Spontaneous testicular tumor regression: case report and historical review
Juan C Astigueta1,2, Milagros A Abad-Licham2,3,4, Folker M Agreda5, Benjamin A Leiva1, Jorge L De la Cruz6
1Oncological Urology Service, Regional Neoplastic Disease Institute, Trujillo 13007, Peru
2School of Medicine, Antenor Orrego Private University, Trujillo 13007, Peru
3Oncological Pathology Service, Regional Neoplastic Disease Institute, Trujillo 13007, Peru
4Centre of Excellence in Pathological Oncology, Trujillo 13007, Peru
5Department of Oncological Surgery, Virgen de la Puerta Hospital, Essalud, Trujillo 13007, Peru
6Oncological Medicine Service, Regional Hospital of Lambayeque, Chiclayo 882, Peru
Correspondence to: Milagros A Abad-Licham. Email: milagrosabadlicham@gmail.com
Abstract
Spontaneous regression of a primary testicular germ-cell tumour (GCT), over time known as ‘Burned out’, ‘Shrinking Seminoma’, ‘pT0’, ‘Burnout’ or ‘Spontaneous Regression’, is an uncommon, generally metastatic phenomenon, which may present elevated tumour markers and a suspicious testicular ultrasound image. The histological study of the testicle demonstrated morphological changes of complete or partial tumour regression and found fibrous scarring and other characteristic changes of this phenomenon, which in some cases include vestiges of GCT.
There are few publications on testicular GCT tumour regression and those that exist present limited data on the biology of the disease and its etiopathogenesis. This entity was recently recognised in the latest edition of the World Health Organization’s (WHO) Classification of Tumours.
We present our clinical, imaging, laboratory, cytohistological and management experience, as well as a historical review of the literature.
Keywords: testicle, spontaneous regression, burned out, germ-cell tumour
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 18/12/2018; Received: 30/06/2018
Introduction
Spontaneous tumour regression has been reported in various neoplasias [1, 2]. In testicular cancer, it is defined as a germ-cell tumour (GCT) that has completely or partially regressed, without any intervention, leaving a scar in the parenchyma with or without vestiges of GCT [3]. The etiopathogenesis of the regression is not defined and it is thought that less than 5% of all testicular GCTs undergo spontaneous regression [4]. It usually presents as metastatic disease and is manifested by symptoms secondary to it. It may have high tumour markers, depending on the histological lineage. Historically, many cases have been classified as primary extragonadal GCTs (EGCTs) but most subsequent studies found evidence of regression of a primary testicular [5–24].
We present our experience and carry out a historical review of the literature.
Materials and methods
Sample
Clinical records of patients from the Urology Service of the Regional Institute of Neoplastic Diseases North, in Trujillo, Peru, were reviewed, from January 2010 to June 2018. We identified the cases with a diagnosis of testicular GCT regression and proceeded to collect the data in a digital card developed for this purpose.
Epidemiological and clinical study
We obtained data such as age, pathological history, time of illness, signs, symptoms and information from the physical examinations.
Imaging study and clinical laboratory
Imaging information obtained was confirmed by evaluating existing material in the Radiology services’ records (ultrasound, x-rays, CT scans and others). Tumour marker data such as alpha fetoprotein (AFP), human chorionic gonadotrophin (HCG) and lactic dehydrogenase (LDH) were collected and correlated with the histological findings.
Management, evolution and current status of the disease
We collected data regarding the type of treatment (surgery, chemotherapy, radiotherapy and other), as far as primary, metastasis and/or recurrence.
Cytological and anatomopathological material
(a) Of the testicle.
(b) Of the metastasis.
We reviewed the cytological and histological material, classifying it according to the World Health Organization’s (WHO’s) 2016 classification of testicular tumours and paratesticular tissue. This edition recognises GCT regression as an entity [4].
Staging
We used the 8th Edition American Joint Committee on Cancer (AJCC) staging system based on the study of tumour (T), lymph node involvement (N), the presence of metastasis (M) and serum tumour marker (S) [25].
Current state of the disease
Upon reviewing the medical history, we obtained the dates of the last check-up and the state of the disease. In the cases with no recent data, we located patients.
Review of existing literature
Bibliographic searches were conducted on Scopus, Medline, EBSCO and BVS from 2000 to the present. The data obtained were analysed, compared and discussed.
Results
Sample
In the review of the medical records, five [5] cases were identified with a diagnosis of primary testicular GCT regression, all metastatic with complete regression.
Case Report
Case 1
A 54-year-old patient without any significant history, with a 6-month disease time, characterised by weight loss, abdominal tumour and lumbar and abdominal pain. Upon physical examination, no peripheral adenopathies were found, a hard fixed mass was felt in the abdomen located in the mesogastrium and testicles with no notable particularities. On the CT scan of the chest, abdomen and pelvis (CT-CAP), a retroperitoneal 17 cm × 10 cm × 8 cm tumour was observed. It encompassed the abdominal aorta, collapsed the vena cava and projected to the iliac arteries; it was initially classified as lymphoma. A core percutaneous biopsy was performed and had inconclusive pathological results. As part of imaging studies, a scrotal ultrasound was requested and a 23 mm × 26 mm hypoechogenic nodule was found in the right testicle. The AFP and HCG tumour markers were found in normal parameters and the LDH in 2480UI. Radical orchiectomy was performed and fibrous scarring associated with histological changes of regression was found. He underwent exploratory laparotomy and subtotal resection of the retroperitoneal tumour with a cytological, histological and immunohistochemical study consistent with a seminoma-type GCT. He received chemotherapy (chemo) with complete tumour remission and tumour markers within normal values; during treatment, he developed deep vein thrombosis (DVT) in both lower extremities (LEs), which was managed medically. At the 60-month follow-up, he shows no evidence of disease.
Case 2
A 58-year-old patient with a 2-month disease time, characterised by bilateral lumbar pain, sensation of thermal rise, volume increase in LEs and weight loss. Physical examination revealed a left supraclavicular lymph node conglomerate of hard consistency, a mass in mesogastrium, hard edema in both legs and no other peripheral adenopathies. The abdomen and testicles were without any particular features. Supraclavicular, subclavian, mediastinal and retroperitoneal adenopathies were observed via CT-CAP, the latter being in a 15 cm × 13 cm × 9 cm conglomerate, which includes the great vessels, as well as thrombus in vena cava and iliac vessel. It was also initially classified as lymphoma. A fine-needle aspiration biopsy (FNAB) of the supraclavicular adenopathy was performed, revealing cytological features of a malignant round-cell neoplasm, likely seminoma. The scrotal ultrasound showed the right testicle with microcalcifications and an 8 mm × 7 mm hypoechogenic nodule. Tumour markers were AFP 4.5UI/l, HCG 8.02UI/L and LDH 3637UI/L. A radical orchiectomy was performed, with a histological report of the fibrous nodule and regression changes, among them germinal neoplasia in situ. A supraclavicular tumour biopsy was performed with histological and immunohistochemical study consistent with seminoma-type GCT. The patient received chemo with complete tumour remission at the supraclavicular and mediastinal level; the remission was partial in the retroperitoneum, therefore, a positron emission tomography was performed, reporting para-aortic tissue of 35 mm, inactive residual appearance, which diminished in subsequent tests. At the 42-month follow-up, there is no evidence of active disease.
Case 3
A 23-year-old patient with a history of thoracic trauma and haemoptysis underwent a thoracic tomography, in which multiple nodules were observed in both pulmonary fields. Subsequently, he developed hemiplegia on the left; in the supplemental CT, a 48-mm hypodense image in the brain was found in the right fronto-parietal region; also in the retroperitoneum, a 11cm × 7 cm × 4cm ganglion conglomerate. Physical examination revealed no peripheral adenopathies, resistance to palpation in the abdomen at the level of mesogastrium and testicles without particular features. The scrotal ultrasound revealed a left testicle with multiple microcalcifications and a 9 mm × 8 mm heterogeneous, hypoechogenic nodule. The tumour markers were AFP 0.9UI/L, HCG 19609UI/L and LDH 561UI/L. Radical orchiectomy was performed with pathology that showed the testicle with fibrous scar associated with histological regression changes. The patient underwent a pulmonary nodule FNA with cytological study compatible with choriocarcinoma-type GCT. Due to brain metastasis, he received radiotherapy (RT) and started chemo. During the treatment, he presented seizures, anaemia and febrile neutropenia; the poor clinical response was observed in imaging studies (hepatic metastasis, increased dimensions of brain metastasis with perilesional haemorrhage) and in the tumour markers (AFP 3.73UI/L, HCG 214UI/L and LDH 734UI/L). Chemo was not completed due to complications and the patient died 7 months after the initial diagnosis with the evidence of disease progression.
Case 4
A 36-year-old patient with no relevant history, with a 7-month disease time characterised by left lumbar pain, abdominal mass, weight loss and increase in volume of the left leg. Upon physical examination, no peripheral adenopathies were found. In the abdomen, a hard and fixed mass was felt in the mesogastrium, testicles had no particularities and an increase in volume in the left leg. On the CT-TAP, a retroperitoneal tumour of 16 cm × 9 cm × 9 cm was observed, which includes the aorta and the cava. Doppler ultrasonography reported DVT in the iliac, femoral and popliteal veins of the left leg. On the scrotal ultrasound, a 30-mm hypoechoic nodule was found in the left testicle, associated with multiple microcalcifications. The tumour markers were AFP 0.69UI, HCG 0.44UI and LDH 1240UI. Radical orchiectomy was performed with pathology that reported parenchyma with a fibrous scar and tubular hyalinization. Percutaneous biopsy of the retroperitoneal tumour with histological and immunohistochemical exam was compatible with the seminoma-type GCT. Full chemo with partial remission of the disease and tumour markers were within normal parameters. The residual tissue has progressively decreased in volume. Currently, at 20 months of follow-up, there is no evidence of active disease.
Case 5
A 20-year-old patient without any significant history, with a 6-month disease time, characterised by weight loss, abdominal tumour and pain. Upon physical examination, no peripheral adenopathies were found, a hard fixed mass was felt in the abdomen located in the mesogastrium, testicles had no notable particularities. On the chest, abdomen and pelvis CT, a retroperitoneal tumour was observed predominantly in the left iliac region measuring 16 cm × 15 cm × 9 cm, which includes iliac vessels and left renal agenesis. Suspecting a metastatic GCT, a scrotal ultrasound was performed and found a 5 mm × 10 mm isoechogenic nodule in the left testicle. Tumour markers were 1745UI AFP, HCG 3705UI and LDH 2948UI. Radical orchiectomy was performed and fibrous scarring associated with histological changes of regression was found. A percutaneous biopsy of the retroperitoneal tumour was performed, with a cytological and histological report of mixed GCT (embryonal carcinoma, teratoma and yolk sac tumour). During the evolution of the disease, one of the complications presented was a bowel obstruction, resolved with sigmoidectomy and block retroperitoneal lymphadenectomy. Patient was currently in chemo with partial remission.
Epidemiological and clinical study
The cases presented between 20 and 58 years with an average age of 38 years. No significant data were found with regard to history. The average disease duration was 3.8 months (range 1–7 months), mainly characterised by abdominal and/or lumbar pain, weight loss and abdominal tumour in three cases and one supraclavicular tumour. On examination of testes, tumours were not felt on palpation in any patient. In two cases, the initial diagnosis was lymphoma Table 1.
Imaging and laboratory study
The results of the imaging studies conducted are summarised in Table 2. All five cases were metastatic, with retroperitoneal tumours larger than 10 cm; in four cases, the tumour encompassed the aorta, cava and/or iliac Figure 1. Two had a diagnosis of DVT. As to tumour markers, in all cases, the LDH was high and, in the other two, HCG and AFP.
Table 1. Epidemiological and clinical data.

Table 2: Imaging and tumour marker data at the initial diagnosis.

Management, evolution and current status of the disease
The initial clinical suspicion in the first three cases was different from primary testicular metastatic GCT, therefore, the diagnostic work-up included aspiration and/or surgical biopsies of retroperitoneal, supraclavicular and lung masses, respectively. Laboratory studies and images were supplemented with histological findings and radical orchiectomy was performed. In the last two cases, where GCT was suggested from the beginning, the pathology of the orchiectomy was consistent with tumour regression, having found evidence of germinal neoplasia in the study of the metastasis.
With the anatomopathological diagnosis of testicular tumour regression and metastatic staging, all patients received chemo and had favourable responses corroborated through imaging studies and MT, except for the third case, who also received RT due to brain metastasis and progressed to death (Table 3).
Anatomopathological material
The existing material was reviewed and classified according to WHO classification of testicular tumours and paratesticular tissue (4).
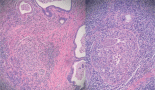
In the testicle product of radical orchiectomy, macroscopically, all cases presented a whitish fibrous scar, located close to the to rete testis. The surrounding testicular parenchyma did not present significant alterations. For histological interpretation, we divide the testicles into two regions: the scar and the area adjacent to the scar (paracicatricial), whose characteristics are described in Figure 2 and shown in micrographs in Figure 3.

Figure 1. Imaging studies: (A) Ultrasound showing heterogeneous nodule and microcalcifications in the testicular parenchyma. (B) Tomography with retroperitoneal mass that encompasses large vessels.
Table 3. Data on the management, anatomopathological diagnosis and state of the disease.

With regard to metastasis, four of these were evaluated initially with cytology, two with FNAB and two with intraoperative cytology. The results of these showed germ-tumour cytology, allowing identification of the types seminoma, choriocarcinoma and embryonal carcinoma. Subsequently, all cases were subjected to conventional histological and immunohistochemical study, corroborating diagnoses of germinal neoplasia; the latter presented teratoma and yolk sac tumour in addition to embryonal carcinoma. Table 4 presents the anatomopathological results in correlation with the tumour markers.
Staging
The five cases were classified as metastatic stage pT0, with findings of testicular tumour regression.
Literature review
Performing a search from January 2000 to June 2018, 159 cases were found in 57 articles. The cases arose between 17 and 67 years old with an average age of 35.96 years; only in nine cases (15.8%), cryptorchidism was reported as a precedent. 96.8% of the patients had metastatic disease and 71.7% had complete regression.
Likewise, the time frame of the review found that there is a discrete increased frequency of regression in the right testicle (47%) and the more common histological type is seminoma (50.8%). The publications by author’s locations were as follows: Europe 22 (38.6%), Asia 18 (31.6%) and America 17 (29.8%). The summary of the data is presented in Tables 5 and 6.
In the review conducted, there are some publications of cases reported as ‘burned out’, the same as the clinic and/or imaging studies and/or laboratory are compatible with GCT; however, they were not considered because they do not have complete data, especially histological findings of the testicle.
Discussion
Over time, the ‘phenomenon’ of tumour regression has been described in different pathologies such as melanoma, breast cancer, lymphoma, renal carcinoma, among others [1, 2]. It is currently known that the process that keeps tumours alive does not only depend on their ability to multiply and block apoptosis, but there is also a close relationship with the immune environment in which the tumour develops, the so-called tumour microenvironment [4, 26–28].

Figure 2. Histomorphological characteristics of testicular tumour regression: scar (blue) and paracicatricial area (orange). The case numbers are on the ‘Y’ axis.

Figure 3. Microphotographs of the histomorphological characteristics of the testicular tumour regression: (A) Fibrous scar with increased vascularity. (B) Increase in vascularity and microcalcifications. (C) Tubular hyalinosis and presence of Leydig cells. (D) Microliths in paracicatric area. (E) NGIS-type embryonal carcinoma. (F) NGIS and intratubular calcifications.
Table 4. Anatomopathological results of the metastasis, diagnostic procedure and correlation with tumour markers.

Table 5. General data from the 2000–2018 literature review.

There are not many publications on the regression of testicular GCTs; this entity has only recently been recognised in the last edition of the WHO’s book on Tumours of the Urinary System and Male Genital Organs (2016), in the chapter on testicular tumours and paratesticular tissues [4]. It is considered that the first to describe this phenomenon was Prim in 1927; he reported the case of a 51-year-old patient who died with multivisceral and retroperitoneal lymph node metastasis, with ‘chorionephiteliomatösen’ histology and no known primary. At the autopsy, he found a scar on the right testicle and posed the question of whether it may have been the primary one and presented ‘spontaneous healing’ [29]. In 1954, Rather et al [30] reported six new cases and reviewed the bibliography, finding 18 additional cases. In the final histological analysis, they described testicles as seven having only one scar, nine having germ-cell tumours and eight having fibrosis, tubular and cystic structures, hemosiderin deposits and calcification. The characteristics of testicular tumour regression have been defined this way for over six decades, with a high approximation for existing approaches, same as have been identified in our sequence.
In 1955, Slater et al [31] reported a case where a patient with a retroperitoneal mass with ‘seminoma and chorioepithelioma’ histology. He underwent bilateral orchiectomy and a small, solid nodule in the left testicle was found, which the microscope identified as scarred teratoma, confirming that they were dealing with a ‘burned out primary…’. This is likely the first publication to use this terminology to define tumour regression in germ cells.
Table 6. Publications on spontaneous testicular GCT regression (2000–2018)

In 1961, Azzopardi et al [32] published a series of 17 cases of young patients that passed away due to disseminated metastatic illnesses, eight with choriocarcinoma histology, five with embryonal carcinoma and four mixed. All of the testicle exams were normal but the pathology study found fibrous scarring in the majority of cases with haemotoxylin deposits in the seminiferous tubules, consistent with the burnt out phenomenon. In 1965, in a second publication on the subject, the same author presented a case that illustrated the pattern of regression in a testicular seminoma with viable metastasis. The author provides a comprehensive description of typical histological findings, as well as a comparison with choriocarcinoma [33].
In 1970, Veragut et al [34] reported two cases of young patients with a diagnosis of retroperitoneal seminoma with no evidence of a primary. In their discussion, they describe the ‘necrobiosis phenomenon’ as a means to explain the spontaneous involution of testicular tumours, indicating that it occurs more frequently in choriocarcinoma and is rare in seminoma.
In 1990 and 2000, two works were published, titled ‘Shrinking Seminoma’ and ‘Shrinking Seminoma—Fact or Fiction?’, describing volume reduction in the testicle with seminoma, where the mechanism is fundamentally ischemia—necrosis secondary to intermittent testicular torsion. Other possible causes also described were chronic inflammation and hormonal disorder. In the aforementioned phenomenon, depending on the stage at which it is diagnosed, a testicle ‘shrunken’ in size, with or without a residual tumour may be found, which is why in a patient with a retroperitoneal, mediastinal or other mass that also presents testicular shrinking, a GCT should be suspected [35, 36].
Near the end of the 20th century, various publications report on probable EGCT, in which lesions were found at the testicular level, consistent with spontaneous regression, correlating to the primary [5–9]. According to different publications, 90% of EGCTs occur between ages 20 and 35 and represent less than 5% of total GCTs; most commonly found in the anterior mediastinum, followed by the retroperitoneum and rarely in the pineal gland, presacral region or in another organ [7, 17, 20, 37, 38]. In general, every extragonadal tumour with GCT histology is considered a metastasis of hidden gonadal GCT until proven otherwise, with some authors even questioning the existence of EGCT [7].
Although the mechanism behind primary tumour regression has not been determined, there are several hypotheses. The two main hypotheses are: those related to an immunological response mediated by cytotoxic T lymphocytes that recognise tumour antigens and destroy malignant neoplastic cells, with subsequent fibrosis replacement; and those related to an ischemic response in the neoplasia, secondary to the blood supply deficit due to high metabolic rates and/or intermittent testicular torsion (Shrinking Seminoma). Another hypothesis indicates that when seminomas become metastatic, the organism produces antibodies that attack the metastasis, as well as the primary testicular tumour, which shrinks and may even be destroyed, leaving only traces. This foundation refers to the immunological theory of regression [26, 39–43].
Clinical manifestations generally depend on the metastatic disease [44–52]; only a few non-metastatic cases were diagnosed with signs and local symptoms such as pain in the scrotal sack, testicular shrinking and infertility studies. [26, 36, 43, 53, 54]. In accordance with the histology review performed, the most frequent symptoms were lumbar, abdominal and abdominal mass pain, very similar to the findings in our series, in addition to reports of weight loss. During the clinical evaluation of our case series, the first two patients were initially listed as having the lymphoproliferative syndrome and the third as having pulmonary metastasis of unknown origin. In various revised reports, due to various symptoms, the diagnostic work was also directed to pathology different from that of GCT [10–12, 14, 21, 24, 44, 54–57].
Examination of the scrotal sacs through palpation is insufficient to exclude testicular tumours. The findings depend on the size of the tumour, its relation to the size of the testicle, the placement, consistency and/or associated pathologies, such as hydrocele, cysts or others [7, 26, 28, 56, 58]. In our casuistry, the physical exam found no testicular tumours, supplemented with ultrasound.
The sensitivity of the testicular ultrasound for diagnosing GCT is close to 100%, so all young patients with a retroperitoneal or mediastinal mass should undergo this test. The characteristics of testicular tumour regression are not specific, as there have been findings of hyperechogenic, hypoechogenic and mixed lesions, nodular and linear areas, signs of testicular atrophy and/or acoustic shadowing reflecting calcifications or fibrosis [3, 26, 59, 60]. If the ultrasound results are inconclusive, scrotal magnetic resonance imaging (sMRI) can be useful in better defining these findings that are not necessarily malignant (infarction, ischemia, trauma or infection) [43, 59, 61]. Patel and Patel [53] reported that, when using the sMRI, a finding that suggests neoplasia is the appearance of a rapid higher height peak at the lesion. In the collection of histological data, it was found that the ultrasound findings most frequently related to regression were hypo- and hyperechogenic lesions and microlithiasis. Another useful imaging test for metastatic lesion detection that is also essentially in the control and follow-up after chemotherapy is positron emission tomography, along with multislice tomography (PET/CT) through radiopharmaceutical administration of F18-FDG [24, 28, 37, 62].
Tumour markers are fundamental to the diagnostic approach, staging, treatment and follow-up; they show up in varying forms depending on the histological lineage and the response to treatment. In our report, during the initial diagnostic, LDH was increased in all of the cases in relation to the metastasis and tumour burden, in two patients with HCG and in one with AFP [4, 5, 25, 38, 60].
The five reported cases were metastatic and diagnosed using the cytohistological results of the lesions, which, in addition to the clinical findings, laboratory and imaging studies, allowed us to formulate the primary testicular diagnosis and to indicate the corresponding radical orchiectomy. In the various reports, the macroscopic description of the testicles showing the partial or total tumour regression phenomenon, the presence of lesions that are hardened, whitened, fibrose, of scar aspect, in the form of nodules (singular or multiple), banded, linear or starred, is reported [3, 4, 7, 38, 63–65]. We found fibrosis scarring in every one of our cases.
Microscopically, our findings coincide with the WHO description defining the diagnostic criteria for testicular tumour regression, including inflammatory lymphoplasmacytic infiltrate (present in around 90% of cases), tubular hyalinization (around 70%), increase in vascularity (50%), hemosiderin (44%) and thick intratubular calcifications. The peripheral area was observed to have atrophy and sclerosis of the seminiferous tubules (100%), germinal cell malignancy in situ (approximately 50%), hyperplasia in Leydig cells (45%), intratubular microlitres (30%). The literature mentions intertubular calcifications and germinal neoplasia in situ as pathognomonic signs [3, 4, 38].
It is known that all germinal tumours have the potential for regression; however, there is evidence in the literature that disagrees on the frequency of the histological subtypes that demonstrate this phenomenon. Historically, choriocarcinoma has been considered the most prone to regression, but recent reports, as well as ours, confirm that seminoma has the most common histology, with exception of the spermatocytic type, which is now a separate entity. The teratomas are classified as the histology group with the lowest probability of regression [3, 4, 8, 38, 66, 67].
Various publications agree that chemotherapy is not completely effective for the testicles due to the haemato-testicular barrier and thus the necessity of surgical resection (orchiectomy) on the ‘regressed’ primary is essential and represents the cornerstone of the burned out definition, in addition to being the foundation for proper treatment. The management is, in general, similar to that for primary GCT testicles [6, 7, 56, 58, 59, 68–71].
For a large majority of patients, the diagnostic work was performed based on the symptomatology of the metastasis, not infrequently with an approach different to that of burned out GCT that shows the delay in proper diagnosis, the risk of complications due to time and progression of the disease, as well as procedures that some may have undergone. The incorrect extragonadal GCT diagnosis implies not treating the primary testicle, which may present partial regression, and, thus, in not responding to the systemic treatment and being maintained in a safe haven by the haemato-testicular barrier, it may become one of the most important factors determining recurrence and prognosis.
In our review of the literature, we found no conclusive publications on survival, the persistence of the disease or recurrence when compared to burned out GCT versus Gonadal and/or extragonadal GCT. In our case, the reported patients began a long-term follow-up protocol in order to carry out collaborative projects with other institutions in an attempt to answer the questions raised.
Conclusions
Throughout time, the evidence regarding the ‘phenomenon’ of testicular tumour regression has been described in various publications, which has allowed for its current definition as an entity with its own diagnostic criteria.
Etiopathogenesis is still not well defined, nor if the tumoural regression tumour itself has some value in the prognosis. What is well defined is the indication for treatment in accordance with GCT protocols.
‘Burned out’ GCTs are classified as metastatic or non-metastatic with complete or partial regression. The most frequent ones were metastatic with complete regression and the most common histological type was seminoma.
In all tumours with GCT clinical and/or histology, the primary testicular tumour should be ruled out before classifying it as an extragonadal GCT.
Conflicts of interest
The authors declared no conflicts of interest.
Authors’ contributions
The main idea and literature review were by JCA and MAA; the collection of data was done by FMA, BAL and JLD. Manuscript revision and approval of the final copy was done by all.
Acknowledgments
We thank the pathology laboratory staff: Medical Technologist Cinthya Ortiz and Biological Cytologist Oriana Vasquez.
References
1. Everson T and Cole W (1956) Spontaneous regression of cancer Ann Surg 144(3) 366–380 https://doi.org/10.1097/00000658-195609000-00007 PMID: 13363274 PMCID: 1465423
2. Salman T (2016) Spontaneous tumor regression J Oncol Sci 2(1) 1–4
3. Balzer B and Ulbright T (2006) Spontaneous regression of testicular germ cell tumors: an analysis of 42 cases Am J Surg Pathol 30(7) 858–865 https://doi.org/10.1097/01.pas.0000209831.24230.56 PMID: 16819328
4. Ulbright T, Amin M, and Balzer B, et al (2016) Tumours of the testis and Paratesticular tissue WHO Classification of Tumours of the Urinary System and Male Genital Organs 4th edn (Lyon: IARC) pp 185–258
5. Burt M and Javadpour N (1981) Germ-cell tumors in patients with apparently normal test Cancer 47(7) 1911–1915 PMID: 6164475
6. Comiter C, Renshaw A, and Benson C, et al (1996) Burned out primary testicular cancer: sonographic and pathological characteristics J Urol 156 85–88 https://doi.org/10.1016/S0022-5347(01)65947-0 PMID: 8648846
7. Scholz M, Zehender M, and Thalmann M, et al (2002) Extragonadal retroperitoneal germ cell tumor: evidence of origin in the testis Ann of Oncol 13 121–124 https://doi.org/10.1093/annonc/mdf003
8. Extramiana J, De La Rosa F, and Madero S, et al (1986) “Burned-out” testicular tumor Actas Urol Esp 10(5) 289–294 PMID: 3030064
9. Trias I, Algaba F, and Hocsman H (1991) “Intratubular germ cell tumor: relation with “burned out” tumor and testicular germinal neoplasia Eur Urol 19(1) 81–84 https://doi.org/10.1159/000473586 PMID: 1848823
10. Yamamoto H, Deshmukh N, Gourevitch D, Taniere P, Wallace M, Cullen M (2007) Upper gastrointestinal hemorrhage as a rare extragonadal presentation of seminoma of testis Int J Urol 14 261–263 https://doi.org/10.1111/j.1442-2042.2007.01685.x PMID: 17430271
11. Coulier B, Lefebvre Y, and de Visscher L, et al (2008) Metastases of clinically occult testicular seminoma mimicking primary JBR–BTR 91 139–144
12. Mesa H, Rawal A, and Rezcallah A, et al (2009) “Burned out” testicular seminoma presenting as a primary gastric malignancy Int J Clin Oncol 14 74–77 https://doi.org/10.1007/s10147-008-0804-0 PMID: 19225929
13. Musser J, Przybycin C, and Russo P (2010) Regression of metastatic seminoma in a patient referred for carcinoma of unknown primary origin Nat Rev Urol 7 466–470 https://doi.org/10.1038/nrurol.2010.99 PMID: 20606623
14. Herrera J, Baztarrica G, and España S, et al (2011) Extragonadal germ-cell tumours with testicular “burned out” phenomenon Rev Arg deUrol 76(1) 22–27
15. Kar H, Kamer E, and Ekinci N, et al (2011) Upper gastrointestinal bleeding as initial presentation of burned-out testicular tumor UHOD 4(21) 245–248 https://doi.org/10.4999/uhod.09113
16. Gonzalez R, Montoto P, and Iglesias E, et al (2012) Tumor de células germinales extragonadal con fenómeno “burned-out” simulando tumor retroperitoneal de estirpe neurogénico Arch Esp Urol 65(10) 900–902 PMID: 23269339
17. Albany C and Einhorn L (2013) Extragonadal germ cell tumors: clinical presentation and management Curr Opin Oncol 25 261–265 PMID: 23422328
18. Onishi K, Tomioka A, and Maruyama Y, et al (2014) Burned-out testicular tumor diagnosed triggered by paraneoplastic neurological syndrome: a case report Hinyokika Kiyo 60(12) 651–655
19. George S, Al-Taleb A, and Hussein S (2015) Retrogressed (burned-out) testicular germ cell tumor disguising as duodenal gastrointestinal stromal tumor Oncology Gastroenterology and Hepatology Reports 4(2) 114–115 https://doi.org/10.4103/2348-3113.152335
20. Makino T, Konaka H, and Namiki M (2016) Clinical features and treatment outcomes in patients with extragonadal germ cell tumors: a single-center experience Anticancer Res 36 313–318 PMID: 26722059
21. Ishikawa H, Kawada N, and Taniguchi A, et al (2016) Paraneoplastic neurological syndrome due to burned-out testicular tumor showing hot cross-bun sign Acta Neurol Scand 133 398–402 https://doi.org/10.1111/ane.12469
22. Juul M and Rasmussen E (2017) A burned-out seminoma lymph node metastasis to the neck of a patient treated for colon cancer Ugeskr Laeger 179(20) 2–3
23. Nishisho T, Sakaki M, and Miyagi R, et al (2017) Burned-out seminoma revealed by solitary rib bone metastasis Skeletal Radiol 46(10) 1415–1420 https://doi.org/10.1007/s00256-017-2701-y PMID: 28634622
24. Freifeld Y, Kapur P, and Chitkara R, et al (2018) Metastatic “Burned Out” Seminoma Causing Neurological Paraneoplastic Syndrome—Not Quite “Burned Out” Front Neurol 9(20) https://doi.org/10.3389/fneur.2018.00020
25. Brimo F, Srigley J, and Ryan C, et al (2017) Testis AJCC Cancer Staging Manual 8th edn (Chicago: Springer) pp 727–735
26. Fabre E, Jira H, and Izard V, et al (2004) Burned-out primary testicular cancer BJU Int 94(1) 74–78 https://doi.org/10.1111/j.1464-410X.2004.04904.x PMID: 15217435
27. Sengupta N, MacFie T, and MacDonald T, et al (2010) Cancer immunoediting and spontaneous tumor regression Pathol Res Pract 206(1) 1–8 https://doi.org/10.1016/j.prp.2009.10.001
28. Mosillo C, Scagnoli S, and Pomati G, et al (2017) Burned-Out Testicular Cancer: Really a Different History? Case Rep Oncol 10 846–850 https://doi.org/10.1159/000480493 PMID: 29071000 PMCID: 5649263
29. Prym P (1927) Spontanheilung eines bösartigen, wahrscheinlich chorionephiteliomatösen gewächses im hoden Virchows Arch Pathol Anat 265 239–258 https://doi.org/10.1007/BF01894164
30. Rather L, Gardiner W, and Frerichs J (1954) Regression and maturation of primary testicular tumors with progressive growth of metastases Stanford Med Bull 12(1) 12–25 PMID: 13135788
31. Slater G, Schultz H, and Kreutzmann W (1955) Occult testicular tumor J Am Med Assoc 157(11) 911–912 https://doi.org/10.1001/jama.1955.02950280035010e PMID: 13233056
32. Azzopardi J, Mostofi F, and Theiss E (1961) Lesion of testis observed in certain patients with widespread choriocarcinoma and related tumors Am J Pathol 38 207–225 PMID: 13685483 PMCID: 1945003
33. Azzopardi J and Hoffbrand A (1965) Retrogression in testicular seminoma with viable metastases J Clin Path 18 135–141 https://doi.org/10.1136/jcp.18.2.135 PMID: 14276145 PMCID: 472854
34. Veraguth P, Maillard G, and MacGee W (1970) Retroperitoneal seminomas without evidence of primary growth Oncology 24 194–209 https://doi.org/10.1159/000224520
35. Simpson A, Calvert D, and Codling B (1990) The shrinking semonima J R Soc Med 83 187 https://doi.org/10.1177/014107689008300321
36. Naseem S, Azzopardi A, and Shrotri N, et al (2000) The shrinking seminoma – fact or fiction? Urol Int 65 208–210 https://doi.org/10.1159/000064878
37. Shinagare A, Jagannathan J, and Ramaiya N, et al (2010) Adult extragonadal germ cell tumors Am J Roentgenol 195(4) 274–280 https://doi.org/10.2214/AJR.09.4103
38. Angulo J, González J, and Rodríguez N, et al (2009) Clinicopathological study of regressed testicular tumors (Apparent Extragonadal Germ Cell Neoplasms) J Urol 182 2303–2310 https://doi.org/10.1016/j.juro.2009.07.045 PMID: 19762049
39. Bissen L, Brasseur P, and Sukkarieh F (2003) Spontaneous regression of testicular tumor JBR-BTR 86(6) 319–321
40. Kontos S, Doumanis G, and Karagianni M, et al (2009) Burned-out testicular tumor with retroperitoneal lymph node metastasis: a case report J Med Case Rep 3(8705) https://doi.org/10.4076/1752-1947-3-8705 PMID: 19830238 PMCID: 2737757
41. Sahoo P, Mandal P, and Mukhopadhyay S, et al (2013) Burned Out Seminomatous Testicular Tumor with Retroperitoneal Lymph Node Metastasis: A Case Report Indian J Surg Oncol 4(4) 390–392 https://doi.org/10.1007/s13193-012-0207-6
42. Miacola C, Colamonico O, and Bettocchi C, et al (2014) Burned-out in a mixed germ cell tumor of the testis: The problem of pT 0. Case report Archivio Italiano di Urologia e Andrologia 86(4) 389–390 https://doi.org/10.4081/aiua.2014.4.389
43. Ichiyanagi O, Nagaoka A, and Izumi T, et al (2013) Suspicion of primary testicular germ cell tumor regressed completely before metastasis Int Canc Conf J 3(2) 87–90 https://doi.org/10.1007/s13691-013-0121-y
44. Leleu O, Vaylet F, and Debove P, et al (2000) Pulmonary metastasis secundary to Burned-Out testicular tumor Respiration 67 590 https://doi.org/10.1159/000029579
45. Perimenis P, Athanasopoulos A, and Geraghty J, et al (2005) Retroperitoneal seminoma with ‘burned out’ phenomenon in the testis Int J Urol 12 115–116 https://doi.org/10.1111/j.1442-2042.2004.00987.x PMID: 15661068
46. Curigliano G, Magni E, and Renne G, et al (2006) “Burned out” phenomenon of the testis in retroperitoneal seminoma Acta Oncologica 45 335–336 https://doi.org/10.1080/02841860500401175
47. Ha H, Jung S, and Park S, et al (2009) Retroperitoneal seminoma with the ‘Burned out’ phenomenon in the testis Korean J Urol 50 516–519 https://doi.org/10.4111/kju.2009.50.5.516
48. Yucel M, Kabay S, and Saracoglu U, et al (2009) Burned-out testis tumour that metastasized to retroperitoneal lymph nodes: a case report J Med Case Rep 3(7266) https://doi.org/10.1186/1752-1947-3-7266 PMID: 19830158 PMCID: 2726527
49. Yucel M, Saracoglu U, and Yalcinkaya S, et al (2009) Burned-out testicular seminoma that metastasized to the prostate Cent European J Urol 62(3) 195–197 https://doi.org/10.5173/ceju.2009.03.art15
50. Orlich C and Jiménez E (2010) Tumor testicular quemado. Regresión espontánea de un seminoma testicular (reporte de un Caso) Revista médica de Costa Rica y Centroamerica LXVII(595) 445–447
51. Gaytán-Escobar E, Muñoz-Islas E, and Colorado-García A, et al (2010) Tumor testicular quemadocon metástasis pulmonares y retroperitoneales; informe de un caso Rev Mex Urol 70(5) 301–304
52. Jaber S (2010) Retroperitoneal mass and burned out testicular tumor Saudi J Kidney Dis Transpl 21(3) 542–543 PMID: 20427889
53. Patel M and Patel B (2007) Sonographic and Magnetic Resonance imaging appearance of a burned-out testicular germ cell neoplasm J Ultrasound Med 26 143–146 https://doi.org/10.7863/jum.2007.26.1.143
54. Chung H, Woon M, and Lo F (2014) Burned-out testes seminoma without distant metastasis Clinical Practice 3(1) 4–6
55. Budak S, Celik O, and Turk H, et al (2015) Extragonadal germ cell tumor with the “burned out” phenomenon presented a multiple retroperitoneal masses: a case report Asian J Androl 17 163–164 https://doi.org/10.4103/1008-682X.137481
56. Vasquez L, Frattini G, and Fernández M (2008) Burned out testis tumor, revision of their characteristics and presentation of three new cases Rev Arg Urol 73(1) 19–24
57. Balalaa N, Selman M, and Hassen W (2011) Burned-Out testicular tumor: A case report Case Rep Oncol 4 12–15 https://doi.org/10.1159/000324041 PMID: 21691568 PMCID: 3114614
58. Gurioli A, Oderda M, and Vigna D, et al (2013) Two cases of retroperitoneal metastasis from a completely regressed burned-out testicular cancer Urologia 80(1) 74–79 https://doi.org/10.5301/RU.2013.10768 PMID: 23504866
59. Tasu J, Faye N, and Eschwegw P, et al (2003) Imaging of burned-out testis tumor: five new cases and review of the literature J Ultrasound Med 22(5) 515–521 https://doi.org/10.7863/jum.2003.22.5.515 PMID: 12751863
60. Peroux E, Thome A, and Geffroy Y, et al (2012) Burned-out tumour; Retroperitoneal metastases; Ultrasound Diagn Interv Imaging 93 796–798 https://doi.org/10.1016/j.diii.2012.03.023 PMID: 22819268
61. El Sanharawi I, Correas J, and Glas L, et al (2016) Non-palpable incidentally found testicular tumors: Differentiation between benign, malignant, and burned-out tumors using dynamic contrast-enhanced MRI Eur J Radiol 85(11) 2071–2082 https://doi.org/10.1016/j.ejrad.2016.09.021
62. Nguyen B, Roarke M, and Yang M (2015) Intestinal metastases as an unusual presentation of a burned-out testicular seminoma: PET/CT imaging Rev Esp Med Nucl Imagen 34(4) 266–267
63. McCarthy W, Cox B, and Laucirica R, et al (2015) Fine Needle Aspiration Diagnosis of a Metastatic Mixed Germ Cell Tumor from a “Burned Out” Testicular Primary with Florid Leydig Cell Hyperplasia Ann Clin Cytol Pathol 1(3) 1013
64. Parada D, Peña K, and Moreira O, et al (2007) Extragonadal retroperitoneal germ cell tumor: primary versus metastases? Arch Esp Urol 60(6) 713–719 https://doi.org/10.4321/S0004-06142007000600020 PMID: 17847753
65. Nakazaki H, Tokuyasu H, and Takemoto Y, et al (2016) Pulmonary metastatic choriocarcinoma from a burned-out testicular tumor Intern Med 55 1481–1485 https://doi.org/10.2169/internalmedicine.55.5679 PMID: 27250057
66. López J and Angulo J (1994) Burned-out tumour of the testis presenting as Retroperitoneal choriocarcinoma Int Urol Nephrol 26 549–553 https://doi.org/10.1007/BF02767657 PMID: 7860203
67. Tejido A, Villacampa F, and Martín M, et al (2000) Tumor testicular fundido Arch Esp Urol 53(6) 447–452
68. Castillo C, Krygier G, and Carzoglio J, et al (2005) Gastrointestinal bleeding as the first manifestation of a burned-out tumour of the testis Clin Transl Oncol 7(10) 458–463 https://doi.org/10.1007/BF02716597 PMID: 16373055
69. Mola M, Gonzalvo V, and Torregrosa M, et al (2005) Tumor testicular bilateral “quemado” (“burn out”) Actas Urol Esp 29(3) 318–321 https://doi.org/10.1016/S0210-4806(05)73247-2
70. Preda O, Nicolae A, and Loghin A, et al (2011) Retroperitoneal seminoma as a first manifestation of a partially regressed (burnt-out) testicular germ cell tumor Rom J Morphol Embryol 52(1) 193–196 PMID: 21424055
71. Qureshi J, Feldman M, and Wood H (2014) Metastatic “Burned-Out” germ cell tumor of the testis J Urology 192 936–937 https://doi.org/10.1016/j.juro.2014.06.038
72. Kebapci M, Can C, and Isiksoy S, et al (2002) Burned-out tumor of the testis presenting as supraclavicular lymphadenopathy Eur Radiol 12 371–373 https://doi.org/10.1007/s003300101038 PMID: 11870436
73. Womeldorph C, Zalupski M, and Knoepp S, et al (2010) Retroperitoneal germ cell tumor diagnosed by endoscopic ultrasound-guided fine needle aspiration World J Gastrointest Oncol 2(12) 443–445 https://doi.org/10.4251/wjgo.v2.i12.443 PMID: 21191538 PMCID: 3011098
74. Gomis C, Alcoba M, and González R, et al (2015) Tumor testicular quemado o burn-out tumor XXX Reunión Nacional del Grupo de Urología Oncológica, España. En: [www.aeu.es/aeu_webs/reuniones/oncologia2015/resumenGR.aspx?Sesion=4&Numero=P-23]
75. Hu B, Shah S, and Shojaei S, et al (2015) Retroperitoneal lymph node dissection as first-line treatment of node-positive seminoma Clin Genitourin Cancer 13(4) e265–e269 https://doi.org/10.1016/j.clgc.2015.01.002 PMID: 25682512
76. Iwatsuki S, Naiki T, and Kawai N, et al (2016) Nonpalpable testicular pure seminoma with elevated serum alpha-fetoprotein presenting with retroperitoneal metastasis: a case report J Med Case Rep 10(114) https://doi.org/10.1186/s13256-016-0906-7 PMID: 27150447 PMCID: 4858825
77. Ulloa–Ortiz O, Garza-Garza R, and Hernán G, et al (2017) Tumor extragonadal de células germinales con fenómeno de burned-out testicular Gaceta Mexicana de Oncología 16(5) 307–310
78. El-Sharkawy M and Al-Jibali A (2017) Burned-out metastatic testicular tumor: choriocarcinoma Int J Health Sci 11(2) 81–82