An evaluation of the adequacy of Indian national and state essential medicines lists (EMLs) for palliative care medical needs—a comparative analysis
Disha Agrawal1,2†, Divya Shrinivas1†, Parth Sharma1,2, Muttacaud Ramakrishnan Rajagopal3, Arun Ghoshal4 and Siddhesh Zadey1,5,6,7
1Association for Socially Applicable Research (ASAR), Pune 411007, Maharashtra, India
2Maulana Azad Medical College, New Delhi 110002, India
3Trivandrum Institute of Palliative Sciences, Trivandrum, Kerala 695009, India
4Department of Palliative Medicine and Supportive Care, Kasturba Medical College and Hospital, Manipal 576104, Karnataka, India
5Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth, Pune 411018, Maharashtra, India
6Global Emergency Medicine Innovation and Implementation (GEMINI) Research Center, Duke University School of Medicine, Durham, NC 27708, USA
7Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY 10032, USA
†Contributed equally.
Abstract
Objectives: Essential medicines lists (EMLs) guide the public sector procurement and supply of medications to impact access to adequate and appropriate palliative care drugs. This study evaluates the adequacy of India’s national and sub-national EMLs that can directly impact palliative care for 5.4 million patients.
Methods: In this qualitative document review, we compared Indian national, and state EMLs acquired from official government websites with the International Association for Hospice and Palliative Care (IAHPC) EML recommendations. We analysed data on the indication and formulation of drugs under the different categories of formulations present (all, some and no), and drugs absent. Literature review and inputs from palliative care experts provided alternatives of absent medications to assess the adequacy of lists in managing the symptoms listed by IAPHC.
Results: We analysed 3 national and 27 state lists for 33 recommended drugs. The Central Government Health Services list had the maximum availability of all formulations of drugs (16 [48%]) nationally. Among states and union territories, the Delhi EML was the closest to IAHPC with 17 (52%) drugs with all formulations present. Karnataka had the most incomplete EML with only 3 (9%) drugs with all formulations present. No EML had all the recommended formulations of morphine. In one national and seventeen state EMLs, oral morphine was absent.
Conclusion: While Indian EMLs lack drugs for palliative care when compared with the IAHPC EML, symptom management is adequate. There is a need for countries with limited resources to modify the IAPHC list for their settings.
Keywords: palliative care, palliative medicine, public health, delivery of healthcare, patient care management, morphine, opioid, essential medicines, controlled substances
Correspondence to: Parth Sharma
Email: parth.sharma25@gmail.com
Published: 05/02/2025
Received: 22/09/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Palliative care deals with reducing serious health-related suffering through the early identification and treatment of physical, psychosocial or spiritual problems related to acute or chronic diseases [1]. Adequate access to and provision of palliative care improves quality of life [2], enables informed treatment decision-making [3] and reduces hospital readmissions and healthcare costs [4]. Although access to palliative care is a right to health globally, only 14% of the 40 million people in need can access it [1].
Although 5.4 million Indians need palliative care annually, merely 1% can access it [5]. In Indian patients with end-stage cancers, the unmet need for palliative care was reported to be 98.3% [6]. Several obstacles disrupt effective palliative care delivery, including poor geographical access, limited awareness, lack of workforce training, restrictive prescription policies for pain medications and limited policy prioritisation, among others [5].
The World Health Organisation (WHO) introduced the concept of essential medicines in 1977 to address priority health needs [7]. These medications are chosen based on their public health importance, efficacy, safety and cost-effectiveness and should be consistently available in sufficient quantities in public health centres. Thus, the implementation of a thoughtfully curated essential medicines list (EML) can enhance the quality of care, management practices and resource allocation, and ensure the availability of medicines by streamlining procurement and distribution processes. While countries can determine their EMLs, the WHO model list serves as a reference for national and institutional lists [8].
India has multiple EMLs. Health is a state subject as per the Indian Constitution. Hence, states draft their EMLs to match the local needs [9]. Two national health insurance schemes – the Employees’ State Insurance Scheme (ESIS) and the Central Government Health Scheme (CGHS) have their EMLs. A third national EML exists for the states without their unique EML to follow. These EMLs guide the procurement of drugs that are dispensed at government-run healthcare institutions at the state and national levels. As the vast majority of Indians reside in rural areas and rely on public (government-run) healthcare facilities, the appropriateness of palliative care service delivery partly depends on the adequacy of the national and state EMLs.
In 2007, responding to a request from the WHO Cancer Control Program, the International Association for Hospice and Palliative Care (IAHPC) collaborated with other organisations to develop a list of essential medicines for the 16 most common palliative care symptoms [10]. The IAHPC is a public charity serving as an international platform to improve access to palliative care and global standards of care [11]. This list, thus, became the model list to serve as a reference for nations globally.
The objective of this study was to evaluate the adequacy of India’s EMLs at the national and state/Union Territory (UT) levels by comparing Indian lists with the list curated by IAHPC. By identifying alternatives to missing drugs in the national and state lists, we also aimed to assess the adequacy of the lists for managing common palliative care symptoms listed by IAHPC.
Methods
Data sources
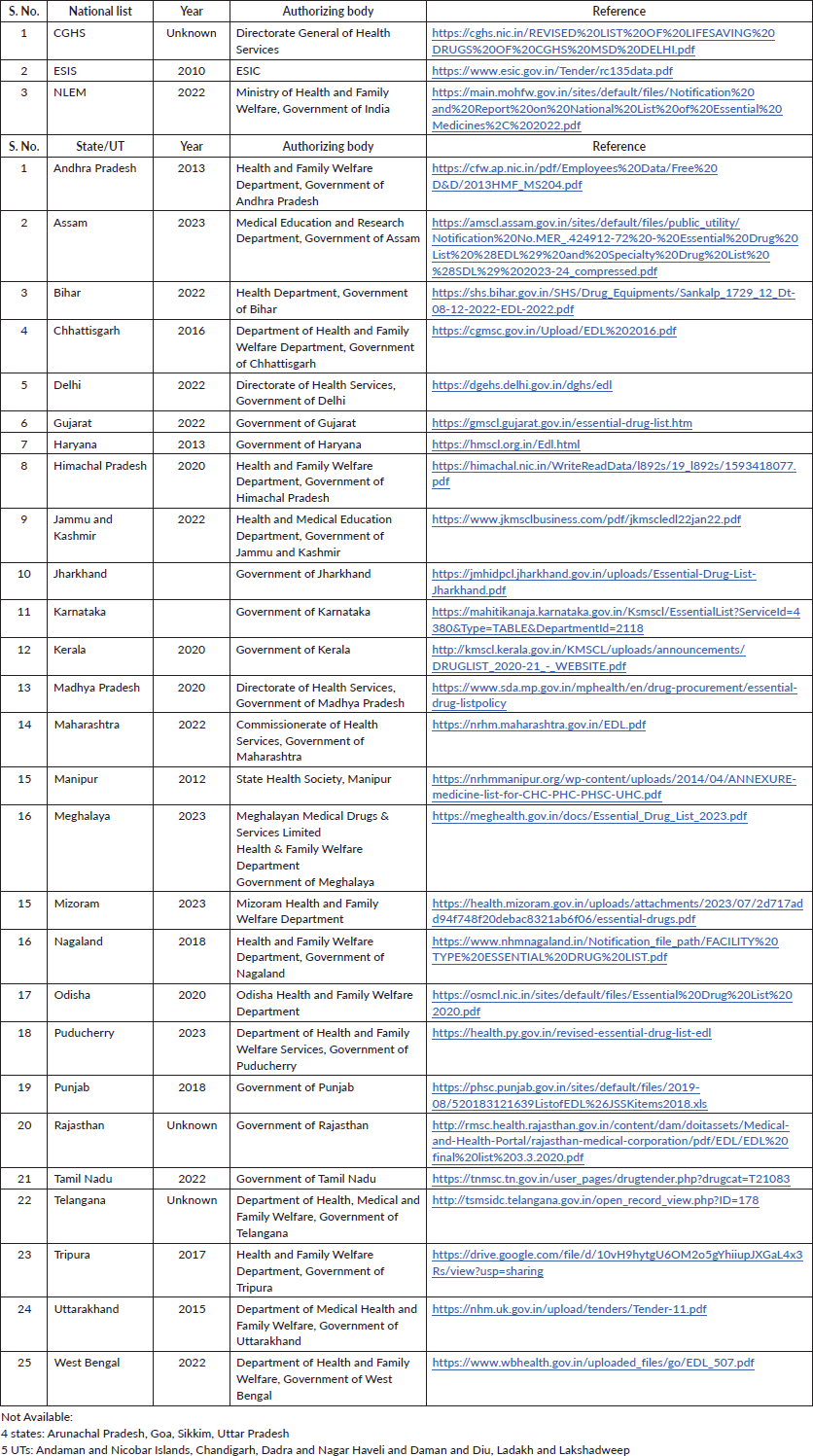
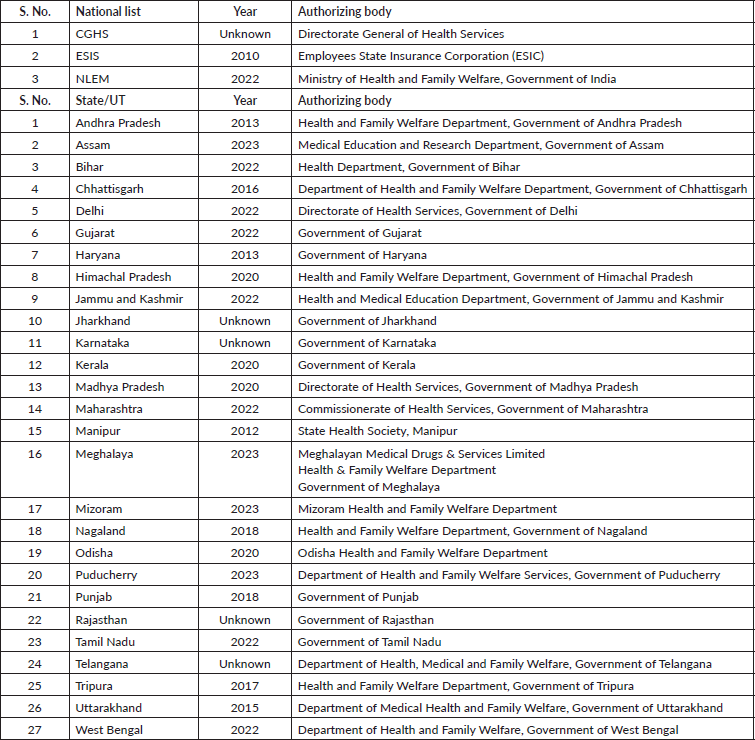
The National List of Essential Medicines (NLEM), last updated in 2022, was designed by the Ministry of Health and Family Welfare, Government of India [12]. The ESIS EML caters to the population employed in factories and other establishments such as hotels, shops and restaurants [13]. The CGHS covers current employees and pensioners of the national (central) government [14]. The state/UT lists were designed for the respective state-level public health facilities.
The IAHPC EMLs were drafted through the consensus of international physicians and pharmacologists. After identifying the most common symptoms in palliative care, a final list of appropriate medications was devised using a modified Delphi process [15]. The IAHPC list included 33 essential drugs, which were looked for in individual national and state EMLs. We accessed the most recent versions of three national and 27 state/UT EMLs from the government websites (Table 1). The links to access the individual EMLs are provided in Supplementary Table 1. The EMLs of 4 states (Arunachal Pradesh, Goa, Sikkim Uttar Pradesh) and 5 UTs (Andaman and Nicobar Islands, Chandigarh, Dadra and Nagar Haveli and Daman and Diu, Ladakh and Lakshadweep) were unavailable.
Table 1. National and state EMLs.
Data extraction
We followed the readying material, extracting data, analysing data and distilling findings approach to evaluate national and state (including union territories) EMLs and compared them with the IAHPC EML of essential medicines for palliative care [16]. The drugs’ names and formulations present in the Indian EMLs were compiled in a Microsoft Excel spreadsheet and matched with the IAHPC list to assess their adequacy.
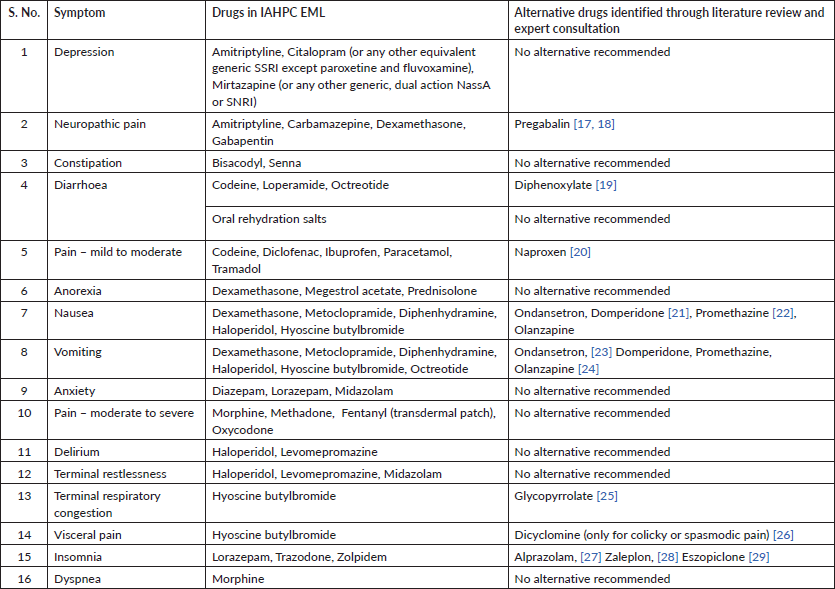
If the EML did not have the drug, we looked for alternative drugs that may be prescribed for the same indication (Table 2). Acceptable alternatives were determined by reviewing the literature and by consulting two experienced palliative care experts (MRR with 31 years of experience and AG with 12 years of experience). Recommendations for alternatives to any drug included in the IAHPC EML, but not present in an Indian EML, were taken from both experts individually in the first stage. Subsequently, both experts were invited to reach a consensus on any differences in recommendations in the second stage. The experts decided on alternative drugs after considering drug efficacy, safety, cost-effectiveness, availability in the Indian market and secondary effects of the drug that would be useful in a patient receiving palliative care. In situations where the drug recommended by IAHPC was considered efficacious, cost-effective and available in the Indian market, the experts did not offer any alternatives.
Table 2. Drugs identified for specific symptoms.
Data analysis
To assess the adequacy of the lists, drugs present in the national and state EMLs were categorised as follows: all formulations present, some formulations present, no recommended formulation present and drug absent. We calculated the percentage of drugs present in each category in national and state EMLs using Equation 1. The different formulations that were mentioned for drugs included tablets, capsules, oral solutions, injectables, suppositories and salts.
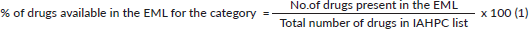
We also looked at the adequacy of the EMLs to manage palliative care symptoms using alternative drugs identified using expert consensus and literature review as mentioned previously. The IAHPC EML mentions 16 common palliative care symptoms, which include depression, neuropathic pain, constipation, anorexia, nausea, vomiting, anxiety, mild to moderate pain, moderate to severe pain, delirium, terminal restlessness, terminal respiratory congestion, visceral pain, dyspnea, diarrhoea and insomnia. We assessed whether the national or state EMLs had at least one drug that could be prescribed for each of these indications. For diarrhoea, the presence of oral rehydration solution (ORS) in the list was considered necessary to adequately manage the symptoms. For terminal restlessness, we considered haloperidol essential for management, with midazolam as an add-on drug [30]. We reported this result as the proportion of EMLs that were adequate to manage all the aforementioned symptoms.
The most recent National Programme for Prevention and Control of Non-Communicable Diseases (NPNCD) guidelines evaluate access to palliative care by assessing morphine-equivalent consumption of strong opioid analgesics (except methadone) per cancer death [31]. Therefore, we specifically looked at the inclusion of various formulations of morphine (oral solution, oral tablet and injectable) in the EMLs as well. Subsequently, we calculated the proportion of EMLs, which included both oral and injectable morphine, only oral morphine, only injectable morphine and no formulation of morphine.
Results
A total of 3 national and 27 state/UT EMLs were analysed in the study. Although India is a union of 28 states and 8 UTs, the remaining EMLs were unavailable in the public domain.
Adequacy of drugs
Among national EMLs, the CGHS had the highest number of all formulations of drugs (16 [48%]). Ten (30%) drugs had some formulations present, one drug had no recommended formulation present and six (19%) drugs were absent. The NLEM contained all formulations of 15 (46%) drugs, some formulations of 5 (15%) drugs, no recommended formulations of 1 (3%) drug and 12 (36%) drugs were absent. The ESIS EML had the least number of drugs with all formulations present (6 [18%]), some formulations were present of 7 (21%) drugs, no recommended formulations were present of 2 (6%) drugs and 18 (55%) drugs were absent (Figure 1).
Among states and UTs, Delhi’s EML was the closest to the IAHPC EML and included all formulations required for 17 (52%) drugs. Eight (24%) drugs had some formulations present, one drug had no recommended formulation present, and seven (21%) drugs were absent. Karnataka’s EML included all recommended formulations of only 3 (9%) drugs, and some formulations of 6 (18%) drugs, while 24 (73%) drugs were absent. The adequacy analysis of all EMLs is presented in Figure 2 and Table 3. The presence of viable alternatives to absent drugs was variable across the EMLs, ranging from 4% of absent drugs in the Karnataka EML to 100% of absent drugs in CGHS, Delhi and Uttarakhand EMLs.
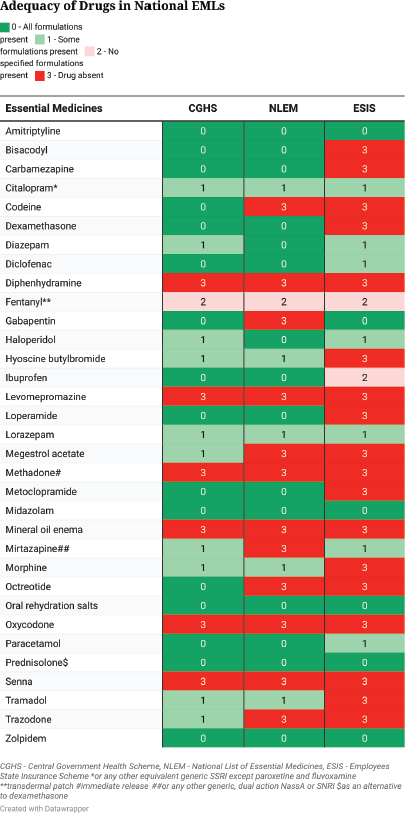
Figure 1. Adequacy of Indian national EMLs compared with the IAHPC EML for palliative care.
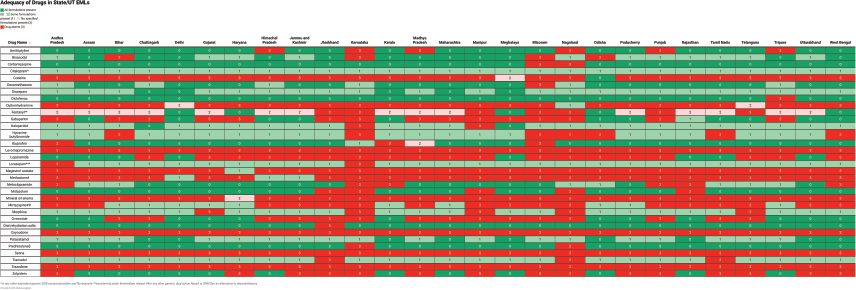
Figure 2. Adequacy of state/UT EMLs compared with the IAHPC EML for palliative care.
Table 3. Drug availability (%) compared with IAHPC recommendations.
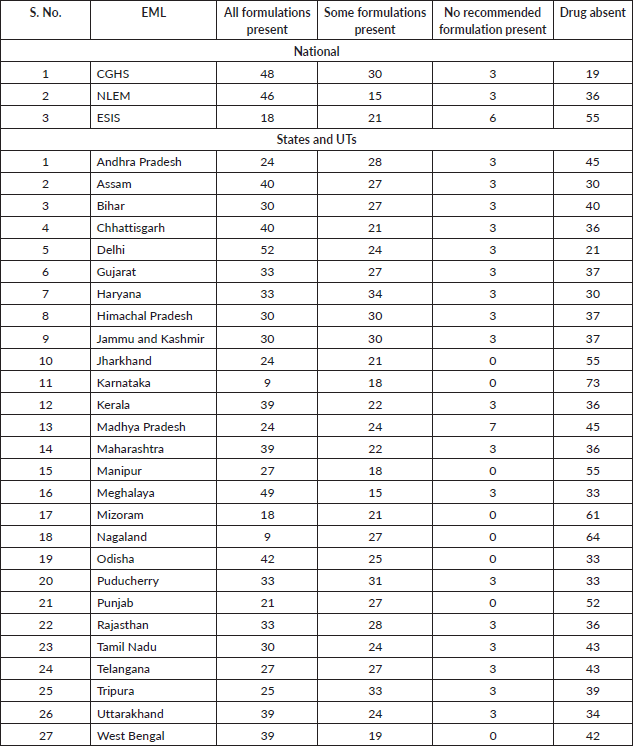
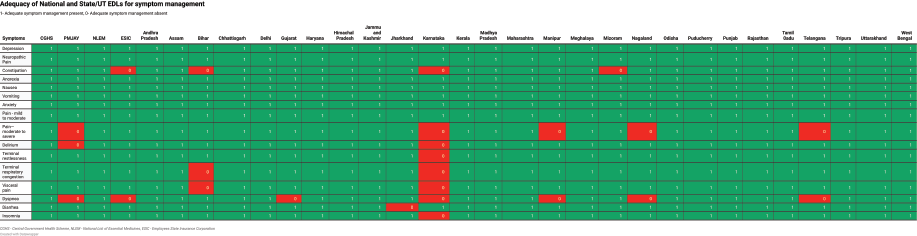
Figure 3. Adequacy of Indian EMLs for managing palliative care symptoms listed by IAHPC.
No Indian EML had all recommended formulations of morphine as oral solutions, tablets and injectables. Nationally, the CGHS EML and NLEM included morphine injectables and tablets, while the ESIS EML did not include morphine in any form. Nine (33%) state/UT EMLs included injectables and tablets. Twelve (44%) included only injectable morphine, and one (4%) included only tablets. Morphine was absent in five (16%) EMLs - Gujarat, Karnataka, Manipur, Nagaland and Telangana.
Adequacy for symptom management
All of the 16 symptoms listed by IAHPC could be managed by at least one drug present in two (67%) national lists – CGHS and NLEM and 19 (70%) state/UT EMLs. The analysis for symptom management in EMLs is presented in Figure 3. Among the inadequately managed symptoms, constipation was not addressed by the EMLs of ESIC, Bihar, Karnataka and Mizoram. Moderate to severe pain management was insufficient in the EMLs of Karnataka, Manipur, Nagaland and Telangana. Manipur’s EML did not manage terminal restlessness effectively, Bihar’s EML did not manage both terminal respiratory congestion and visceral pain, while Karnataka’s EML did not manage any of these symptoms. Dyspnea was inadequately managed in the EMLs of ESIC, Gujarat, Karnataka, Manipur, Nagaland and Telangana. Jharkhand’s EML was the only one that left diarrhoea unmanaged, while the EML of Karnataka was the only EML inadequate to manage delirium and insomnia. However, depression, neuropathic pain, anorexia, nausea, vomiting, anxiety and mild to moderate pain were adequately managed by all state and national EMLs.
Discussion
In our study, we report that India's national and state EMLs did not completely align with the recommendations of IAHPC. The Delhi EML was the closest to IAHPC recommendations, followed by the NLEM. Some drugs recommended by the IAHPC, such as levomepromazine, senna, trazodone and oxycodone, were absent from all state and national lists, except trazodone, which was present in the NLEM. Others, such as megestrol and mineral oil enema, were present in only one state list each, and only the NLEM included codeine and megestrol. While EMLs include fentanyl in the injectable form, the commonly prescribed formulation for pain management – transdermal patch, was included by only two states (Haryana and Meghalaya). The experts in the study felt that morphine was a more cost-effective and efficient substitute for transdermal fentanyl in the Indian setting due to the difficulty of titration and the high cost of the latter, which restricts access to it. Additionally, even though all EMLs, barring one, contained ORS for the management of diarrhoea, Indian EMLs were found to be inadequate to treat intractable diarrhoea since the presence of loperamide is integral for non-infective intractable diarrhoea management [32, 33]. Dyspnea, constipation and moderate to severe pain are other symptoms poorly managed by Indian EMLs. States such as Bihar, Karnataka, Telangana and the north-eastern states of Manipur, Nagaland and Mizoram lag behind the rest of the country in the inclusion of recommended drugs, as well as symptom management, even after including viable alternatives present in the EMLs.
Essential drugs cater to any population’s priority needs, availability and affordability. Given the rising number of patients needing palliative care, essential drugs must be made available and accessible to them. EMLs are instrumental in improving access to essential drugs through the public health system and reducing the financial burden on patients and caregivers for conditions that are relatively common in the community [34, 35]. The implementation of the National Essential Medicines Policy in China led to better prescription practices and a decline in average prescription costs. Another study looking at the impact of the essential drugs programme in peripheral health units in Yemen showed similar results, with improved availability and more rational use of drugs [36, 37]. Thus, limited global evidence highlights the impact of implementing EMLs in terms of improving access to treatment, enhancing drug availability, optimising prescription practices and reducing costs.
While we used the IAHPC EML, published in 2007, as the benchmark against which all other EMLs were compared, we noticed that certain updates based on recent evidence need to be incorporated. For example, in 2010, Fosbøl et al [38] reported an increased risk of cardiovascular events in patients receiving treatment with diclofenac which has been mentioned in the IAHPC list for the management of mild to moderate pain. Additionally, we propose a further classification of nausea and vomiting into that caused by gastroparesis, radiotherapy and chemotherapy to simplify prescription, as their treatments widely differ [39].
The increasing advocacy for fentanyl transdermal patches, touted for their potency in pain management, is raising concerns, particularly in low- and middle-income countries (LMICs) like India. While a 100-mg dose of injectable fentanyl is equivalent to 10 mg of morphine [40] in India, fentanyl costs 12 times as much as morphine per day. The high cost of fentanyl, coupled with its limited accessibility, makes it impractical for widespread use in LMICs [40, 41]. Indian EMLs did not include all the recommended formulations of morphine, a practical alternative to fentanyl. Morphine’s importance is highlighted by the fact that an objective of the National Program for Palliative Care (NPPC) is to increase its availability. However, while the IAHPC recommends the inclusion of oral morphine solution in addition to tablets and injectables, it is often not commercially available in India due to its short shelf life and limited manufacturers [42]. Hence, it may not be a feasible inclusion in an Indian EML for palliative care. Morphine usage is also used as an indicator to assess the coverage of palliative care services in the NPNCD [43]. Emphasis should be placed on ensuring that all formulations of morphine are available and affordable. Despite the 2014 amendment to the NDPS Act, which reduced the number of required licenses for opioid possession from six to one, complex regulatory procedures and a lack of prescription awareness among physicians continue to hinder patients' access to essential pain management medications like morphine. In 2014, India's total morphine consumption was a mere 278 kg. Considering that a patient with terminal cancer requires 75 mg of morphine per day for approximately 90 days, this amount is only sufficient to adequately treat 40,000 patients [44, 45]. Addressing these barriers is crucial for improving pain management in countries like India, where the need for affordable and effective pain relief is paramount. Simplifying regulatory processes and enhancing physician education on opioid prescriptions are key steps toward ensuring that patients receive the pain management they need.
The Sustainable Development Goal 3.8 mentions access to quality essential healthcare services and access to safe, effective, quality and essential medicines for the achievement of Universal Health Coverage (UHC). We noted the presence of different national EMLs, meant effectively for different sections of the population, with the CGHS EML meant for government employees performing better than the ESIS EML meant for other workers. This highlights redundancy in the system and a potential source for inequitable healthcare delivery. Additionally, the EMLs devised by individual states should ideally include all the drugs present in the national list, with the addition of drugs considered necessary based on the local epidemiology of diseases. Establishing a single national EML, aligned with global standards is integral to achieving UHC, especially for palliative care provision. By implementing EMLs tailored to include drugs required to deliver palliative care, healthcare systems can effectively address the diverse needs of patients while promoting equitable and cost-effective healthcare delivery [35].
India launched NPPC in 2012 to make high-quality palliative care accessible across all levels of health care [43]. This included making drugs for pain relief and other symptoms available at the primary healthcare level [46]. Thus, it is imperative that the benefits of drug inclusion in the EML are not restricted to tertiary care setups and that drugs are made available in primary and secondary care centres as well [43]. While national and state EMLs do not conform to the IAHPC recommendations, they contain alternatives for the management of symptoms commonly encountered in palliative care. However, some states, such as Bihar, Gujarat, Jharkhand, Karnataka, Mizoram, Manipur, Nagaland and Telangana, lack these alternatives, rendering them inadequate in managing some of these symptoms. It is crucial to incorporate essential medications from the IAHPC list or adopt alternatives to address these gaps. For instance, adding morphine to Nagaland’s EML would improve the management of both moderate to severe pain and dyspnea. Furthermore, Jharkhand should include ORS in its EML for the management of diarrhoea, as it is the only EML currently lacking this essential treatment.
Our findings highlight that there is a need for an EML tailored to palliative care in India and similarly in other countries, with drugs thoughtfully included as per existing procurement, storage and distribution constraints. EMLs specific for settings with different levels of available resources will help guide countries that currently do not have an EML or are looking to update existing EMLs.
Strengths and limitations
Our study is the first to analyse the presence of specific medications essential for palliative care across the diverse EMLs of India. This contributes significantly to evaluating their readiness to manage the prevalent symptoms encountered by patients receiving palliative care. We were also able to delve deeper into analysing the management of common symptoms experienced by patients receiving palliative care in cases where the drugs recommended by the IAHPC were unavailable, and alternative options were explored.
Our study has a few limitations. First, while we identified the inclusion of medications for palliative care across multiple EMLs, we lacked the resources to assess their availability at the grassroots level and within hospitals. A comprehensive evaluation, combining our study with grassroots-level investigations, would provide a more accurate measure of access to essential drugs. Second, we did not compare the available drug doses in EMLs with the recommendations of IAHPC as the required dose can be attained by modifying the quantity consumed of the available drug. Finally, the costs of the alternate drugs listed by experts were not compared. However, it was a criterion that the experts considered when deciding the alternatives.
Conclusion
Indian EMLs are not entirely in line with the IAHPC recommendations for essential palliative care drugs. However, they contain a range of drugs adequate to treat most symptoms requiring palliation. Considering the importance of morphine, both in palliative care symptom management and monitoring of palliative care-related national programs in India, the national and state/UT EMLs should be updated to incorporate oral and injectable formulations of morphine. There is a need to update the IAHPC list using recent evidence, and there is also a need to design a list based on different levels of available resources to guide countries in formulating their EMLs for palliative care service delivery.
Conflicts of interest
The authors have no competing interests to declare.
Funding
None.
Ethical approval
The study did not involve any human or animal subjects and, therefore, did not require any ethical clearance.
Data sharing
The data are available upon reasonable request that can be made to Dr Parth Sharma (Email ID: parth.sharma25@gmail.com).
Author contributions
Corresponding author: PS
Joint authorship: DA and DS contributed equally to this paper.
Conceptualisation: PS; Reviewed study proposal: PS, SZ, MRR, AG; Data extraction: DA, DS; Data analysis: DA, DS; Scientific advisors: MRR, AG, SZ; Writing of the original draft: DA, DS; Review and editing of the final draft: All authors; Project supervision: PS, SZ.
References
1. WHO (2024) Palliative Care [Internet] (Geneva: WHO) [https://www.who.int/news-room/fact-sheets/detail/palliative-care] Date accessed: 17/01/24
3. Noble H, Brazil K, and Burns A, et al (2017) Clinician views of patient decisional conflict when deciding between dialysis and conservative management: qualitative findings from the palliative care in chronic kidney disease (PACKS) study Palliat Med 31(10) 921–931 https://doi.org/10.1177/0269216317704625 PMID: 28417662
4. Lalani N and Cai Y (2022) Palliative care for rural growth and wellbeing: identifying perceived barriers and facilitators in access to palliative care in rural Indiana, USA BMC Palliat Care 21(1) 25 https://doi.org/10.1186/s12904-022-00913-8 PMCID: 8857623
5. Bag S, Mohanty S, and Deep N, et al (2020) Palliative and end of life care in India –current scenario and the way forward J Assoc Physicians India 68(11) 61–65 PMID: 33187039
6. Patil S, Sharma P, and Arora A, et al (2024) Unmet need for cancer palliative care in India BMJ Support Palliat Care https://doi.org/10.1136/spcare-2024-004978 PMID: 38834235
7. WHO (2024) Model Lists of Essential Medicines [Internet] (Geneva: WHO) [https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists] Date accessed: 7/6/24
8. Kar SS, Pradhan HS, and Mohanta GP (2010) Concept of essential medicines and rational use in public health Indian J Community Med 35(1) 10–13 https://doi.org/10.4103/0970-0218.62546 PMID: 20606912 PMCID: 2888334
9. Constitution of India Seventh Schedule
10. IAHPC Essential Medicines for Palliative Care (2024) International Association for Hospice & Palliative Care [Internet] [https://hospicecare.com/what-we-do/projects/palliative-care-essentials/iahpc-essential-medicines-for-palliative-care/] Date accessed: 17/02/24
11. IAHPC (2024) Vision and Mission –International Association for Hospice & Palliative Care [Internet]
12. Ministry of Health and Family Welfare, Government of India (2022) National List of Essential Medicines [Internet] [https://main.mohfw.gov.in/sites/default/files/Notification%20and%20Report%20on%20National%20List%20of%20Essential%20Medicines%2C%202022.pdf] Date accessed: 13/6/24
13. Employees State Insurance Corporation (ESIC) (2010) Employee’s State Insurance Corporation: Medical Rate Contract [Internet] [https://www.esic.gov.in/Tender/rc135data.pdf] Date accessed: 13/6/24
14. Directorate General of Health Services (2024) CGHS List of Life Saving Drugs [Internet] [https://cghs.nic.in/REVISED%20LIST%20OF%20LIFESAVING%20DRUGS%20OF%20CGHS%20MSD%20DELHI.pdf] Date accessed: 13/6/24
15. De Lima L, Krakauer EL, and Lorenz K, et al (2007) Ensuring palliative medicine availability: the development of the IAHPC list of essential medicines for palliative care J Pain Symptom Manage 33(5) 521–526 https://doi.org/10.1016/j.jpainsymman.2007.02.006 PMID: 17482041
16. Dalglish SL, Khalid H, and McMahon SA (2021) Document analysis in health policy research: the READ approach Health Policy Plan 35(10) 1424–1431 https://doi.org/10.1093/heapol/czaa064 PMCID: 7886435
17. Jiang J, Li Y, and Shen Q, et al (2018) Effect of pregabalin on radiotherapy-related neuropathic pain in patients with head and neck cancer: a randomized controlled trial J Clin Oncol 37(2) 135–143 https://doi.org/10.1200/JCO.18.00896 PMID: 30457920
18. Mishra S, Bhatnagar S, and Goyal GN, et al (2012) A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study Am J Hosp Palliat Care 29(3) 177–182 https://doi.org/10.1177/1049909111412539
19. Bugaev N, Bhattacharya B, and Chiu WC, et al (2019) Antimotility agents for the treatment of acute noninfectious diarrhea in critically ill patients: a practice management guideline from the Eastern Association for the Surgery of Trauma J Trauma Acute Care Surg 87(4) 915–921 https://doi.org/10.1097/TA.0000000000002449 PMID: 31574060
20. Wood H, Dickman A, and Star A, et al (2018) Updates in palliative care –overview and recent advancements in the pharmacological management of cancer pain Clin Med 18(1) 17–22 https://doi.org/10.7861/clinmedicine.18-1-17
21. Esseboom EU, Rojer RA, and Borm JJ, et al (1995) Prophylaxis of delayed nausea and vomiting after cancer chemotherapy Neth J Med 47(1) 12–17 https://doi.org/10.1016/0300-2977(95)00027-K PMID: 7651559
22. Rao KV and Faso A (2012) Chemotherapy-induced nausea and vomiting: optimizing prevention and management Am Health Drug Benefits 5(4) 232–240 PMID: 24991322 PMCID: 4046471
23. Currow DC, Coughlan M, and Fardell B, et al (1997) Use of ondansetron in palliative medicine J Pain Symptom Manage 13(5) 302–307 https://doi.org/10.1016/S0885-3924(97)00079-1 PMID: 9185436
24. Glare P, Miller J, and Nikolova T, et al (2011) Treating nausea and vomiting in palliative care: a review Clin Interv Aging 6 243–259 https://doi.org/10.2147/CIA.S13109 PMCID: 3180521
25. Hindmarsh J, Everett P, and Hindmarsh S, et al (2020) Glycopyrrolate and the management of “death rattle” in patients with myasthenia gravis J Palliat Med 23(10) 1408–1410 https://doi.org/10.1089/jpm.2019.0598 PMID: 31976808
26. Jewell R (2008) Dicyclomine xPharm: The Comprehensive Pharmacology Reference eds Bylund DB and Enna SJ (Amsterdam: Elsevier)
27. Sanabria E, Cuenca RE, and Esteso MÁ, et al (2021) Benzodiazepines: their use either as essential medicines or as toxics substances Toxics 9(2) 25 https://doi.org/10.3390/toxics9020025 PMID: 33535485 PMCID: 7912725
28. Bhandari P and Sapra A (2024) Zaleplon (Treasure Island: StatPearls Publishing)
29. Hair PI, McCormack PL, and Curran MP (2008) Eszopiclone: a review of its use in the treatment of insomnia Drugs 68(10) 1415–1434 https://doi.org/10.2165/00003495-200868100-00005 PMID: 18578559
30. Zaporowska-Stachowiak I, Stachowiak-Szymczak K, and Oduah MT, et al (2020) Haloperidol in palliative care: indications and risks Biomed Pharmacother 132 110772 https://doi.org/10.1016/j.biopha.2020.110772 PMID: 33068931
31. Ministry of Health and Family Welfare, Government of India (2024) Operational Guidelines National Programme for Prevention and Control of Non-Communicable Diseases [Internet] [https://media.licdn.com/dms/document/media/D4D1FAQFUE2ll_2hsmg/feedshare-document-pdf-analyzed/0/1684375232945?e=1717632000&v=beta&t=Zt6cZJnsCoNPy__OrsopQ9-tswx99fQXtsJDDUgN1lA] Date accessed: 28/5/2024
32. Maroun JA, Anthony LB, and Blais N, et al (2007) Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on chemotherapy-induced diarrhea Curr Oncol 14(1) 13–20 https://doi.org/10.3747/co.2007.96 PMID: 17576459 PMCID: 1891194
33. Clay PG and Crutchley RD (2014) Noninfectious diarrhea in HIV seropositive individuals: a review of prevalence rates, etiology, and management in the era of combination antiretroviral therapy Infect Dis Ther 3(2) 103–122 https://doi.org/10.1007/s40121-014-0047-5 PMID: 25388760 PMCID: 4269634
34. Jhaj R, Banerjee A, and Kshirsagar NA, et al (2022) Use of drugs not listed in the National list of essential medicines: findings from a prescription analysis by the Indian Council of Medical Research-Rational use of medicines centres network in tertiary care hospitals across India Indian J Pharmacol 54(6) 407–416 https://doi.org/10.4103/ijp.ijp_878_21
35. Wirtz VJ, Hogerzeil HV, and Gray AL, et al (2017) Essential medicines for universal health coverage Lancet 389(10067) 403–476 https://doi.org/10.1016/S0140-6736(16)31599-9
36. Chao J, Gu J, and Zhang H, et al (2018) The impact of the national essential medicines policy on rational drug use in primary care institutions in Jiangsu province of China Iran J Public Health 47(1) 24–32 PMID: 29318114 PMCID: 5756597
37. Hogerzeil HV, Walker GJ, and Sallami AO, et al (1989) Impact of an essential drugs programme on availability and rational use of drugs Lancet 1(8630) 141–142 https://doi.org/10.1016/S0140-6736(89)91152-5 PMID: 2563055
38. Fosbøl EL, Folke F, and Jacobsen S, et al (2010) Cause-specific cardiovascular risk associated with nonsteroidal antiinflammatory drugs among healthy individuals Circ Cardiovasc Qual Outcomes 3(4) 395–405 https://doi.org/10.1161/CIRCOUTCOMES.109.861104 PMID: 20530789
39. Ang SK, Shoemaker LK, and Davis MP (2010) Nausea and vomiting in advanced cancer Am J Hosp Palliat Care 27(3) 219–225 https://doi.org/10.1177/1049909110361228 PMID: 20197557
40. Ramos-Matos CF, Bistas KG, and Lopez-Ojeda W (2024) Fentanyl (Treasure Island: StatPearls Publishing)
41. Neighbors DM, Bell TJ, and Wilson J, et al (2001) Economic evaluation of the fentanyl transdermal system for the treatment of chronic moderate to severe pain J Pain Symptom Manage 21(2) P129–P143 https://doi.org/10.1016/S0885-3924(00)00247-5
42. Lin CY, Shen LJ, and Huang CF et al (2013) Beyond-use date of extemporaneous morphine hydrochloride oral solution J Food Drug Anal 21(2) 142–146 https://doi.org/10.1016/j.jfda.2013.05.002
43. Ministry of Health and Family (2024) Directorate General of Health Services [Internet] [https://dghs.gov.in/content/1351_3_NationalProgramforPalliativeCare.aspx] Date accessed: 8/5/24
44. Jacob A and Mathew A (2017) End-of-life care and opioid use in India: challenges and opportunities JCO Global Oncol 3(6) 683–686 https://doi.org/10.1200/JGO.2016.008490
45. UNODC (2024) India: The Principle of Balance to Make Opioids Accessible for Palliative Care [Internet] (Vienna: UNODC) [https://www.unodc.org/southasia/frontpage/2011/april/interview-m-r-rajagopal-access-to-opioids-for-palliative-care.html] Date accessed: 7/7/24
46. Ministry of Health and Family Welfare, and Government of India (2024) Operational Guidelines: Palliative care at Health and Wellness Centers [Internet] [https://nhsrcindia.org/sites/default/files/2021-06/Operational%20Guidelines%20for%20Palliative%20Care%20at%20HWC.pdf] Date accessed: 29/5/24
Supplementary material
Supplementary Table 1. National and state EMLs.