Basophils may be a risk factor for upper gastrointestinal cancer: a Mendelian randomization (MR) study
Pengkhun Nov1†, Wandan Li1†, Duanyu Wang1†, Socheat Touch2, Samnang Kouy2, Peizan Ni1, Qianzi Kou1, Ying Li1, Chongyang Zheng1, Arzoo Prasai1, Wen Fu1, Kunpeng Du1, Syphanna Sou2 and Jiqiang Li1
1Department of Radiation Oncology, Oncology Center, Zhujiang Hospital of Southern Medical University, No 253 Mid Gongye Ave, Haizhu District, Guangzhou 510282, Guangdong Province, China
2Department of Radiation Oncology, Luang Mè Hospital of University of Health Sciences, Street 109, Phnom Penh 120110, Cambodia
ahttps://orcid.org/0000-0002-0684-7291
bhttps://orcid.org/0000-0002-585-5911
†These authors contributed equally to this work
Abstract
Objective: Upper gastrointestinal (UGI) cancers, including esophageal (EC) and gastric (GC) cancers, pose a significant global health challenge. Previous studies have indicated a fundamental correlation between basophil count and the risk of UGI cancer. However, confirming a causal relationship demands further investigation. Mendelian randomization (MR) provides a critical method for evaluating the possible causal connections between peripheral circulating blood cells (PCBCs) and UGI cancer.
Method: Our study comprehensively employed a two-sample MR analysis. We used publicly available genetic data to survey the causal association between PCBC and UGI cancer. We used inverse variance weighting and weighted median for MR analyses and sensitivity analyses to assess heterogeneity and pleiotropy.
Results: In terms of the association between PCBCs and UGI cancer, we found that basophils count (EC: OR = 1.416, 95% CI = 1.125−1.783, p = 0.003; GC: OR = 1.623, 95% CI = 1.052−2.505, p = 0.029) were all strongly correlated with both EC and GC. Interestingly, Basophil count was a risk factor for both EC and GC. However, no significant correlations were seen between eosinophil, monocyte, lymphocyte or white blood cell count and UGI cancer.
Conclusion: The findings of this research corroborate the idea that basophils might serve as a fundamental risk factor for UGI cancer. Further exploration of the underlying mechanisms driving this relationship could provide crucial understanding helpful in creating prospective preventive and treatment methods for UGI cancer.
Keywords: esophageal cancer, gastric cancer, peripheral circulating blood cells, Mendelian randomization (MR), genome wide association study (GWAS)
Correspondence to: Jiqiang Li, Syphanna Sou, and Kunpeng Du
Email: ljq821028@126.com, syphannasou@gmail.com, and dkp321098@smu.edu.cn
Published: 14/11/2024
Received: 08/07/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Upper gastrointestinal (UGI) cancers, particularly esophageal cancer (EC; 510,716 new cases in 2022) and gastric cancer (GC; approximately 1 million new cases in 2022), present substantial global health challenges, marked by significant morbidity and mortality rates [1]. Identifying novel risk factors and underlying mechanisms is crucial for developing effective prevention and early detection strategies [2, 3]. While numerous studies have explored the correlation between various types of peripheral circulating blood cells (PCBCs) and cancer risk, the causal nature of these relationships remains under active investigation [4, 5].
Basophils, a type of granulocyte involved in immune responses and allergic reactions, have been highlighted in recent research as potentially linked to an increase risk of UGI cancer [6]. Previous observational studies have suggested that a higher basophil count is related to a higher risk of UGI cancer [7]. However, these observational studies are susceptible to deviation and confounders, thus limiting their ability to confirm causality [6, 7]. Mendelian randomization (MR), a method that provides conclusive causal evidence by analysing the association of genetic variation with exposure and discovery, offers a promising replacement for randomised controlled trials (RCTs) [8]. By utilising genetic variants that affect basophil levels, MR facilitates a more rigorous examination of basophils’ role in UGI cancer development, minimising biases commonly present in observational studies [9–11].
In the present study, we investigated the histophysiological and pathophysiological engagement of PCBCs in the carcinogenesis of cancers of the GI tract using MR analysis, which was achieved through a recent Genome-Wide Association Study (GWAS) focusing on PCBCs and UGI cancers and statistical summaries from the UK Biobank [12]. Our study is dedicated to exploring the causal association between PCBCs and UGI cancers, with a special focus on those in tumor initiation, progression and treatment resistance. We present an extensive MR study that not only identifies specific PCBCs associated with UGI cancers but also addresses the constraints in current research. Our goal is to provide valuable insights that could refine future methodologies and advance etiological research. This work is intended to support precision prevention, control and the development of innovative therapeutic approaches. Additionally, we aim to develop personalised treatment strategies targeting the specific PCBCs vulnerabilities of UGI cancer.
Materials and methods
Study design
Causal associations between PCBC and UGI cancer were assessed using a two-sample MR analysis. MR uses genetic variants as a proxy for risk factors. To ensure reliable causal inferences, the instrumental variables (IVs) used in MR must meet three key assumptions: (1) the genetic variant must be directly related to the exposure; (2) the genetic variant is not related to potential confounders between the exposure and the finding; and (3) the genetic variant affects the finding only through the exposure and not through other pathways Figure 1.
Data sources for exposure and outcome
GWAS statistical summary for each PCBCs trait is publicly available from the GWAS catalogue (accession numbers: GCST0001391 to GCST0002121) [12]. We used keywords for each cancer to find IDs from (https://gwas.mrcieu.ac.uk/). Cancer IDs included: ebi-a-GCST90018841 (EC), ukb-saige-151 (GC). Finally, we used summary statistics from the latest large-scale Blood Cell Characterisation Genome Study (BCX), conducted by the blood cell consortium with participants of European ancestry [12]. The GWAS database is a fully comprehensible gathering of genetic variants and their connections to a diversity of traits or diseases. It provides a powerful resource for researchers and clinicians who are interested in developing an understanding of the genetic basis of complex traits and diseases. From this GWAS, we derived genetic variants associated with circulating leukocyte, lymphocyte, monocyte, neutrophil, eosinophil and basophil count levels. Based on EC IDs, we downloaded EC data for 476,306 Europeans (n = 998 case patients and 475,308 control participants) from the GWAS (<a href=https://www.ebi.ac.uk/gwas/>https://www.ebi.ac.uk/gwas/</a>). The UK Biobank is a large-scale biomedical database and research resource that is designed to allow researchers to seeking the hereditary, environmental and lifestyle factors that influence various diseases and health outcomes. The database includes health and genetic information from over 500,000 participants in the United Kingdom, making it one of the most comprehensive biomedical resources of its kind. Based on the ID of GC, we downloaded the data from UK Biobank including 393,926 European individuals (n = 554 case patients and 393,372 control participants) for EC (https://<a href=https://www.ukbiobank.ac.uk/>www.ukbiobank.ac.uk</a>).
Instrument selection
We implemented a more stringent correlation threshold (p < 5 × 10^-9) for the selection of genetic IVs, given the large number of single nucleotide polymorphisms (SNPs) that have genome-wide significance (p < 5 × 10^-8) for PCBCs traits [12]. These IVs were identified by grouping them according to the 1,000 Genomes Project’s Linkage Disequilibrium (LD) reference panel, with a threshold of R^2 < 0.001 at a distance of 1,000 kilobases (kb). Given the relatively limited size of the GWAS data from PCBCs, we employed a p value threshold of 5 × 10^ -8 p-value threshold and a less significant clustering threshold (R2 < 0.001 at a distance of 1,000 kb) [13]. To ensure the reliability of the tool, we selected IVs with an F-statistic of more than 10, identifying them as strong tools for subsequent analyses. We then extracted these IVs from the summary statistics regarding UGI cancer outcomes according to the methodology of previous studies [14], excluding any IVs with a potential pleiotropic effect (p < 10^-5) on UGI cancer. To maintain consistency in the analyses, we synchronised the SNPs in the exposure dataset and in the findings dataset to ensure that the effect estimates of the same effect allele remained consistent. Alleles with intermediate effect frequencies (EAFs >0.42) or SNPs incompatible with the allele were excluded from our analyses [13].
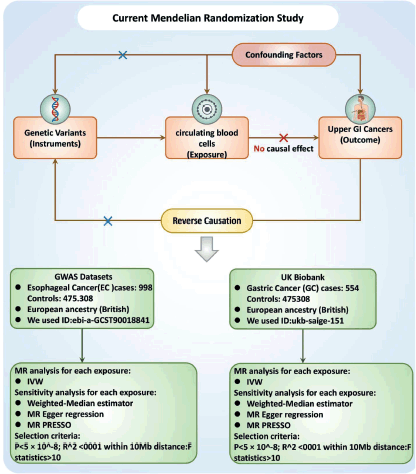
Figure 1. Study design flowchart. The first assumption is that the instrument variables are strongly related to the exposure. The second assumption specifies that the instrument variables are not associated with any confounders. The third assumption establishes that the instrument variables influence the outcome solely through the exposure. Abbreviations include SNPs for single-nucleotide polymorphisms, LD for linkage disequilibrium, IVW for inverse variance weighted and weighted median, MR-Egger and MR-PRESSO.
Statistical analysis
In our research, we used a series of Genetic Variants as IVs rather than relying solely on an allele score. This approach was chosen to thoroughly examine key assumptions, uncover potential pleiotropy and facilitate more effective sensitivity and multivariable MR analyses [11]. To assess the consistency of our findings under different assumptions about heterogeneity and pleiotropy, we utilised four distinct MR methodologies: The inverse variance weighted (IVW), Weighted Median, MR-Egger and MR Pleiotropy Residual Sum and Outlier (MR-PRESSO). The IVW method, employing a random-effects model, served as the primary analysis framework for all four sets of IVs. Cochran’s Q statistical measurements and the appropriate p-values were used to test for heterogeneity among the elected IVs.
Our study also included analyses with more stringent conditions. In the IVW approach, under the hypothesis that all genetic variants are effective, if many SNPs are influenced by horizontal pleiotropy, bias will occur [15]. Conversely, the weighted median approach, effective when less than 50% of variants exhibit horizontal pleiotropy, presumes most genetic variants are valid [16]. In cases where over 50% of variants are affected by horizontal pleiotropy, we evaluated the strength of our genetic instruments through F statistics, considering a mean F < 10 indicative of weak IVs [17].
Furthermore, to rule out the effect of horizontal multidimensionality, we used a frequently utilised approach (MR-Egger), which implores the presence of horizontal multidimensionality if the interception term is statistically significant [18]. We also used the powerful (MR-PRESSO method to eliminate potential horizontal pleiotropic outliers, which could have affected the estimation results of the MR-PRESSO package [19]. Additionally, Steiger-filtering analyses were conducted to detect and eliminate genetic variants more strongly associated with the outcome than the exposure, demonstrating possible reverse causality [20].
All statistical analyses were conducted with R software 4.3.1 (R Foundation) and specific R packages (‘TwoSampleMR’ and ‘MR’) tailored for MR analysis [21, 22].
Results
The causal role of PCBCs and UGI cancers
The causal role of PCBCs and EC
Figure 2 summarises the causal effect estimates of circulating blood count on susceptibility to EC. We found a strong positive causal association between EC risk circulating basophil cell count (OR = 1.416, 95% CI = 1.125–1.783, p = 0.003). However, no significant correlations were observed between monocyte, lymphocyte, eosinophil or neutrophil counts and EC susceptibility.
Neither the MR-Egger intercept test nor Cochran’s Q test showed pleiotropy or heterogeneity (Tables S1 and S2). Additionally, scatter plots Figure 3A and funnel plots Figure 3B were employed to analyse the data, effectively reducing the likelihood of potential outliers and horizontal pleiotropy affecting any of the identified basophil cells.
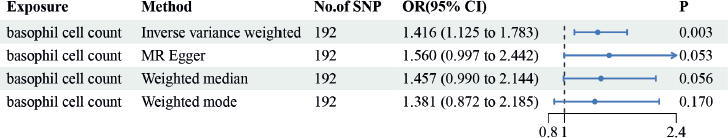
Figure 2. The causal estimation between basophil cell count and EC. We selected IVW as a primary method p < 0.05 showed statistical significant; OR value >1 indicated a risk factor.
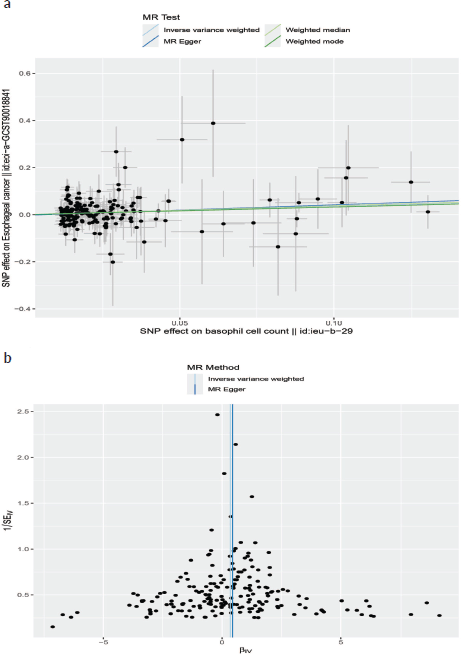
Figure 3. (a): The scatter plot demonstrating the genetic associations of basophil cell count on the risk of EC. (b): The funnel plot represents IVs for each significant causal relation between basophil cell count and EC.
The causal role of PCBCs and GC
IVW results suggested a risk effect of basophil cell count (OR = 1.623, 95% CI = 1.052–2.505, p = 0.029) on GC risk (Figure 4). However, no significant correlations were seen between basophil cell count, monocyte cell count, lymphocyte cell count or neutrophil cell count and GC. Neither the MR-Egger intercept test nor Cochran’s Q test revealed pleiotropy or heterogeneity (Tables S3 and S4). Additionally, scatter plots Figure 5A and funnel plots Figure 5B were employed to analyse the data, effectively reducing the likelihood of potential outliers and horizontal pleiotropy affecting any of the identified basophil cells.
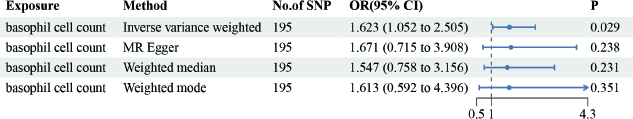
Figure 4. The causal estimation between basophil cell count and GC. We selected IVW as a primary method p < 0.05 showed statistically significant; OR value >1 indicated a risk factor.
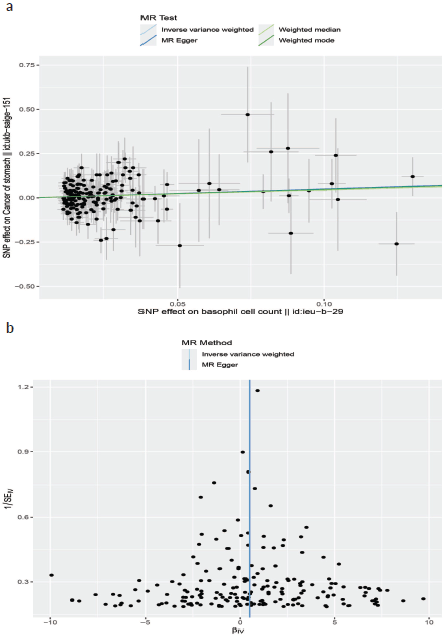
Figure 5. (a): The scatter plot demonstrating the genetic associations of basophil cell count on the risk of GC. (b): The funnel plot represents IVs for each significant causal relation between basophil cell count and GC.
Discussion
MR analysis has been frequently employed to illustrate possible causality between risk factors and diseases [19]. In this MR study, we used MR to investigate the causal correlations between PCBCs and UGI cancer. The results indicate that higher basophil levels may elevate the risk of UGI cancer, thus expanding our comprehension of the potential cancer-causing roles of these cells. Basophils, a type of white blood cell, are naturally present in small quantities in the bloodstream. They are essential for the immune system and play a crucial role in both hypersensitivity reactions and fighting against parasitic infections. Basophils are recognised for their ability to produce and release inflammatory substances like histamine and cytokines, which contribute to the body’s immune response [23, 24]. Elevated and reduced levels of basophils in peripheral blood may be linked to the progression of specific types of solid tumors in humans [25, 26].
Basophils derived from stem progenitor cells are found in the bone marrow [27–29]. In both humans and mice, interleukin-3 (IL-3) serves as the primary growth factor essential for basophils maturation [30–33]. Several studies have indicated that basophils in these species can be cultured in vitro. This is achieved by incubating bone marrow cells or, in humans, CD34* precursors with IL-3 for a duration of 10–14 days [30, 34–36]. Although IL-3 plays a crucial role in the establishment of basophils from their precursors, additional growth factors contribute to their expansion and function. For instance, the combination of Feline McDonough Sarcoma-like tyrosine kinase 3 ligand with IL-3 has been demonstrated to enhance basophil production in cultures [37]. Moreover, research by Siracusa et al [38] revealed that mouse basophils could be produced using thymic stromal lymphopoietin (TSLP), which involves the heterodimeric TSLP receptor (TSLPR/IL-7Ro) [38]. This study further suggests that IL-3 and TSLP can stimulate the polarization of two different types of basophils in mice, each characterised by unique gene expression profiles and functions [39].
In terms of the function of PCBCs in UGI cancer, basophils have not generated much interest due to their scarce dissemination and relatively unexplored functions. Basophils perform an essential role in hypersensitivity diseases and type 1 hypersensitivity responses [40]. In addition, they are recruited to tissues following parasitic, bacterial and viral infections. Subsequent establishment of basophil removal antibodies and basophil-deficient mouse models has recently provided a greater understanding of basophil biology beyond allergic reactions [41]. Consequently, a potential relationship between basophils and tumor immunity may also exist [42]. Basophils not only enhance humoral immune function but also liberate intracellular agents causing anti-tumor immunity [43–45], including the chemokines CCL3 and CCL4, which expedite CD8+ T-cell infiltration [43], and the chemokines TNF-α and IL-6, which enhance inflammatory anti-tumor responses [46, 47]. Some studies have reported that basophils have favorable survival outcomes in melanoma [48, 49], ovarian cancer [50], endometrial [51], sarcoma cancers [52], non-small cell lung cancer [53] and glioblastoma [54], while basopenia is correlated with a worse prognosis for colorectal cancer [55, 56]. On the other hand, basophilia has been linked to better outcomes in patients with melanoma who undergoing immunotherapy treatment [49]. The above findings are inconsistent with our results demonstrating that basophils were strongly associated with UGI cancer and that increased basophil counts may contribute to reduced survival.
Dvorak’s [57] seminal discovery of piecemeal degranulation in basophils in human pancreatic cancer (PC) set the stage for subsequent investigations. Extensive research has been conducted to explore basophils’ involvement in human pancreatic ductal adenocarcinoma (PDAC) [58]. In those patients with PDAC, basophils expressing IL4 have been found in tumor-draining lymph nodes (TDLNs), and their presence emerged as an independent negative prognosis indicator for patient survival. Investigating basophils’ function in PC was further conducted in basophil-deficient Mcpt8-Cre mice [59] and wild-type (WT) mice. Following PC implantation, cancer was observed in 80% of WT mice but was notably absent in the Mcpt8-Cre mice. The basophils in TDLNs were observed, and the role of cancer-associated fibroblasts in releasing TSLP was noted, which in turn activated dendritic cells (DCs) to generate IL-3 from CD4* T cells. Both DCs and CD14* monocytes secreted CCL7, triggering the migration of basophils into TDLNs. Once activated by IL-3, the basophils assumed a pro-tumorigenic role, secreting IL-4 and thereby promoting Th2 and M2 polarization. In another interesting study, in the advanced stage of chronic myeloid leukemia (CML), basophilia was also found to be accumulated [60] and a notable reduction in the IKAROS, a transcription factor in these patient’s bone marrow, was observed [61]. CML patient-derived basophils were found to express HIGF, promoting the expansion of CML cells [62]. Furthermore, in a mouse CML model, basophil-derived CCL3 was implicated in promoting CML progression [63]. Basophilia was recognised as an independent predictor of acute myeloid leukemia that progressed from myelodysplastic syndrome [64, 65]. These observations are supported by our results that basophils are the risk factors in UGI cancer, where elevated basophil counts may correlate with diminished survival.
In other solid tumor types, such as GCs [7] and prostate [4], adverse effects related to tissue infiltrating or circulating basophils have been reported. Notably, in bladder cancer patients, the initial basophil count has been identified as a predictor of recurrence following tumor resection and bacillus Calmette–Guérin therapy [5]. In contrast, a study using a mouse model of breast cancer (BC) revealed that basopenia, or a low basophil count, correlated with an increased number of pulmonary metastases [66]. However, no significant correlation has been established between basophils and prognosis in BC patients [67]. This variation in findings suggests that the local tumor microenvironment might dictate whether basophils exhibit pro-tumor or anti-tumor effects, potentially explaining their inconsistent impact on survival across different cancer types [26]. Basophils play a crucial role in supporting humoral immunity, mainly by secreting molecules that modulate B-cell function. Activated basophils can express CD40L, IL-4 and IL-6, which are vital in sustaining B-cell proliferation and boosting IgM and IgG1 production. Research by Rodriguez Gomez et al [45] have demonstrated that basophils, both in vitro and in vivo, are instrumental in promoting plasma cell survival [24, 45]. Our research aligns with these findings, particularly highlighting a strong association of basophils with UGI cancer, supporting the broader implications of basophils in cancer dynamics.
Strength and limitations
Our two-sample MR analysis was designed to analyse the causal relationships between PCBCs in UGI cancer using large-sample GWAS and UK Biobank data. This design reduced the limitations of traditional observational studies by eliminating the influence of confounding factors and reverse causality. In addition, MR mitigates the representativeness and feasibility issues inherent to RCTs. However, there are several limitations to this study. First, it depended on publicly accessible GWAS and UK Biobank data, which prevented further exploration of the impact of other relevant factors on UGI cancers, such as gender, age and body mass index [68]. Second, these findings can only be generalised to European populations, as that was the sample present in the original GWAS and UK Biobank, and further study is needed in different ethnic subgroups [69]. Third, even with multi-sensitivity analysis, horizontal pleiotropy could not be comprehensively evaluated. Finally, we employed wider thresholds for assessing the findings, which may grow false positives but also enable a more critical assessment of the association between circulating blood cell characteristics and UGI cancer. In addition, this study covered a wide range of circulating blood cells, but the functions and mechanisms of circulating blood cells in disease are not yet fully understood, which limits our interpretation of the findings from the MR analysis.
Conclusion
In summary, our MR analysis offers evidence suggesting a potential causal relationship between PCBCs and the risk of UGI cancer. The results highlight the possible role of basophils as a risk factor for UGI cancer, emphasising the importance of further research to elucidate the underlying mechanisms. Understanding the causal link between basophils and UGI cancer may offer valuable insights for the development of targeted preventive and therapeutic strategies, potentially contributing to improved outcomes for individuals at risk of this disease.
Acknowledgments
The authors thank medical engineering research team (yigongkeyan tuandui) very much for their guiding and providing suitable coding to analyse this MR study.
Conflicts of interest
No competing interests.
Funding
The present study was supported by the Natural Science Foundation of Guangdong Province (Grant No.2023A1515012548) and Science and Technology Program of Guangzhou (Grant No.2024A04J4802).
Ethics approval
Not applicable because the present study extracted data from database.
Author contributions
- Pengkhun Nov design, analysis and interpretation of data.
- Wandan Li revise and agree to publish.
- Duanyu Wang drafting of the paper.
- Syphanna Sou revise and agree to publish.
- Socheat Touch revise and agree to publish.
- Samnang Kouy revise and agree to publish.
- Qianzi Kou revise and agree to publish.
- Ying Li revise and agree to publish.
- Kunpeng Du revise and agree to publish.
- Jiqiang Li revise and agree to publish.
Data availability
All the data for this paper is available in the GWAS and UK biobank database.
Patients consent for publication
Not available because we used public database analyse this study.
References
1. Bray F, Laversanne M, and Sung H, et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 74(3) 229–263 https://doi.org/10.3322/caac.21834 PMID: 38572751
2. Huang RJ, Laszkowska M, and In H, et al (2023) Controlling gastric cancer in a world of heterogeneous risk Gastroenterology 164(5) 736–751 https://doi.org/10.1053/j.gastro.2023.01.018 PMID: 36706842 PMCID: 10270664
3. Wadhwa V, Patel N, and Grover D, et al (2023) Interventional gastroenterology in oncology CA Cancer J Clin 73(3) 286–319 https://doi.org/10.3322/caac.21766
4. Hadadi A, Smith KE, and Wan L, et al (2022) Baseline basophil and basophil-to-lymphocyte status is associated with clinical outcomes in metastatic hormone sensitive prostate cancer Urol Oncol 40 271.e9–271.e18 https://doi.org/10.1016/j.urolonc.2022.03.016 PMID: 35466038 PMCID: 9117505
5. Ferro M, Di Lorenzo G, and Vartolomei MD, et al (2020) Absolute basophil count is associated with time to recurrence in patients with high grade T1 bladder cancer receiving bacillus Calmette-Guérin after transurethral resection of the bladder tumor World J Urol 38 143–150 https://doi.org/10.1007/s00345-019-02754-2
6. Maruyama S, Okamura A, and Kanie Y, et al (2023) Prognostic significance of circulating basophil counts in patients who underwent esophagectomy for esophageal cancer Langenbecks Arch Surg 408(1) 235 https://doi.org/10.1007/s00423-023-02977-3 PMID: 37329456
7. Wu C, Oiu Y, and Zhang R, et al (2022) Association of peripheral basophils with tumor M2 macrophage infiltration and outcomes of the anti-PD-1 inhibitor plus chemotherapy combination in advanced gastric cancer Trans Med 20 386 https://doi.org/10.1186/s12967-022-03598-y
8. Pingault JB, O’Reilly PF, and Schoeler T, et al (2018) Using genetic data to strengthen causal inference in observational research Nat Rev Genet 19(9) 566–580 https://doi.org/10.1038/s41576-018-0020-3 PMID: 29872216
9. Emdin CA, Khera AV, and Kathiresan S (2017) Mendelian randomization JAMA 318(19) 1925–1926 https://doi.org/10.1001/jama.2017.17219 PMID: 29164242
10. Smith GD and Ebrahim S (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32(1) 1–22 https://doi.org/10.1093/ije/dyg070 PMID: 12689998
11. Davies NM, Holmes MV, and Davey SG (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians BMJ 362 k601 https://doi.org/10.1136/bmj.k601 PMID: 30002074 PMCID: 6041728
12. Orru V, Steri M, and Sidore C, et al (2020) Complex genetic signatures in immune cells underlie autoimmunity and inform therapy Nat Genet 52(10) 1036–1045 https://doi.org/10.1038/s41588-020-0684-4 PMID: 32929287 PMCID: 8517961
13. Cai J, Li X, and Wu S, et al (2022) Assessing the causal association between human blood metabolites and the risk of epilepsy J Transl Med 20(1) 437 https://doi.org/10.1186/s12967-022-03648-5 PMID: 36180952 PMCID: 9524049
14. Zeng P, Wang T, and Zheng J, et al (2019) Causal association of type 2 diabetes with amyotrophic lateral sclerosis: new evidence from Mendelian randomization using GWAS summary statistics BMC Med 17(1) 225 https://doi.org/10.1186/s12916-019-1448-9 PMID: 31796040 PMCID: 6892209
15. Hartwig FP, Davey Smith G, and Bowden J (2017) Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption Int J Epidemiol 46(6) 1985–1998 https://doi.org/10.1093/ije/dyx102 PMID: 29040600 PMCID: 5837715
16. Bowden J, Davey Smith G, and Haycock PC, et al (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator Genet Epidemiol 40(4) 304–314 https://doi.org/10.1002/gepi.21965 PMID: 27061298 PMCID: 4849733
17. Allen RJ, Porte J, and Braybrooke R, et al (2017) Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study Lancet Respir Med 5 869–880 https://doi.org/10.1016/S2213-2600(17)30387-9 PMID: 29066090 PMCID: 5666208
18. Burgess S, Bowden J, and Fall T, et al (2017) Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants Epidemiology 28 30–42 https://doi.org/10.1097/EDE.0000000000000559
19. Verbanck M, Chen CY, and Neale B, et al (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases Nat Genet 50 693–698 https://doi.org/10.1038/s41588-018-0099-7 PMID: 29686387 PMCID: 6083837
20. Hemani G, Tilling K, and Davey SG (2017) Orienting the causal relationship between imprecisely measured traits using GWAS summary data PLoS Genet 13 e1007081 https://doi.org/10.1371/journal.pgen.1007081 PMID: 29149188 PMCID: 5711033
21. Hemani G, Zheng J, and Elsworth B, et al (2018) The MR-base platform supports systematic causal inference across the human phenome Elife 7 e34408 https://doi.org/10.7554/eLife.34408 PMID: 29846171 PMCID: 5976434
22. Yavorska OO and Burgess S (2017) MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data Int J Epidemiol 46(6) 1734–1739 https://doi.org/10.1093/ije/dyx034 PMID: 28398548 PMCID: 5510723
23. Yamanishi Y, Miyake K, and Iki M, et al (2017) Recent advances in understanding basophil-mediated Th2 immune responses Immunol Rev 278 237–245 https://doi.org/10.1111/imr.12548 PMID: 28658549
24. Liu Q, Luo D, and Cai S, et al (2020) Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer Clin Transl Med 9 6 https://doi.org/10.1186/s40169-019-0255-4 PMID: 32037496 PMCID: 7008108
25. Varricchi G, Ameri P, and Cadeddu C, et al (2018) Antineoplastic drug-induced cardiotoxicity: a redox perspective Front Physiol 9 167 https://doi.org/10.3389/fphys.2018.00167 PMID: 29563880 PMCID: 5846016
26. Marone G, Schroeder IT, and Mattei F, et al (2020) Is there a role for basophils in cancer? Front Immunol 11 2103 https://doi.org/10.3389/fimmu.2020.02103 PMID: 33013885 PMCID: 7505934
27. Arinobu Y, Iwasaki H, and Gurish MF, et al (2005) Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis Proc Natl Acad Sci USA 102 18105–18110 https://doi.org/10.1073/pnas.0509148102 PMID: 16330751 PMCID: 1312421
28. Hamey FK, Lau WWY, and Kucinski I, et al (2021) Single-cell molecular profiling provides a high-resolution map of basophil and mast cell development Allergy 76 1731–1742 https://doi.org/10.1111/all.14633
29. Wanet A, Bassal MA, and Patel SB, et al (2021) E-cadherin is regulated by GATA-2 and marks the early commitment of mouse hematopoietic progenitors to the basophil and mast cell fates Sci Immunol 6 eaba0178 https://doi.org/10.1126/sciimmunol.aba0178 PMID: 33547048 PMCID: 8261706
30. Gambardella AR, Poto R, and Tirelli V, et al (2022) Differential effects of alarmins on human and mouse basophils Front Immunol 13 894163 https://doi.org/10.3389/fimmu.2022.894163 PMID: 35693823 PMCID: 9177950
31. Varricchi G, Poto R, and Marone G, et al (2021) IL-3 in the development and function of basophils Semin Immunol 54 101510 https://doi.org/10.1016/j.smim.2021.101510 PMID: 34756806
32. Schroeder IT, Chichester KL, and Bieneman AP (2009) Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease J Immunol 182 2432–2438 https://doi.org/10.4049/jimmunol.0801782 PMID: 19201898 PMCID: 2704022
33. Kirshenbaum AS, Goff JP, and Dreskin SC, et al (1989) IL-3-dependent growth of basophil-like cells and mastlike cells from human bone marrow J Immunol 142 2424–2429 https://doi.org/10.4049/jimmunol.142.7.2424 PMID: 2647850
34. Valent P, Besemer J, and Muhm M, et al (1989) Interleukin 3 activates human blood basophils via high-affinity binding sites Proc Natl Acad Sci USA 86 5542–5546 https://doi.org/10.1073/pnas.86.14.5542 PMID: 2473472 PMCID: 297659
35. Valent P, Schmidt G, and Besemer J, et al (1989) Interleukin-3 is a differentiation factor for human basophils Blood 73 1763–1769 https://doi.org/10.1182/blood.V73.7.1763.1763 PMID: 2469498
36. Yoshimoto T and Nakanishi K (2012) Generation and characterization of mouse basophils from bone marrow and purification of basophils from spleen Curr Protoc Immunol 3 3.24.1–3.24.16
37. MacGlashan D Jr (2020) Modulating the human basophil phenotype during its development and maturation: basophils derived from In vitro cultures of CD34(+) progenitor cells Methods Mol Biol 2163 69–83 https://doi.org/10.1007/978-1-0716-0696-4_6 PMID: 32766967
38. Siracusa MC, Saenz SA, and Hill DA, et al (2011) TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation Nature 477 229–233 https://doi.org/10.1038/nature10329 PMID: 21841801 PMCID: 3263308
39. Siracusa MC, Wono ED, and Artis D (2012) Functional heterogeneity in the basophil cell lineage Adv Immunol 115 141–159 https://doi.org/10.1016/B978-0-12-394299-9.00005-9 PMID: 22608258 PMCID: 3675451
40. Salter BM, Oliveria JP, and Nusca G, et al (2015) Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent J Allergy Clin Immunol 136 1636–1644 https://doi.org/10.1016/j.jaci.2015.03.039 PMID: 25962901
41. Salabert-Le Guen N, Hemont C, and Delbove A, et al (2018) Thymic stromal lymphopoietin does not activate human basophils J Allergy Clin Immunol 141 1476–1479 https://doi.org/10.1016/j.jaci.2017.11.012
42. Schroeder IT and Bieneman AP (2017) Activation of human basophils by A549 lung epithelial cells reveals a novel IgE-dependent response independent of allergen J Immunol 199 855–865 https://doi.org/10.4049/jimmunol.1700055 PMID: 28652400 PMCID: 5541892
43. Sektioglu IM, Carretero R, and Bulbuc N, et al (2017) Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells Cancer Res 77 291–302 https://doi.org/10.1158/0008-5472.CAN-16-0993
44. Denzel A, Maus UA, and Rodriguez Gomez M, et al (2008) Basophils enhance immunological memory responses Nat Immunol 9 733–742 https://doi.org/10.1038/ni.1621 PMID: 18516038
45. Rodriguez Gomez M, Talke Y, and Goebel N, et al (2010) Basophils support the survival of plasma cells in mice J Immunol 185 7180–7185 https://doi.org/10.4049/jimmunol.1002319 PMID: 21068399
46. Gordon JR and Galli SJ (1990) Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin Nature 346 274–276 https://doi.org/10.1038/346274a0 PMID: 2374592
47. Gooch JL, Lee AV, and Yee D (1998) Interleukin 4 inhibits growth and induces apoptosis in human breast cancer cells Cancer Res 58 4199–4205 PMID: 9751635
48. Wagner NB, Luttermann F, and Gassenmaier M, et al (2020) Absolute and relative differential blood count predicts survival of AJCC stage I-II melanoma patients scheduled for sentinel lymph node biopsy Australas J Dermatol 61 310–318 https://doi.org/10.1111/ajd.13248
49. Rosner S, Kwong E, and Shoushtari AN, et al (2018) Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma Cancer Med 7 690–697 https://doi.org/10.1002/cam4.1356 PMID: 29468834 PMCID: 5852343
50. Bax HJ, Chauhan J, and Stavraka C, et al (2020) Basophils from cancer patients respond to immune stimuli and predict clinical outcome Cells 9 1631 https://doi.org/10.3390/cells9071631 PMID: 32645919 PMCID: 7408103
51. Yu L, Davis IJ, and Liu P (2023) Regulation of EWSR1-FLI1 function by post-transcriptional and post-translational modifications Cancers (Basel) 15(2) 382 https://doi.org/10.3390/cancers15020382 PMID: 36672331 PMCID: 9857208
52. Balikani L and Policarpio-Nicolas MLC (2023) Undifferentiated component of dedifferentiated endometrial carcinoma presenting as a “small round cell” malignancy in a mediastinal lymph node, mimicking small cell carcinoma Diagn Cytopathol 51(8) 519–524 https://doi.org/10.1002/dc.25184 PMID: 37318779
53. Hiltbrunner S, Spohn ML, and Wechsler R, et al (2021) Comprehensive statistical exploration of prognostic (bio-)markers for responses to immune checkpoint inhibitor in patients with non-small cell lung cancer Cancers (Basel) 14 75 https://doi.org/10.3390/cancers14010075
54. Zheng L, Yu M, and Zhang S (2021) Prognostic value of pretreatment circulating basophils in patients with glioblastoma Neurosurg Rev 44 3471–3478 https://doi.org/10.1007/s10143-021-01524-2 PMID: 33765226
55. Wei Y, Zhang X, and Wang G, et al (2018) The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage colorectal cancer Asia Pac J Clin Oncol 14 243–251 https://doi.org/10.1111/ajco.12871
56. Wu J, Ge XX, and Zhu W, et al (2019) Values of applying white blood cell counts in the prognostic evaluation of resectable colorectal cancer Mol Med Rep 19 2330–2340 PMID: 30664202
57. Dvorak AM (1995) Ultrastructural analysis of human mast cells and basophils Chem Immunol 61 1–33 PMID: 7662139
58. De Monte L, Wörmann S, and Brunetto E, et al (2016) Basophil recruitment into tumor-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients Cancer Res 76 1792–1803 https://doi.org/10.1158/0008-5472.CAN-15-1801-T PMID: 26873846
59. Ohnmacht C, Schwartz C, and Panzer M, et al (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths Immunity 33 364–374 https://doi.org/10.1016/j.immuni.2010.08.011 PMID: 20817571
60. Denburg JA and Browman G (1988) Prognostic implications of basophil differentiation in chronic myeloid leukemia Am I Hematol 27 110–114 https://doi.org/10.1002/ajh.2830270208
61. Beer PA, Knapp DJ, and Miller PH, et al (2015) Disruption of IKAROS activity in primitive chronic-phase CML cells mimics myeloid disease progression Blood 125 504–515 https://doi.org/10.1182/blood-2014-06-581173 PMCID: 4300391
62. Cerny-Reiterer S, Ghanim V, and Hoermann G, et al (2012) Identification of basophils as a major source of hepatocyte growth factor in chronic myeloid leukemia: a novel mechanism of BCR-ABLI-independent disease progression Neoplasia 14 572–584 https://doi.org/10.1593/neo.12724 PMID: 22904675 PMCID: 3421954
63. Baba T, Naka K, and Morishita S, et al (2013) MIP-1alpha/CCL3-mediated maintenance of leukemia-initiating cells in the initiation process of chronic myeloid leukemia J Exp Med 210 2661–2673 https://doi.org/10.1084/jem.20130112 PMID: 24166712 PMCID: 3832924
64. Matsushima T, Handa H, and Yokohama A, et al (2003) Prevalence and clinical characteristics of myelodysplastic syndrome with bone marrow eosinophilia or basophilia Blood 101 3386–3390 https://doi.org/10.1182/blood-2002-03-0947
65. Wimazal F, Germing U, and Kundi M, et al (2010) Evaluation of the prognostic significance of eosinophilia and basophilia in a larger cohort of patients with myelodysplastic syndromes Cancer 116 2372–2381 https://doi.org/10.1002/cncr.25036 PMID: 20209617
66. Wang C, Chen YG, and Gao IL, et al (2015) Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model Oncol Lett 10 754–760 https://doi.org/10.3892/ol.2015.3304 PMID: 26622565 PMCID: 4509112
67. Chan YB, Arslan A, and Cetindag MF, et al (2014) Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer Asian Pac Cancer Prev 15 4225–4231 https://doi.org/10.7314/APJCP.2014.15.10.4225
68. Tian D, Zhou Y, and Chen Y, et al (2023) Genetically predicted ankylosing spondylitis is causally associated with psoriasis Front Immunol 14 1149206 https://doi.org/10.3389/fimmu.2023.1149206 PMID: 37483619 PMCID: 10357290
69. Zhao N, Guo P, and Tang M, et al (2023) Evidence for a causal relationship between psoriasis and cutaneous melanoma: a bidirectional two-sample mendelian randomized study Front Immunol 14 1201167 https://doi.org/10.3389/fimmu.2023.1201167 PMID: 37503344 PMCID: 10368886
Supplementary tables
Table S1. The pleiotropy of causal relationship between basophil and EC. The p-values for the MR-Egger intercept were above 0.05, suggesting that no significant pleiotropy effects were found.
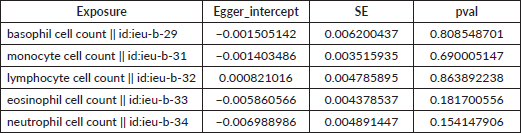
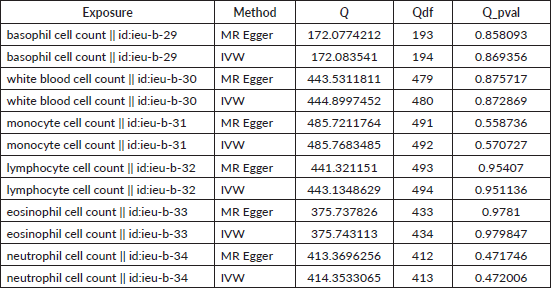
Table S2. The heterogeneity of causal relationship between basophil and EC. The p-values for the Cochran’s Q yielded were above 0.05, suggesting that no significant heterogeneity effects were found.
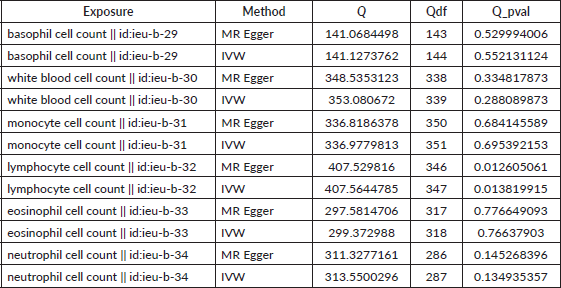
Table S3. The pleiotropy of causal relationship between basophil and GC. The p-values for the MR-Egger intercept were above 0.05, suggesting that no significant pleiotropy effects were found.
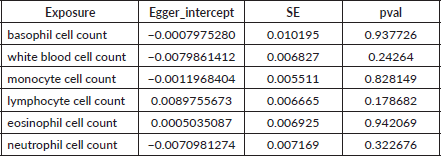
Table S4. The heterogeneity of causal relationship between basophil and GC. The p-values for the Cochran’s Q yielded were above 0.05, suggesting that no significant heterogeneity effects were found.