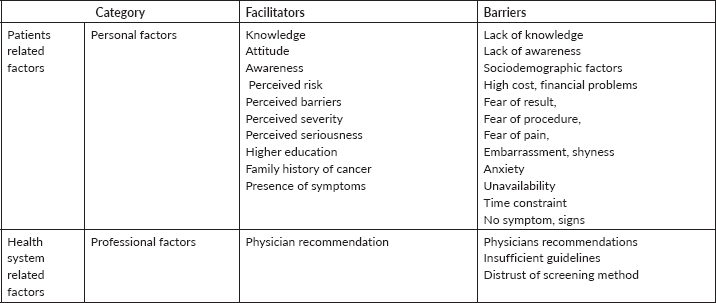
Barriers and facilitators are shown in Table 3.
Table 1. Characteristics of included studies in the review.
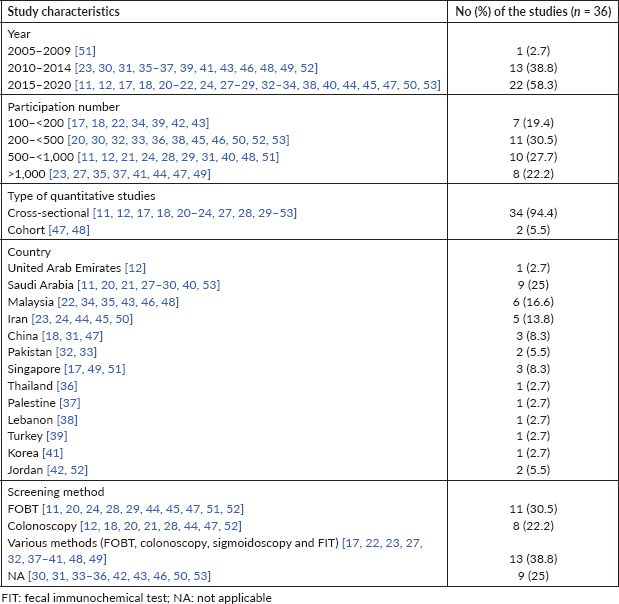
Table 2. Characteristics of included studies in the review.
Barriers and facilitators
Knowledge of screening
In any screening programme, especially CRC screening, knowledge and awareness are considered as a crucial element. Knowledge of risk factors and screening methods leads to increased use of screening [12, 28, 30]. General lack of knowledge of CRC screening methods and risk factors were known as barriers in five studies of different countries [20, 22, 28, 34, 47]. Althobaiti and Jradi [28] showed that low participation in screening is related to lack of knowledge of screening and symptoms of CRC. Low level of knowledge stems from low level of education in relation to low level of awareness and attitudes. Another study among older Saudis showed that prior information about signs and risk factors had positive influence on awareness and intention to screening [29]. In a study in United Arab Emirates, overall evaluation of knowledge revealed a poor level of knowledge on risk factors, and only 40% of adults identified FOBT as a main screening test for CRC prevention [12].
In a study in China, individuals who have knowledge of screening tests were six times more likely to perform CRC screening rather than those who do not have any knowledge (high: AOR: 6.68, 95% CI = (4.36, 10.24), p < 0.001) [15]. Positive attitude that screening can be effective in early detection and reducing treatment time leads to decision to participate in CRC screening. Results of three studies demonstrate that when persons are aware of signs and risk factors of CRC, their participation in CRC screening increases [12, 29, 51]. According to Tfaily et al [38] study in Lebanon, people with higher awareness of risk factors were 2.2 times more likely to participate in CRC screening (OR = 2.221, 95% CI = (1.023, 4.820), p-value = 0.04). Believing that CRC is preventable is about (73.3%) and curable (70.5%) effected on CRC screening two times more strongly for choosing FOBT method as test (OR = 2, 95% CI: 1.04–2.29) [21].
Perceived severity, seriousness, barriers, risk, susceptibility, benefit
Other motivators of participation in CRC screening are perceived risk, severity and seriousness barriers. In many studies, results showed that perceived severity, seriousness and susceptibility leads to screening, and results of perceived barriers had a negative effect on screening [18, 42, 46, 48, 50–52]. In a study, perception towards sub scales and health motivators was seen. Results showed that there was a significant positive correlation between knowledge of CRC screening and perceived susceptibility, seriousness and perceived barriers. Knowledge of CRC screening has a greater effect on perceived susceptibility to CRC, the seriousness of CRC, barriers for CRC and health motivation than those without knowledge about it [46, 52]. Participants who perceived fewer barriers (OR = 0.37; 95% CI: 0.21–0.89), perceived more susceptibility (OR = 2.99; 95% CI: 1.23–5.45) were more likely to utilise screening tests [50]. Studies showed that some facilitators such as knowledge, awareness, sociodemographic factors, self-efficacy, perceived barriers, susceptibility, severity and benefits had positive influence on CRC screening [12, 20, 27, 28].
Higher self-efficacy intent for screening was 1.14 times higher in participants (OR = 1.14, 95% CI: 1.04–1.26) [45].
Fears of result, fear of procedure
Common psychological barriers have been shown such as fear, embarrassment, anxiety and pain in most studies [20, 22, 31, 37–39, 41–43]. Results of five studies showed fear of the painful medical procedures [11, 20, 38, 39, 41]. Fear of tumour detection and test result subsequent fear of developing
Table 3. Facilitators and barriers of CRC screening in Asian countries.
CRC and fear of complications cause ignorance of screening [11, 22, 29, 33, 41, 43, 46, 49]. Of the included studies, 51.6% reported fear of painful medical procedures as perceived barriers [11]. Procedure of screening may be embarrassment for participants. Al-naggar et al [22] showed that participants did not want to do screening, because of shyness (55.1%), painful procedure in FOBT (53.5%), embarrassment (55.1%) in sigmoidoscopy and then 32.1% fear of cancer detection.
Professional factors
After patient related barriers, professional factors as healthcare system barriers were categorised as common barriers. These barriers include the following: lack of recommendation by doctor or medical health staff [12, 20, 22, 23, 28, 31, 34, 35, 46, 48], lack of integrated and updated guidelines in health care centres [28, 31, 37, 42], lack of resources [12, 28].
According to Althobaiti and Jradi’s [28] study, it was described that among medical students, knowledge of CRC factors and screening modalities was poor (52.47% and 57.83%, respectively). On the other hand, increase in medical education increased knowledge of screening three-fold (OR = 3.23; 95% CI: 2.01–5.18) and attitudes toward low level of medical science education were increased two (OR = 2.74; 95% CI: 1.86–4.03) times higher [28].
Results of Chen’s study [31] showed that majority of physicians’ barriers toward CRC were identified as lack of knowledge of colorectal guidelines (46.7%) and lack of sufficient information about CRC patients for early screening (43.8%). A study in Singapore on motivators such as presence of symptoms (92%), physician’s recommendation (81.4%) and family history (70.7%) reported increased screening. Physicians recommendation had 7.15 times higher influence (OR = 7.10 (95% CI: 3.08–16.4), p < 0.001) on screening among survivors [17]. Recommendation by a doctor has a positive effect on screening, while believing that the screening process is painful has a negative effect on screening. The results of a study show that 95% of people report lack of doctor’s advice as a barrier to screening [12, 49]. Physician recommendations and advice [12, 37, 40], promoting knowledge and awareness of medical staff and students, reconciling guidelines of CRC screening are the strong motivating factors of CRC screening in different studies [24, 31, 34, 37, 48]. In a study among Saudi patients, 43% of the participants got knowledge of screening by regular awareness programmes from health care system [11].
Costs of screening
Medical costs associated with screening were a barrier in six studies. Huang et al [17] reported that cost of screening is too expensive and caused 50% of barrier of screening.
Time constraints
Lack of time [12, 20, 21, 36, 41, 43], accessibility to CRC screening were illustrated as more barriers reported by studies [17, 31, 32, 36, 43, 49, 51].
Long waiting times in public hospitals is one of the barriers in Saudi Arabia, Korea and Malaysia [12, 36, 41, 43].
Accessibility
One of the important barriers for screening was about accessibility, lack of transportation and screening availability which differ from area residency in a country. More barriers have been reported from participants who live in rural areas [20]. In a study in Saudi Arabia, general lack of unavailability of FOBT was the only important barrier of CRC screening [20].
Socio demographic factors
Demographic specifications influenced the use of screening modalities and affected on barriers and facilitators. Characteristics such as age [12, 29, 32, 36–39, 46, 48, 51], gender [12, 20, 22, 27, 29, 32, 45, 47, 48], level of education [11, 12, 14, 23, 28–30, 32, 37, 39, 40, 47, 51], socioeconomic status and employment status [12, 17, 23, 37], marital status [29], ethnicity [12, 48] have been examined.
In a study in Singapore, younger participants (OR = 3.21, 95% CI: 1.01–5.41, p < 0.01) and more educated (OR = 1.54, 95% CI: 0.48–2.61), p < 0.01) had the highest rate of screening [51]. In a study of Al-Hajeili et al [14], level of education (p = 0.001) and region of residence (p = 0.02) significantly associated with knowledge of screening, knowledge about colonoscopy was associated with gender (p = 0.03), educational level (p < 0.01) and family history of CRC (p = 0.04). According to the study by Alhuzaim et al [11], the level of education has a positive role in the knowledge, behavior and self-efficacy of the participants. In this study, 65% of educated people are more inclined to be screened.
According to this study, increased age > 50 and level of education below secondary school were associated with decreased odds of CRC screening, odds ratio of age 0.9 (OR = 0.9, 95% CI: 0.50–0.99, p = 0.002) indicated low CRC screening than younger participants and about educational level 0.7 (OR = 0.7, 95% CI: 0.53–0.95, p = 0.02) below secondary school had lower CRC screening compared with high level of education [37]. On the other hand, study of Tfaily et al [38] demonstrated that older participants (above 50 years of age) had two times more knowledge and 55% awareness about CRC screening and 43% willingness to do screening. The study of Galal et al [29] showed that gender, unmarried and having less than college education were considered reducing predictors of CRC screening. Unmarried participants had 0.11 times lower CRC screening-rate (OR = 0.11; 95% CI: 0.10–0.23; p = 0.001) than married participants for screening. In a study among adults in United Arabs Emirates, knowledge of participations between UAE nationals and non-UAE nationals had significantly differences (p < 0.001), non-UAE nationals had better knowledge [12].
People over the age of 50 were more aware of the signs and symptoms than other participants in the study [38]. One of the important motivators that influences CRC screening is self-efficacy [11, 45]. In a study in Iran, self-efficacy (OR = 1.17, 95% CI: 1.08–1.27) plays a role as a motivator variable about CRC screening among other participants [45].
Lack of signs and symptoms
One of the barriers of screening CRC reported by participants included lack of symptoms. Six studies reported that participants with no symptoms lead to lower screening history of symptoms and believed sickness caused more participation in screening [3, 4, 20, 22–23, 29–31]. In a study in Saudi Arabia, 73.4% participants reported absence of signs and symptoms as the most important barrier [20].
Social factors and communications
Family and friends and relatives’ recommendations have a role in raising sufficient awareness.
History of cancer in family members motivated others for screening by increasing knowledge of screening [11, 14,17, 29, 31, 40, 46, 48, 49]. A Saudi-Arabian study having relatives diagnosed with CRC screening (OR = 1.67, 95% CI: 0.99–2.81, p-value < 0.0001) leads to believing in the effect of screening to detect cancer [14].
Media and social networks, physician’s recommendations are the main sources of encouragement [11, 12, 17, 21, 23, 29, 38, 45, 49, 50]. On the other hand, a study showed that risk groups having positive family history of CRC, screening did not have increased clinical knowledge and awareness [23]. Awareness of CRC screening was two times higher in participants with clinical recommendation (OR = 2.384, 95% CI = (1.20–4.70), and p-value = 0.012), and those who undergo regular physician check-ups have three times higher (OR = 3.167, 95% CI = (1.88–5.32), p-value < 0.0001) level of awareness [38].
Ways reported to increase knowledge and information were through media (such as books, newspapers, magazines, TV, radio, Internet, knowledge from health care staff, family members and friends information) [23, 24, 29, 44, 45, 48, 49, 51, 52].
Comparisons of countries
Studies from Saudi Arabia and Palestine demonstrated that one of most common barriers was lack of physician recommendation, absence of signs and symptoms and lack of knowledge of CRC [11, 20, 21, 30, 37]. In the eastern region of Saudi Arabia, lack of provider’s knowledge of recommended screening and lack of public awareness of CRC screening were most common barriers [29]. Financial problems had no effect on participating in CRC
screening, because a large population had access to free screening tests that covered by the ministry of health [11, 20, 21, 29, 30, 40, 53]. In south East of Asia, Malaysia and Singapore, fear of cancer, avoiding doing screening after lack of knowledge, lack of recommendation by physician were the most common barriers of CRC screening [22, 34, 35, 43, 46, 48, 49]. In a study from Iran, more than 90% population did not have any knowledge of CRC risk factors, symptoms and screening tests [23]. The rate of FOBT screening was 29.9% [24]. Lack of awareness and limited literacy, lack of physician recommendation were as the most common barriers [23, 24]. In South Korea, only 31.7% of target population participated in screening programmes and one of the barriers was cost of screening that most of the cost paid by participants [41].
Discussion
Our systematic review found a literature evaluating barriers and facilitators to CRC screening of participants from cross-sectional studies from Asia. Different modalities of CRC screening such as FOBT, colonoscopy, sigmoidoscopy have been introduced for diagnosing CRC in different countries of Asia for many years. Reports demonstrated that in many countries doing CRC screenings, there is a poor level and score of knowledge, and attitude of doing screening is low. More awareness and recommendations about screening tests are needed, as well as further investigation. Our study compared Asian countries for different barriers of CRC screening.
This review of quantitative studies is relevant to the general population, physicians and medical students who are providing CRC screening need to be promoted about CRC screening factors. Results of 36 studies demonstrated that factors influencing the decision to participate in CRC screening are knowledge, attitude of CRC as a curable disease and lack of knowledge about CRC and CRC screening modalities. Knowledge was revealed as important point relating to participating in screening. Increased knowledge had a positive impact on attitude toward CRC, then had a stronger intent for screening. Obviously factors such as education level, cultural and social barriers affect on searching for CRC risk factors and then tendency to CRC screening. Using information sources such as media, videos, books and physician recommendation was found to have an impact on CRC screening. On the other hand, the main factors for ignoring the screening are the lack of knowledge, cost, fear of diagnosis, fear of screening procedures, lack of time, embarrassment. Remarkably, the difference in facilitators and barriers results in different groups with different sociodemographic factors and different guidelines which use the maximum reported score of doing screening modalities is 73% and the lowest is 0.7%. Information on factors on CRC screening such as knowledge, attitude and barriers are poor and need to be further considered, raising knowledge and awareness are equal for reducing barriers. However, appropriate guidelines and protocols must be developed.
Lack of integrated guidelines in countries and low level of knowledge among medical students are also common barriers. Asian population had a poor knowledge-rate and low rate of screening in comparison to western and American societies [54, 55]. On the other hand, media and social communications and family history of cancer, physician recommendation played an effective role on screening-participation. The most common barriers in Asian countries were lack of knowledge, lack of physician recommendation and fear of screening. In comparison to western countries’ fear of screening results and fear of screening procedure, in American countries cost of screening was the most common barrier of screening. Physician recommendation in Asian countries was low in contrast to the American physician recommendation was 72.6% [47, 55].
Strength and limitations of the review
Our search strategy was inclusive and we searched a wide range of databases and then to enhance sensitivity we retrieved full text of all selected articles. We included studies with a large number of participants, who intended to evaluate screening barriers and facilitators. We compared barriers and facilitators from different regions of Asia.
In this review, we compared countries and demonstrated barriers and facilitators of each studies. A limitation was that we used quantitative studies and we recommended to use both qualitative and quantitative studies.
Conclusion
We found that lack of knowledge and awareness about CRC and CRC screening was preventing participation in CRC screening in Asia. While interventional education and guidelines are concurrent with logistics, cultural and motivational barriers must be overcome for reducing inequities in participation in CRC screening. Awareness programmes by health care officials, governments and health care organisations can lead to increased knowledge and ultimately to regular participation in screening. Our study showed that Asian countries have similar barriers and facilitators.
Funding
Not applicable.
Conflicts of interest
None.
Availability of data
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
References
1. Ferlay J, Shin HR, and Bray F, et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008 Int J Cancer 127(12) 2893–2917 https://doi.org/10.1002/ijc.25516
2. Siegel RL, Miller KD, and Fedewa SA, et al (2017) Colorectal cancer statistics, 2017 CA Cancer J Clin 67(3) 177–193 https://doi.org/10.3322/caac.21395 PMID: 28248415
3. Herbst A and Kolligs FT (2012) Detection of DNA hypermethylation in remote media of patients with colorectal cancer: new biomarkers for colorectal carcinoma Tumor Biol 33(2) 297–305 https://doi.org/10.1007/s13277-012-0346-y
4. Ghoncheh M, Mohammadian M, and Mohammadian-Hafshejani A, et al (2016) The incidence and mortality of colorectal cancer and its relationship with the human development index in Asia Ann Glob Health 82(5) 726–737 [doi: 10.1016/j.aogh.2016.10.004] PMID: 28283123.
5. Zahedi A, Rafiemanesh H, and Enayatrad M, et al (2015) Incidence, trends and epidemiology of cancers in North West of Iran Asian Pac J Cancer Prev 16(16) 7189–7193 [doi: 10.7314/apjcp.2015.16.16.7189] PMID: 26514510
6. Mandel JS, Church TR, and Bond JH, et al (2000) The effect of fecal occult-blood screening on the incidence of colorectal cancer N Engl J Med 343(22) 1603–1607 https://doi.org/10.1056/NEJM200011303432203 PMID: 11096167
7. Winawer SJ, Zauber AG, and Ho MN, et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy N Engl J Med 329(27) 1977–1981 https://doi.org/10.1056/NEJM199312303292701 PMID: 8247072
8. World Health Organization (2009) Towards a Strategy for Cancer Control in the Eastern Mediterranean Region
9. Levin B, Lieberman DA, and McFarland B, et al (2008) Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology Gastroenterology 134(5) 1570–1595 https://doi.org/10.1053/j.gastro.2008.02.002 PMID: 18384785
10. Rafiemanesh H, Mohammadian-Hafshejani A, and Ghoncheh M, et al (2016) Incidence and mortality of colorectal cancer and relationships with the human development index across the world Asian Pac J Cancer Prev 17(5) 2465–2473 PMID: 27268615
11. Alhuzaim W, Alosaimi M, and Almesfer AM, et al (2020) Saudi patients’ knowledge, behavior, beliefs, self-efficacy and barriers regarding colorectal cancer screening Int J Pharm Res Allied Sci 9(1) 14–20
12. Al Abdouli L, Dalmook H, and Akram Abdo M, et al (2018) Colorectal cancer risk awareness and screening uptake among adults in the United Arab Emirates Asian Pac J Cancer Prev 19(8) 2343–2349 PMID: 30141313 PMCID: 6171396
13. Ghoncheh M, Mohammadian M, and Mohammadian-Hafshejani A, et al (2016) The incidence and mortality of colorectal cancer and its relationship with the human development index in Asia Ann Glob Health 82(5) 726–737 https://doi.org/.1016/j.aogh.2016.10.004>10.1016/j.aogh.2016.10.004
14. Al-Hajeili M, Abdulwassi HK, and Alshadadi F, et al (2019) Assessing knowledge on preventive colorectal cancer screening in Saudi Arabia: a cross-sectional study J Family Med Prim Care 8(10) 3140–3146 https://doi.org/10.4103/jfmpc.jfmpc_508_19 PMID: 31742133 PMCID: 6857381
15. Bai Y, Wong CL, and He X, et al (2020) Effectiveness of tailored communication intervention in increasing colonoscopy screening rates amongst first-degree relatives of individuals with colorectal cancer: a systematic review and meta-analysis Int J Nurs Stud 101 https://doi.org/10.1016/j.ijnurstu.2019.103397
16. Almasi Z, Mohammadian-Hafshejani A, and Salehiniya H (2016) Incidence, mortality, and epidemiological aspects of cancers in Iran; differences with the world data J BUON 21(4) 994–1004 PMID: 27685925
17. Huang Y, Soon YY, and Ngo LP, et al (2019) A cross-sectional study of knowledge, attitude and barriers to colorectal cancer screening among cancer survivors Asian Pac J Cancer Prev 20(6) 1817–1824 https://doi.org/10.31557/APJCP.2019.20.6.1817 PMID: 31244305 PMCID: 7021622
18. Bai Y, Wong CL, and Peng X, et al (2020) Colonoscopy screening behaviour and associated factors amongst first-degree relatives of people with colorectal cancer in china: testing the health belief model using a cross-sectional design Int J Environ Res Public Health 17(14) 4927 https://doi.org/10.3390/ijerph17144927 PMCID: 7400103
19. Mozafar Saadati H, Khodamoradi F, and Salehiniya H (2020) Associated factors of survival rate and screening for colorectal cancer in Iran: a systematic review J Gastrointest Cancer 51(2) 401–411 https://doi.org/10.1007/s12029-019-00275-0
20. Alduraywish SA, Altamimi LA, and Almajed AA, et al (2020) Barriers of colorectal cancer screening test among adults in the Saudi Population: a cross-sectional study Prev Med Rep 20 101235 https://doi.org/10.1016/j.pmedr.2020.101235 PMID: 33194537 PMCID: 7645071
21. Almadi MA, Mosli MH, and Bohlega MS, et al (2015) Effect of public knowledge, attitudes, and behavior on willingness to undergo colorectal cancer screening using the health belief model Saudi J Gastroenterol 21(2) 71–77 https://doi.org/10.4103/1319-3767.153814 PMID: 25843192 PMCID: 4392578
22. Al-Naggar RA, Al-Kubaisy W, and Yap BW, et al (2015) Attitudes towards colorectal cancer (CRC) and CRC screening tests among elderly Malay patients Asian Pac J Cancer Prev 16(2) 667–674 https://doi.org/10.7314/APJCP.2015.16.2.667 PMID: 25684505
23. Bidouei F, Abdolhosseini S, and Jafarzadeh N, et al (2014) Knowledge and perception toward colorectal cancer screening in east of Iran Int J Health Policy Manag 3(1) 11–15 https://doi.org/10.15171/ijhpm.2014.48 PMID: 24987716 PMCID: 4075097
24. Dashdebi KG, Noroozi A, and Tahmasebi R (2016) Factors predicting fecal occult blood testing among residents of Bushehr, Iran, based on the health belief model. Asian Pac J Cancer Prev 17 17–22 https://doi.org/10.7314/APJCP.2016.17.S3.17
25. Berkowitz Z, Hawkins NA, and Peipins LA, et al (2008) Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults J Am Geriatr Soc 56 307–314 https://doi.org/10.1111/j.1532-5415.2007.01547.x
26. Sirriyeh R, Lawton R, and Gardner P, et al (2012) Reviewing studies with diverse designs: the development and evaluation of a new tool J Eval Clin Pract 18(4) 746–752. https://doi.org/10.1111/j.1365-2753.2011.01662.x
27. Almadi MA and Alghamdi F (2019) The gap between knowledge and undergoing colorectal cancer screening using the Health Belief Model: a national survey Saudi J Gastroenterol 25(1) 27–39 https://doi.org/10.4103/sjg.SJG_455_18 PMID: 30618441 PMCID: 6373220
28. Althobaiti A and Jradi H (2019) Knowledge, attitude, and perceived barriers regarding colorectal cancer screening practices and risk factors among medical students in Saudi Arabia BMC Med Educ 19(1) 421 https://doi.org/10.1186/s12909-019-1857-7 PMID: 31727029 PMCID: 6854663
29. Galal YS, Amin TT, and Alarfaj AK, et al (2016) Colon cancer among older saudis: awareness of risk factors and early signs, and perceived barriers to screening Asian Pac J Cancer Prev 17(4) 1837–1846 https://doi.org/10.7314/APJCP.2016.17.4.1837 PMID: 27221862
30. Khayyat YM and Ibrahim EM (2014) Public awareness of colon cancer screening among the general population: a study from the Western Region of Saudi Arabia Qatar Med J 2014(1) 17–24 https://doi.org/10.5339/qmj.2014.3 PMID: 25320688 PMCID: 4197369
31. Chen YS, Xu SX, and Ding YB, et al (2013) Colorectal cancer screening in high-risk populations: a survey of cognition among medical professionals in Jiangsu, China Asian Pac J Cancer Prev 14(11) 6487–6491 https://doi.org/10.7314/APJCP.2013.14.11.6487
32. Hasan F, Shah SMM, and Munaf M, et al (2017) Barriers to colorectal cancer screening in Pakistan Cureus 9(7) e1477 PMID: 28944116 PMCID: 5602228
33. Hussain I, Majeed A, and Rasool MF, et al (2020) Knowledge, Attitude, preventive practices and perceived barriers to screening about colorectal cancer among university students of newly merged district, Kpk, Pakistan – a cross-sectional study J Oncol Pharm Pract 1078155220922598
34. Ooi CY, Hanafi NS, and Liew SM (2019) Knowledge and practice of colorectal cancer screening in an urban setting: cross-sectional survey of primary care physicians in government clinics in Malaysia Singapore Med J 60(11) 596–604 https://doi.org/10.11622/smedj.2019011 PMID: 30644527 PMCID: 6875808
35. Yusoff HM, Daud N, and Noor NM, et al (2012) Participation and barriers to colorectal cancer screening in Malaysia Asian Pac J Cancer Prev 13(8) 3983–3987 https://doi.org/10.7314/APJCP.2012.13.8.3983 PMID: 23098504
36. Thanapirom K, Treeprasertsuk S, and Rerknimitr R (2012) Awareness of colorectal cancer screening in primary care physicians J Med Assoc Thailand 95(7) 859–865
37. Qumseya BJ, Tayem YI, and Dasa OY, et al (2014) Barriers to colorectal cancer screening in palestine: a national study in a medically underserved population Clin Gastroenterol Hepatol 12(3) 463–469 https://doi.org/10.1016/j.cgh.2013.08.051
38. Tfaily MA, Naamani D, and Kassir A, et al (2019) Awareness of colorectal cancer and attitudes towards its screening guidelines in Lebanon Ann Glob Health 85(1) 75 https://doi.org/10.5334/aogh.2437 PMID: 31148437 PMCID: 6634322
39. Tastan S, Andsoy II, and Iyigun E (2013) Evaluation of the knowledge, behavior and health beliefs of individuals over 50 regarding colorectal cancer screening Asian Pac J Cancer Prev 14(9) 5157–5163 https://doi.org/10.7314/APJCP.2013.14.9.5157 PMID: 24175793
40. Taha I, et al (2019) Assessment of knowledge about colorectal cancer in Saudi Arabia Indo Am J Pharm Sci 6(1) 1100–1105
41. Park B, Lee H-Y, and Choi KS, et al (2011) Cancer Screening in Korea, 2010: Results from the Korean National Cancer Screening Survey Asian Pac J Cancer Prev 12(8) 2123–2128
42. Omran S, Barakat H, and Muliira JK, et al (2015) Knowledge, experiences, and barriers to colorectal cancer screening: a survey of health care providers working in primary care settings J Cancer Educ 30(1) 53–61 https://doi.org/10.1007/s13187-014-0676-0
43. Norwati D, Harmy MY, and Norhayati MN, et al (2014) Colorectal cancer screening practices of primary care providers: results of a national survey in Malaysia Asian Pac J Cancer Prev 15(6) 2901–2904 https://doi.org/10.7314/APJCP.2014.15.6.2901 PMID: 24761922
44. Salimzadeh H, Bishehsari F, and Delavari A, et al (2016) Cancer risk awareness and screening uptake in individuals at higher risk for colon cancer: a cross-sectional study BMJ Open 6(12) https://doi.org/10.1136/bmjopen-2016-013833 PMID: 27998901 PMCID: 5223631
45. Ramazani AA, Norozi E, and AmirabadiZadeh H, et al (2020) Predictors of Colorectal Cancer Screening Participation in Southern Khorasan (Iran) J Gastrointest Cancer 52(1) 187–191 https://doi.org/10.1007/s12029-020-00379-y PMID: 32125621
46. Al-Dubai SAR, Ganasegeran K, and Alabsi AM, et al (2013) Exploration of risk taking behaviors and perceived susceptibility of colorectal cancer among Malaysian adults: a community based cross-sectional study BMC Public Health 13 930 https://doi.org/10.1186/1471-2458-13-930 PMID: 24093502 PMCID: 3851727
47. Huang J, Choi P, and Pang TWY, et al (2020) Factors associated with participation in colorectal cancer screening: a population-based study of 7200 individuals Eur J Cancer Care 30(2) e13369
48. Hilmi I, Hartono JL, and Goh K (2010) Negative perception in those at highest risk--potential challenges in colorectal cancer screening in an urban asian population Asian Pac J Cancer Prev 11(3) 815–822 PMID: 21039060
49. Wong RK, Wong ML, and Chan YH, et al (2013) Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model BMC Public Health 13 677 https://doi.org/10.1186/1471-2458-13-677 PMID: 23879593 PMCID: 3726512
50. Taheri-Kharameh Z, Noorizadeh F, and Sangy S, et al (2016) Factors associated with adherence to colorectal cancer screening among moderate risk individuals in Iran Asian Pac J Cancer Prev 16(18) 8371–8375 https://doi.org/10.7314/APJCP.2015.16.18.8371 PMID: 26745087
51. Ng EST, Tan CH, and Teo DC, et al (2007) Knowledge and perceptions regarding colorectal cancer screening among Chinese – a community-based survey in Singapore Prev Med 45(5) 332–335 https://doi.org/10.1016/j.ypmed.2007.06.021 PMID: 17707496
52. Omran S and Ismail AA (2010) Knowlednge and beliefs of Jordanians toward colorectal cancer screening Cancer Nurs 33(2) 141–148 https://doi.org/10.1097/NCC.0b013e3181b823f3 PMID: 20145539
53. Al-Thafar AK, Al-Naim AF, and Albges DS, et al (2017) Knowledge attitude and practice of colorectal cancer among school teachers in Al-Ahsa Saudi Arabia Asian Pac J Cancer Prev 18(10) 2771 PMID: 29072408 PMCID: 5747402
54. Waller J, Macedo A, and von Wagner C, et al (2012) Communication about colorectal cancer screening in Britain: public preferences for an expert opinion Br J Cancer 107(12) 1938–1943 https://doi.org/10.1038/bjc.2012.512 PMID: 23175148 PMCID: 3516693
55. Klabunde CN, Schenck AP, and Davis WW (2006) Barriers to colorectal cancer screening among medicare consumers Am J Prev Med 30(4) 313–319 https://doi.org/10.1016/j.amepre.2005.11.006 PMID: 16530618






