Identifying nutritional, functional, and quality of life correlates with male hypogonadism in advanced cancer patients
Domenico Fuoco1, 4, Jonathan di Tomasso4, Caroline Boulos1, Robert D Kilgour1, 2, Jose A Morais1, 3, Manuel Borod1, 4 and Antonio Vigano1, 4
1McGill Nutrition and Performance Laboratory, McGill University Health Centre, Montreal H4A 3S5, Canada
2Department of Exercise Science, Concordia University, Montreal H4B 1R6, Canada
3Department of Geriatric Medicine, McGill University, Montreal H4A 3S5, Canada
4Division of Supportive and Palliative Care Medicine, McGill University Health Centre, Montreal H4A 3S5, Canada
Correspondence to: Antonio Vigano. Email: antonio.vigano@mcgill.ca
Abstract
With the availability of a potential treatment to reverse male hypogonadism (MH), the primary aim of this case series study was to determine independent relationships between this condition and the nutritional, functional, and quality of life characteristics of advanced cancer patients (ACP). Free testosterone levels were measured in 100 male patients with advanced lung and gastrointestinal (GI) cancer. Routine blood markers of nutrition and inflammation, self-reporting questionnaires for symptom, nutrition, and functional status along with handgrip dynamometry were assessed for all patients at bedside. Almost half of this cohort underwent further assessments (body composition, lower body strength, in depth quality of life and fatigue questionnaires) at the McGill Nutrition and Performance Laboratory (mnupal.mcgill.ca). Multiple regression analyses were performed to identify independent correlations between free testosterone and the above measures. Seventy-six percent of patients were diagnosed with MH. Using multiple linear regression, low free testosterone (31.2 pmol/L) was independently associated with lower albumin (B = –3.8 g/L; 95% confidence interval CI –6.8:–0.8), muscle strength (–11.7 lbs; –20.4: –3.0) and mass in upper limbs (–0.8 kg; –1.4: –0.1), overall performance status (Eastern Cooperative Oncology Group Performance Scale, ECOG PS 0.6; 0.1:1.1), cancer-related fatigue (Brief Fatigue Inventory, BFI 16.7; 2.0: 31.3), and overall quality of life (MQoL total score –1.42; –2.5: –0.3). Thus MH seems to be highly prevalent in ACP, and it is independently associated with important nutritional, functional, and quality of life characteristics in this patient population.
Keywords: hypogonadism, male, quality of life, function, testosterone, cachexia, cancer, case series study
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 04/08/2015; Received: 16/02/2015
Introduction
MH is characterised by the presence of low circulating concentrations of androgens, namely testosterone, a hormone mainly produced by the testicles [1]. Androgen deficiency has been found to be highly prevalent in male cancers and it has been suggested as a contributing factor of cachexia sequelae and cancer-related fatigue [2]. In addition, MH could be an important cause of muscle wasting in cancer cachexia and sarcopoenia [2]. Clinical signs such as high levels of inflammation, low appendicular fat and muscle mass, and questionnaire scores indicative of anxiety, depression, and poor functional well-being, have been found to be associated with MH primarily via univariate analyses [2]. Recently, Dev et al (2014) confirmed the association between MH and survival through multivariate analyses [3]. The aim of this case series study is to determine, using multivariate analyses, whether independent relationships exist between MH and nutritional, functional, and quality of life characteristics in ACP.
Methods
Population
The work herein discusses a clinical study classified according to the National Cancer Institute (NCI) in consecutive case series. All patients recruited for this study were male and had a new diagnosis (six months or less) of advanced (stage III–IV) GI or non-small cell lung (NSCL) cancers within the Cancer Mission of the McGill University Health Centre. The patient recruitment and data collection took place between March 2006 and November 2007. Ethical approval was acquired from the Institutional Review Board of the McGill University Health Centre.
Measures
The diagnosis of MH was made on the basis of serum-free testosterone measurement, using the Coat-A-Count (Diagnostic Product Corp., Los Angeles, CA) radioimmunoassay, and ADAM questionnaire [12]. Concentrations lower than 31.2 pmol/L were considered to be clinically significant for MH, as already reported by Gagnon et al [7].
All other assessments took place either in the hospital setting (at bedside or in outpatient clinics) or at the McGill Nutrition and Performance Laboratory (MNUPAL, www.mnupal.mcgill.ca). MNUPAL is a clinical outcome research laboratory where patients affected by multiple chronic diseases such as cancer in advanced stages can receive the best supportive care and medical oversight in a new state-of-the-art facility. In the hospital setting, the following tests were completed: the Edmonton Symptom Assessment System (ESAS) to assess severity of anorexia, weakness, dyspnoea, and decreased feeling of well-being; the abridged Patient Generated Subjective-Global Assessment (aPG-SGA) to evaluate nutritional status; and handgrip strength by Jamar dynamometry (Sammons Preston, Bolingbrook, IL). Selected blood markers were also measured to assess the presence/severity of the inflammatory response (white blood cells count, C-reactive protein) and visceral protein status (serum albumin).
At MNUPAL, the patients completed the BFI [22] and the McGill Quality of Life questionnaire (MQoL) [23]. Body composition was assessed via dual-energy x-ray absorptiometry (DXA) (Lunar Prodigy, GE Healthcare, Madison, WI). Scans were analysed for total lean and fat mass (kg), percentage of fat and appendicular lean tissues, along with bone mineral content and bone mineral density, using the Advance Encore software (GE Healthcare).
All data was stored in the MNUPAL Human Cancer Cachexia Database (HCCD).
Statistical analysis
In order to test for independent relationships between low free testosterone levels (independent variable) with the above clinical and biological outcomes (dependent variables), we performed several multivariate linear regression models (one for each dependent variable), controlling for gender, age, time between diagnosis, and baseline assessment, survival time from baseline assessments to death/loss to follow-up, diagnosis of lung versus GI malignancies, presence/absence of concurrent oncological treatment (radio/chemotherapy), and concurrent medications. For medications potentially impacting on the relationships under investigation, the presence or absence of at least one of the following medications was considered: statins, anti-inflammatories, steroids, angiotensin-converting-enzyme (ACE) inhibitors, anti-hormonal agents, antioxidants, essential amino acids, anabolic hormones, and metformin. All data were analysed using SPSS (version 14.0, 2005, SPSS Inc., Chicago, IL).
Results and discussion
Results
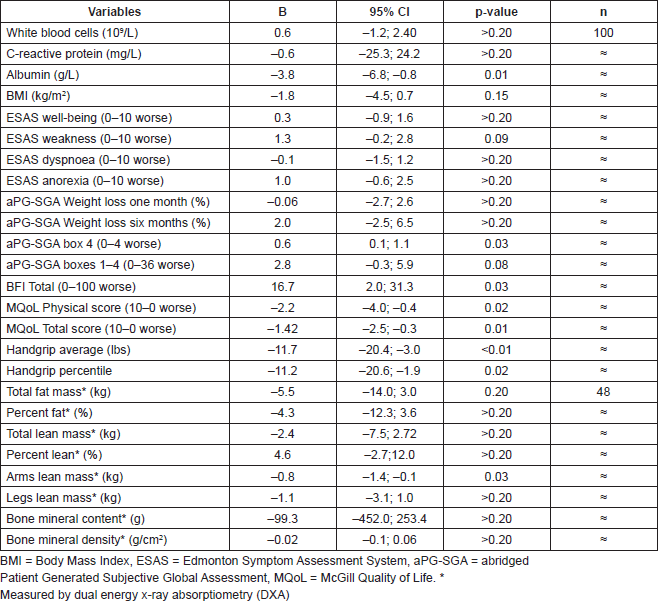
At the time of the final analysis, 100 patients satisfied the inclusion criteria and were recruited for this study. All patients were assessed at the hospital bedside, whereas 48 of them (48%) were also assessed at the MNUPAL. Table 1 indicates the clinical characteristics (n = 100). Patients had an average age of 64 years old (SD ± 11.5) and presented predominantly with GI malignancies (62%). The presence of MH was identified in 76 patients (76%). A statistically significant indirect association was found between MH and albumin levels (p = 0.01). MH was also associated with higher levels of fatigue, poor performance status, and quality of life. Significantly lower handgrip strength correlated with MH as well as decreased muscle mass in the upper limbs. However, there was no statistically significant relationship between MH and symptom severity or biological markers of inflammation (p > 0.2).
Discussion
The study shows that in a sample of 100 patients, there is a high prevalence of hypogonadism in males with advanced cancer. In addition, there is an independent relationship between MH and a worsening of visceral protein status, muscle strength, and mass in upper limbs, overall performance status, cancer-related fatigue, functional, and overall quality of life.
According to Burney and Garcia (2012), MH has been reported between 40% and 90% in ACP [4]. These findings concur with the high prevalence (76%) of MH in the present study. The complex pathophysiology of androgen deficiency in ACP has not been entirely elucidated. The presence of higher levels of circulating inflammatory mediators and/or concurrent oncological treatments have been shown in a combination of primary (i.e. related to testicular failure) and secondary (i.e. related to the hypothalamus–pituitary axis failure) hypogonadism [4]. The decline in testosterone levels observed in ACP may be negatively influenced by various factors such as other comorbidities [13], chronic opioid treatments [14], and changes in concentrations of sex-hormone-binding globulin [2].
Since the late 1980’s, physicians have agreed that MH is a non-specific consequence and a biomarker of chronic illness [13]. The current hypothesis to explain MH is based on the body’s adaptive response to the illness. During the recovery phase from illness, changes in homeostasis reflect a tendency to conserve energy, reduce aggressiveness, depresses libido and sexual activity. [13,15–17].
Table 1. Patient characteristics, demographics, and ongoing treatments present at the assessment visit.
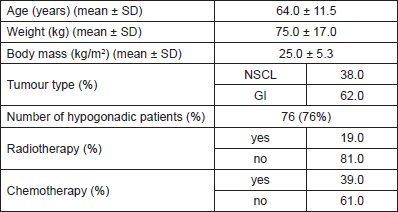
Table 2. Multiple linear regression analysis comparing hypogonadic (free testosterone < 31.2 pmol/L) versus eugonadic patients.
ACP present with symptoms of fatigue and cancer-related pain with the latter being typically treated with long-term opioid therapy [14]. Although cancer-related fatigue and pain were common symptoms in this clinical study, opioid therapy was not monitored. We have considered these facts as limitations of our study. Also because MH is a consequence of chemotherapy [18] and of the cancer per se [21], we did not report the percentage of those treated with or without opioids.
Handgrip strength as well as performance status (as measured by box 4 in the aPG-SGA) were clearly lower in the presence of androgen deficiency and this observation concurred with the presence of decreased lean mass in the arms and fatigue in hypogonadic patients. Kilgour et al (2010) have recently identified an association between muscle strength and muscle mass in ACP such that those who experienced greater fatigue had lower muscle mass and strength [5]. All of the above associations suggest that the lack of circulating testosterone could have a blunted anabolic impact on muscle morphology in cancer patients with MH. This hypothesis has been supported by eight clinical, randomised, double-blinded, and placebo-controlled trials using testosterone but in a rather small cohort population of hypogonadic patients who were sarcopoenic with no diagnosis of cancer (https://clinicaltrials.gov/ct2/results?term=testosterone&cond="Sarcopenia"). To date, only one clinical trial with testosterone administration has been conducted in ACP (3.0.CO;2-N" href="http://dx.doi.org/10.1002/(SICI)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.CO;2-N">10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N PMID: 10091805
23. Cohen SR et al (1996) Existential well-being is an important determinant of quality of life. Evidence from the McGill Quality of Life Questionnaire Cancer 77(3) 576–86 DOI: 10.1002/(SICI)1097-0142(19960201)77:3<576::AID-CNCR22>3.0.CO;2-0 PMID: 8630968






