Laser thermal ablation to treat a recurrent soft-tissue sarcoma of the leg: a case report
Chiara De Angelis1, Paolo Della Vigna2, Gianluca Maria Varano2 and Giovanni Mauri2
1Division of Radiology, IEO, European Institute of Oncology IRCCS, via Ripamonti 435, 20141 Milan, Italy
2Division of Interventional Radiology, IEO, European Institute of Oncology IRCCS, via Ripamonti 435, 20141 Milan, Italy
Abstract
We present the case of a 52-year-old male patient with recurrence of a soft-tissue sarcoma of the left leg treated with percutaneous laser ablation. The patient received the diagnosis of sarcoma for the first time in 2011; further local recurrences and a pulmonary metastatic spread occurred during follow-up, so the patient has been treated several times with chemotherapy, limb-sparing surgery and radiotherapy. In September 2017, a new local recurrence of sarcoma occurred, for which limb amputation was proposed but refused by the patient. Laser ablation with ultrasound guidance was performed, with complete ablation at 6 months and limb salvage.
Keywords: sarcoma, laser ablation, interventional radiology, amputation
Correspondence to: Chiara De Angelis
Email: kirachiara@hotmail.com
Published: 05/03/2019
Received: 30/06/2018
Publication costs for this article were supported by the ecancer Global Foundation.
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sarcomas represent a heterogeneous group of about 100 subtypes of soft-tissue and bone tumours of mesenchymal origin [1]. They are rare in the adult population, accounting for approximately 1% of all newly diagnosed malignancies annually [1–3], while they are more frequent in the paediatric population, accounting for 15% of all the tumours in this age group [3].
The first-line treatment for early stage (non-metastatic) sarcomas is surgery, which must be carried out as early as possible to increase healing probability [1–4]. In fact, if surgically removed, sarcomas can be cured [1].
Even after a complete remission following the primary treatment, 30%–35% of bone sarcoma patients and about 50% of soft tissue sarcoma patients experience local or systemic relapse [3, 4]. The complete removal of all tumours, irrespective of local or systemic recurrence, is advocated to achieve long-term survival [3].
Nevertheless, not all patients benefit from surgical treatment and unfortunately, many patients are metastatic at the onset of the disease while more than 50% of patients develop metastases during the course of their disease [1, 3]. Lungs are the predominating site of metastases and the exclusive site in over 50% of patients at the first metastatic event [1, 3, 5]. In patients with metastatic disease, the standard treatment is neoadjuvant chemotherapy and radiotherapy; chemotherapy generally prolongs survival by a few months [1].
Radiotherapy aims to provide adequate local control, but its role remains controversial in ideal treatment sequence with surgery and improvement in survival [2].
Percutaneous image-guided thermal ablations have been used in different clinical settings, with the aim of achieving the complete ablation of the tumour with reduced invasiveness in comparison with surgical resection. However, few reports have described the clinical application of image-guided thermal ablations in the treatment of recurrent sarcomas [1–10] and the majority of them referred to the treatment of liver, lung or other organs’ metastases. In particular, among the different ablative techniques, percutaneous laser ablation (PLA) seems to represent the ideal technique for treating small lesions in critical structures due to the very small calibre of the applicator and the extreme precision of the ablation area.
In the present paper, we report a case of a patient with recurrent sarcoma successfully treated with the use of PLA.
Case
A 52-year-old man received the first diagnosis of a monophasic fibrous synovial sarcoma of the left popliteal fossa in 2011, at the age of 45.
In the same year, he had the first surgical intervention, which consisted of the enucleation of a lesion on the thigh. Then, despite two subsequent cycles of chemotherapy (Ifosfamide and Epirubicin), the disease locally progressed, so the oncologists decided to perform preoperative radiotherapy and then a second, more radical surgical intervention the year later.
Until the end of 2014, the patient was stable, with no local or systemic recurrences.
He had to be treated again in 2015 and 2016 for local relapses with limb-sparing surgeries, including also a left femoro-popliteal bypass. After these operations, the disease distantly progressed with a pulmonary metastatic spread. Then, the patient was treated with four high-dose, neoadjuvant chemotherapy cycles (Ifosfamide), unfortunately without a complete response: the lung lesions decreased but did not completely disappear. So, in August 2017, the patient underwent microwave ablation of the four larger lung lesions in order to achieve control of the disease.
Moreover, on 2 October 2017, control magnetic resonance imaging (MRI) of the left leg and knee showed a new local relapse consisting of two pathologic nodules: one of 8 mm near the scar of the lateral calf of the gastrocnemius and one of 14 mm near the scar in the soleus muscle. Both nodules were also visible at ultrasound (US) examination (Figure 1A and B).
Lesion surgical excision was considered not feasible due to previous therapies, and the first proposed treatment option for this recurrence was leg amputation, which the patient refused. Thus, together with the patient’s oncologist and radiation oncologist, the interventional radiologist decided to attempt treatment with PLA under US-guidance in order to avoid a major amputation.
The patient agreed and gave informed consent to the treatment.
Procedure
Before starting the procedure, 0.5 mg im of atropine and 2 mg iv of midazolam were given to the patient. Then, the patient was turned into the prone position. US examination, performed also with contrast material (contrast-enhanced ultrasound (CEUS)), showed both the lesions in the soft tissue of the posterior sector of the left leg.
Local anaesthesia with Mepivacain diluted in physiological solution was injected at the level of the more dorsal lesion, also achieving a separation of the lesion from the skin (Figure 2A). Under US-guidance, PLA was performed by using a laser unit (Echolaser, Elesta Srl, Florence, Italy). The laser source is a semiconductor diode with a wavelength of 1,064 nm, and a multi-source device enables the use of up to four 300 μm fibres at once. A 21-gauge Chiba needle was placed in the centre of the lesion. Then, a bare-tip, 300 μm in diameter laser fibre was introduced through the needle. Ablation with one pull-back and a total amount of energy of 2,400 Joules was performed at a fixed power of 3 W (Figure 2A and C).
During the procedure, the patient also underwent continuous monitoring of pulse-oximetry, electrocardiography (ECG) and blood pressure.
At the end of the procedure, a CEUS showed a good devascularisation of both the treated lesions with a good safety margin. In fact, there was no evidence of residual hyper-enhancing foci, suggesting complete necrosis of both nodules (Figures 3 and 4).
No complications were observed during or after treatment.
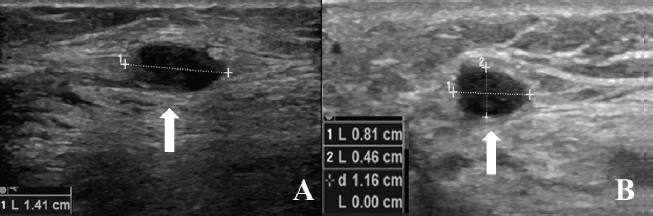
Figure 1. US examination shown in (A): The 14-mm pathologic nodule (arrow) near the scar in the soleus muscle and (B): The 8-mm pathologic nodule (arrow) near the scar of the lateral calf of the gastrocnemius.
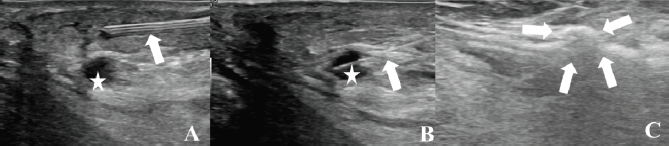
Figure 2. Laser ablation. (A): Injection of local anaesthesia through a small needle (arrow) at the level of the more dorsal lesion (star). (B): A bare-tip, 300 μm in diameter laser fibre, is introduced through a small needle (arrow) into the lesion to be treated (star). (C): The arrows show the gas bubbles produced by the procedure. After another injection of local anaesthesia and hydro-dissection, the medial lesion was treated inserting two parallel 21-gauge Chiba needles and laser fibres. Thermal ablation was performed with one pull-back and a total energy of 3,200 Joule.
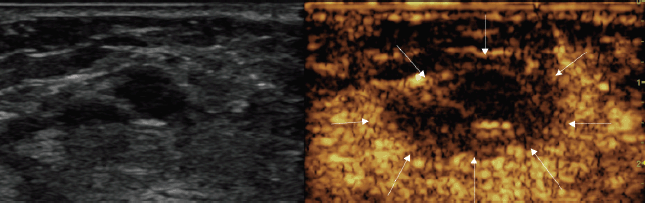
Figure 3. Post-treatment CEUS showing large avascular area, including the 14-mm treated nodule (arrows).
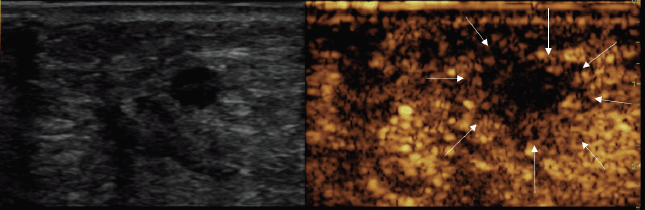
Figure 4. Post-treatment CEUS showing large avascular area, including the 8-mm treated nodule (arrows).
MRI study with and without contrast-enhancement was performed within 2 months after laser ablation to evaluate the antitumour effects. The exam showed the complete disappearance of tumour enhancement of both treated nodules, without signs referable to the persistence of the disease or to the appearance of new solid nodules suspected for active disease. This was regarded as complete tumour ablation.
Two months after PLA, CEUS also confirmed the good outcomes of treatment, showing no enhancement of the treated nodules, smaller than before the treatment (Figure 5).
However, CEUS showed the appearance of two new suspected nodules, cranial and lateral compared to the treated ones, the major 8 mm long. These new nodules were confirmed at US examination 6 months after PLA while previously treated lesions were further reduced.
Discussion
The therapeutic approaches in patients with locally recurrent sarcomas have been largely debated during the last decades [6, 11]: for some authors, the outcome in patients with recurrent extremity soft-tissue sarcomas depends on metastatic disease [12], for others, it relies on the control of the primary tumour [13]. For these reasons, an aggressive approach is warranted in the treatment of local recurrence [6]. Local treatments include surgery and radiotherapy. Percutaneous tumour ablation techniques, especially radiofrequency ablation, have been attempted in few cases to achieve local control of the disease [1–3, 6]. A significant advantage of percutaneous thermal ablation over surgery and radiation includes the repeatability of ablation, as it might be repeated several times in case of recurrence [7]. Moreover, compared with surgery, ablation offers the potential of decreased recovery time, a less invasive procedure, and is often performed in patients deemed not medically fit for surgery [7].
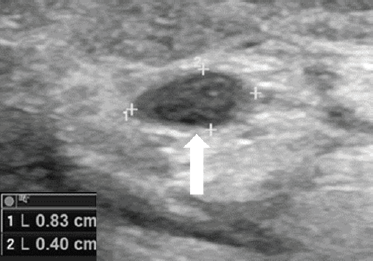
Figure 5. Two months after PLA, the 14-mm nodule appeared smaller (8 × 4 mm) than before the treatment (arrow).
Particularly, as in our case, image-guided thermal ablation offers the possibility to postpone or avoid limb amputation, with a significant advantage for the patients’ quality of life.
To the best of our knowledge, only a few reports have described image-guided ablations in controlling recurrent sarcomas [1–10] and the majority of them referred to the treatment of liver, lung or other organs’ metastases.
In 2014, Yamakado et al [3] published a retrospective study about the clinical utility of radiofrequency ablation (RFA) in 52 inoperable patients (two of whom were children) with recurrent bone and soft-tissue sarcomas. In this study, they showed that RFA was a useful and safe therapeutic option in these patients [3].
In 2015, Belfiore et al [6] also published a case report about the use of RFA under computed-tomography (CT) guidance to treat a 13 cm recurrent malignant fibrous histiocytoma of the right thigh in an 83-year-old female patient. After ablation, a marked shrinkage of the tumour was obtained. Further local recurrences occurred during follow-up and were safely treated by RFA. At the 6-year follow-up, the patient was still alive and presented a local control of disease, without the metastatic spread of the tumour [6]. Belfiore et al [6] reported RFA to be safe, effective and repeatable for soft-tissue sarcoma recurrences, allowing long-term local control of the disease. Even if from these small experiences it is not possible to draw definitive conclusions regarding the role of thermal ablations in soft tissue sarcomas, it seems possible to consider thermal ablations among the toolbox of therapeutic options to be taken into consideration when disease control is the goal of treatment and invasiveness needs to be kept at a minimum.
Jones et al [4] aimed to evaluate the efficacy and safety of RFA for both lung and liver metastases in a series of sarcoma patients, demonstrating that it was feasible, effective and well tolerated if the goal of therapy is local disease control. The role of interventional radiology in treating metastatic soft tissue sarcomas is also mentioned in the works of Gronchi et al [5] and Olivier et al [1]. Moreover, Jones et al [4] and Olivier et al [1] found RFA most effective to treat lesions ≤ 35 mm in inoperable patients.
We chose to treat our patient using PLA because of the number and the dimensions of the lesions. In fact, PLA has been proposed as the ablation technique of choice in patients with multiple and small tumours or for cases with a high-risk location of the lesion to be treated, such as lesions in the neck or renal tumour at high bleeding risk [14–17]. Moreover, the diameter of PLA needles is smaller than RFA electrodes and microwave ablation antennas, making PLA safer and more suitable to ablate lesions with at-risk location and/or difficult to be reached [17–19].
Moreover, both lesions were visible at US examinations, so we decided to treat them under US guidance; as mentioned above, other authors opted for an ablation treatment under CT guidance [3, 6].
In case of poor visibility at US, the use of CEUS has been reported to be extremely helpful to enhance the conspicuity of a lesion in order to better depict it and to enhance the accuracy of image-guided targeting [20].
Moreover, immediate post-procedural CEUS is strongly recommended to assess the completeness of the ablation treatment and to guide the immediate re-treatment in case of residual viable tissue [21–23].
Another option that can help interventional radiologists in cases of lesions being invisible at US and not completely depictable with CEUS is the use of virtual navigations systems and fusion imaging, which has been reported to be a safe, feasible and effective strategy [24–26]. Finally, hydrodissection can be used to achieve a safe distance between the target lesion and surrounding critical structures, thus making it possible to also perform ablation in cases with difficult location [14, 24]. In the presented case, we used hydrodissection to achieve a safe distance between the lesion and the superficial skin and deep structures, such as surgical bypass. This allowed us to perform a complete ablation without damaging surrounding critical structures.
Conclusion
In conclusion, from our very preliminary experience, PLA seems to represent a feasible, tolerable and effective technique for the local control of recurrent sarcomas. Further studies on larger series are needed to confirm these preliminary results. PLA can be considered to be within the therapeutic toolbox for patients with sarcoma when the principal aim is to achieve local disease control by minimising treatment invasiveness.
Conflicts of interest
G Mauri is a consultant for Elesta SrL. All of the other authors have no conflicts of interest.
Funding statement
The authors received no specific funding for this work.
References
1. Olivier T, Pop D, and Chouiter Djebaili A, et al (2015) Treating metastatic sarcomas locally: a paradoxe, a rationale, an evidence? Crit Rev Oncol Hematol 95(1) 62–77 https://doi.org/10.1016/j.critrevonc.2015.01.004 PMID: 25630662
2. Jiang L, Jiang S, and Lin Y, et al (2015) Significance of local treatment in patients with metastatic soft tissue sarcoma Am J Cancer Res 5(6) 2075–2082 PMID: 26269766 PMCID: 4529626
3. Yamakado K, Matsumine A, and Nakamura T, et al (2014) Radiofrequency ablation for the treatment of recurrent bone and soft-tissue sarcomas in non-surgical candidates Int J Clin Oncol 19(5) 955–962 https://doi.org/10.1007/s10147-013-0640-8
4. Jones RL, McCall J, and Adam A, et al (2010) Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma Eur J Surg Oncol 36(5) 477–482 https://doi.org/10.1016/j.ejso.2009.12.005 PMID: 20060679
5. Gronchi A, Guadagnolo BA, and Erinjeri JP (2016) Local ablative therapies to metastatic soft tissue sarcoma Am Soc Clin Oncol Educ B 36 e566–575 https://doi.org/10.14694/EDBK_157450
6. BelfioreMP, Ronza FM, and Della Volpe T, et al (2015) Long-term local disease control in a recurrent soft-tissue sarcoma of the thigh treated by radiofrequency ablation J Surg Oncol 111(6) 708–710 https://doi.org/10.1002/jso.23877 PMID: 25864731
7. Thompson SM, Schmitz JJ, and Schmit GD, et al (2017) Image-guided thermal ablative therapies in the treatment of sarcoma Curr Treat Options Oncol 18(4) 25 https://doi.org/10.1007/s11864-017-0465-1 PMID: 28391423
8. Nakamura T, Matsumine A, and Yamakado K, et al (2009) Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas Cancer 115(16) 3774–3781 https://doi.org/10.1002/cncr.24420 PMID: 19514086
9. Keil S, Bruners P, and Brehmer B, et al (2008) Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma Cardiovasc Intervent Radiol 31(S2) S213–S216 https://doi.org/10.1007/s00270-007-9263-7 PMID: 18175176
10. Hohenberger P, Kasper B, and Ahrar K (2013) Surgical management and minimally invasive approaches for the treatment of metastatic sarcoma Am Soc Clin Oncol Educ B 33 457–464 https://doi.org/10.1200/EdBook_AM.2013.33.457
11. Stotter AT, A’Hern RP, and Fisher C, et al (1990) The influence of local recurrence of extremity soft tissue sarcoma on metastasis and survival Cancer 65(5) 1119–1129 PMID: 2302663
12. Stojadinovic A, Yeh A, and Brennan MF (2002) Completely resected recurrent soft tissue sarcoma: primary anatomic site governs outcomes J Am Coll Surg 194(4) 436–447 https://doi.org/10.1016/S1072-7515(02)01120-1 PMID: 11949749
13. Zagars GK, Ballo MT, and Pisters PWT, et al (2003) Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy Int J Radiat Oncol Biol Phys 57(3) 739–747 https://doi.org/10.1016/S0360-3016(03)00714-4 PMID: 14529779
14. Mauri G, Nicosia L, and Varano GM, et al (2017) Unusual tumour ablations: report of difficult and interesting cases Ecancermedicalscience 11 733 [http://www.ncbi.nlm.nih.gov/pubmed/28487751] Date accessed: 20/8/17 https://doi.org/10.3332/ecancer.2017.733 PMID: 28487751 PMCID: 5406223
15. Mauri G, Cova L, and Monaco CG, et al (2017) Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA) Int J Hyperth 33(3) 295–299 [http://www.ncbi.nlm.nih.gov/pubmed/27701923] Date accessed: 3/10/17 https://doi.org/10.1080/02656736.2016.1244707
16. Pacella CM, Mauri G, and Achille G, et al (2015) Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients J Clin Endocrinol Metab 100(10) 3903–3910 [http://www.ncbi.nlm.nih.gov/pubmed/26274342] Date accessed: 11/10/15 https://doi.org/10.1210/jc.2015-1964 PMID: 26274342
17. Sartori S, Mauri G, and Tombesi P, et al (2018) Ultrasound-guided percutaneous laser ablation is safe and effective in the treatment of small renal tumors in patients at increased bleeding risk Int J Hyperth 35(1) 19–25 [http://www.ncbi.nlm.nih.gov/pubmed/29749271] Date accessed: 18/5/18
18. Mauri G, Cova L, and Ierace T, et al (2016) Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation Cardiovasc Intervent Radiol 39(7) 1023–1030 [http://www.ncbi.nlm.nih.gov/pubmed/26911732] Date accessed: 22/2/17 https://doi.org/10.1007/s00270-016-1313-6 PMID: 26911732
19. Mauri G, Cova L, and Tondolo T, et al (2013) Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results J Clin Endocrinol Metab 98(7) E1203–E1207 [http://www.ncbi.nlm.nih.gov/pubmed/23666969] Date accessed: 28/6/14 https://doi.org/10.1210/jc.2013-1140 PMID: 23666969
20. Dong Y, Wang W-P, and Gan Y-H, et al (2014) Radiofrequency ablation guided by contrast-enhanced ultrasound for hepatic malignancies: preliminary results Clin Radiol 69(11) 1129–1135 [http://www.ncbi.nlm.nih.gov/pubmed/25060936] Date accessed: 9/12/15 https://doi.org/10.1016/j.crad.2014.06.016 PMID: 25060936
21. Mauri G, Porazzi E, and Cova L, et al (2014) Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment Insights Imaging 5(2) 209–216 [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3999370&tool=pmcentrez&rendertype=abstract] Date accessed: 28/6/14 https://doi.org/10.1007/s13244-014-0315-7 PMID: 24563244 PMCID: 3999370
22. Meloni MF, Andreano A, and Franza E, et al (2012) Contrast enhanced ultrasound: should it play a role in immediate evaluation of liver tumors following thermal ablation? Eur J Radiol 81(8) e897–e902 [http://www.ncbi.nlm.nih.gov/pubmed/22658846] Date accessed: 16/12/15 https://doi.org/10.1016/j.ejrad.2012.05.002 PMID: 22658846
23. Prada F, Del Bene M, and Fornaro R, et al (2016) Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection Neurosurg Focus 40(3) PMID: 26926065
24. Mauri G, Nicosia L, and Varano GM, et al (2017) Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation Insights Imaging 8(3) 357–363 Date accessed: 20/8/17 https://doi.org/10.1007/s13244-017-0555-4 PMID: 28500486 PMCID: 5438321
25. Mauri G, Cova L, and De Beni S, et al (2014) Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 Cases Cardiovasc Intervent Radiol 38(1) 143–51 [http://www.ncbi.nlm.nih.gov/pubmed/24806953] Date accessed: 26/6/14
26. Mauri G, De Beni S, and Forzoni L, et al (2014) Virtual navigator automatic registration technology in abdominal application Conference Proceedings Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2014 https://doi.org/10.1109/EMBC.2014.6944889






