Utility of glass slide morphology (GSM) and whole slide image (WSI) in the diagnosis of acute leukemia (AL) by types
Hamisa Iddy1, Ahlam Nasser2, Ally Hussein2,3, Anna Schuh2,4 and Clara Chamba2
1Ocean Road Cancer Institute (ORCI), Dar es salaam 11101, Tanzania
2Muhimbili University of Health and Allied Sciences (MUHAS), Dar es salaam 11103, Tanzania
3Tanzania Field Epidemiology and Laboratory Training Program, Dar es Salaam 11101, Tanzania
4University of Oxford, Oxford OX37DL, UK
Abstract
Acute leukemia (AL) is a diverse group of hematological malignancies characterised by the accumulation of immature blast cells in the bone marrow. Accurate classification into acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) is essential for treatment and prognosis. This study aimed to assess the performance of glass slide morphology (GSM) using a light microscope versus whole slide imaging (WSI) in diagnosing and classifying AL, using flow cytometry as the gold standard test. Peripheral smears and bone marrow aspirates from 97 patients suspected of AL were stained with Romanowsky stain and reviewed by a single hematologist. For GSM, the hematologist was provided with a single slide, which was to be evaluated under a light microscope. For WSI, the Alexapath mobile scanner (ADA1) was used to scan the slides for review by the hematologist. Patient identification was concealed from the interpreting hematologist, and an interval of 2 weeks was set between the review of GSM and WSI of the same patient. The sensitivity and specificity of GSM and WSI were compared to the results of flow cytometry. Out of the 97 patients suspected to have AL, 47 were confirmed to have AL by flow cytometry. Among these, 19 (40.4%) were diagnosed with AML and 28 (59.6%) with ALL. GSM demonstrated high sensitivity (89.4%) and specificity (90.0%) for diagnosing AL, but lower sensitivity in distinguishing AML (57.9%) from ALL (75.0%). Similarly, WSI exhibited a reasonable sensitivity (80.9%) and high specificity (98.0%) for diagnosing AL, but lower sensitivity in differentiating AML (57.9%) and ALL (46.4%). GSM and WSI are reasonable and acceptable techniques for accurately screening AL cases and accelerating referral to tertiary centers of excellence.
Keywords: acute leukemia, glass slide morphology, whole slide image, flow cytometry, sensitivity, specificity, Tanzania
Correspondence to: Ahlam Nasser
Email: dr.ahlamnasser@gmail.com
Published: 31/10/2024
Received: 30/04/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Acute leukemia (AL) is a heterogeneous group of malignancies affecting all age groups with an average annual incidence rate of 4–7 per 100,000 population [1]. It is characterised by the accumulation of immature blast cells in the bone marrow, which replace normal marrow tissue, including hemopoietic precursor cells [2]. Based on precursor cells of origin, AL can be broadly classified into acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). With advancements in treatment, it has become increasingly important to accurately classify the type of AL based on morphology, cytochemistry, immunophenotyping, cytogenetic and molecular genetics studies [3]. This categorization bears prognostic and therapeutic implications, allowing individualization of the type and intensity of treatment according to the leukemia type. Additionally, immunophenotyping of surface antigens expressed by the leukemia cells using flow cytometry is considered the gold standard method for accurately classifying AL as either AML or ALL. However, due to high costs associated with flow cytometry and other sophisticated investigations such as cytogenetic and molecular genetics studies, these tests remain unavailable and inaccessible to the majority of patients in many resource-limited settings, including Tanzania. As a result, morphologic assessment remains the mainstay of diagnosis.
Over the years, morphologic assessment of leukemia blasts has been done using traditional glass slide morphology (GSM) examination under the microscope. With the advancement of technology, whole slide imaging (WSI) and digital pathology have been increasingly used in clinical practice. WSI may be used as a substitute for the traditional GSM examination in areas with limited trained personnel to accurately make the diagnosis of AL. This is particularly important in sub-Sahara African countries including Tanzania where there is a limited number of pathologists and hematologists. The average number of pathologists per head of population in sub-Saharan Africa is 1/1,000,000 compared to 1 pathologist to 15–20,000 in the US and UK [4]. To date, in Tanzania, there are a total number of 40 hematologists serving a population of almost 60 million people [5]. The majority of these are concentrated in the capital city of Dar es salaam where the National Referral Hospital that provides specialist care is located. As a result, there are significant delays in the diagnosis of hematologic disorders including AL. A potential solution to bridge the gap of adequately trained personnel is the use of WSI where technical health care personnel from peripheral hospitals scan and send digital whole slide images of the peripheral blood smears for morphological review by specialist hematologists at the zonal or national hospitals. This will allow faster diagnosis and early referral to the specialised center for definitive diagnosis. This is even more crucial in the setting of AL where timely diagnosis is critical for patients’ survival. Therefore, this study aimed to evaluate the performance of GSM and WSI in diagnosing AL and its types using flow cytometry as the gold standard test.
Methods
Study design and settings and study population
This was a hospital-based cross sectional study conducted at Muhimbili National Hospital (MNH) in Dar es Salaam from January to May 2019. The recruitment point for this study was the bone marrow aspiration room, where all adult and pediatric patients who had been previously reviewed by a hematologist and were suspected of having AL based on clinical presentation, complete blood counts and peripheral smears were enrolled. The exclusion criteria consisted of previously diagnosed AL, relapsed AL and cases where bone marrow aspiration resulted in a dry tap.
Sample collection and processing
All bone marrow samples of the recruited patients were processed for GSM and WSI. Additionally, for each of the consenting patients undergoing a bone marrow aspiration, their previous complete blood count and smear results were reviewed to determine if the patient had peripheral circulating blasts for flow cytometry. For patients with cytopenia and no circulating blasts, a bone marrow aspirate sample was used for flow cytometry.
Morphologic examination of the smears
Bone marrow aspirate smears were evaluated and reviewed by one experienced hematologist after staining with Romanowsky stain. For GSM, the hematologist was provided with a single slide which was to be evaluated under a light microscope. For WSI, the Alexapath mobile scanner (ADA1) was used to scan the slides, acquiring a total of 20 images per patient slide for review by the hematologist. Patient identification was concealed from the interpreting hematologist, and an interval of 2 weeks was set between the review of GSM and WSI of the same patient. The type of AL was classified into AML or ALL according to FAB classification.
Immunophenotyping by flow cytometry
Immunophenotyping was done using multiparametric, eight-colour flow cytometer (Beckman Coulter, FACS Canto II). Panels of monoclonal antibodies from the European Group for the Immunological Characterization of Leukemia criteria were used. Cell suspensions were stained with multiple panels of three monoclonal antibodies and a two-step strategy was used. The samples were then labeled with fluorescein isothiocyanate, Phycoerythrin and Peridin Chlorophyll Protein Complex. Cell suspensions were also stained with panels of identically conjugated isotype controls for the antibodies of each panel. Leukemic samples were considered positive for a particular antigen if 20% or more of leukemic cells reacted with a particular monoclonal antibody. The panels of antibodies included the following: CD34, HLA-DR, CD117, CD13, CD14, CD33, CD19, CD10, CD20, CD2, CD3, CD5, CD7, CD45 and CD64.
A case was considered B ALL if it expressed (CD10+, CD19+, CD20+, CD34+ and HLADR+). The T-cell ALL were considered when the markers for T-cell were positive (CD2+, CD3+, CD5+, CD7+ and CD34+). Myeloid cases were considered AML if expressed progenitor antigens (HLA-DR, CD34 and CD117), as well as myeloid antigens (CD13, CD33, CD14 and CD64).
Statistical analysis
We conducted an analysis to assess the effectiveness of GSM and WSI in diagnosing AL by types, specifically AML and ALL. We evaluated the diagnostic performance of GSM and WSI compared to flow cytometry results, considered the gold standard. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy were calculated and expressed in percentages.
These measures were determined using the following formulas:
Sensitivity (%) = (True positives (TP) / (TP + False negatives (FN))) × 100
Specificity (%) = (True negatives (TN) / (TN + False positives (FP))) × 100
PPV (%) = (TP / (TP + FP)) × 100
NPV (%) = (TN / (TN + FN)) × 100
Accuracy (%) = ((TP + TN) / Total Samples) × 100
TP represents cases where GSM or WSI correctly identified the presence of AL in the sample. FN occurs when GSM or WSI incorrectly indicates the absence of AL when it was actually present. TN are cases where GSM or WSI correctly identified the absence of AL. FP occurs when GSM or WSI incorrectly indicates the presence of AL when it was actually absent.
The analysis was performed using epi info 7.2 statistical software (CDC, USA), and descriptive statistics were used to summarise the results. The analysis findings were presented in tables.
Ethical consideration
Ethical clearance was sought from the Research and Publications Committee of Muhimbili University of Health and Allied Sciences. The permission to conduct the study was sought from the authority of MNH. Written informed consents were obtained from the patients in accordance with the Declaration of Helsinki before recruitment.
Results
A total of 115 patients with clinical suspicion of AL were screened for eligibility; resulting in the exclusion of 18 patients who had not undergone flow cytometry testing. Out of the 97 remaining patients, 47 were confirmed to have AL, of which AML and ALL were 19 (40.4%) and 28 (59.6%) patients, respectively. The GSM and WSI of these patients were evaluated for the diagnosis of AL and its types (Figure 1).
Generally, GSM demonstrated high sensitivity (89.4%) and specificity (90.0%) in diagnosing AL, achieving an accuracy rate of 87.9% (Table 1). However, when distinguishing AML from ALL, GSM sensitivity decreased to 57.9%, while specificity remained high at 89.7%. GSM correctly identified only 11 out of 19 patients with AML. Conversely, in identifying ALL, GSM performed better, accurately identifying 21 out of 28 patients, resulting in a sensitivity of 75% and specificity of 91.3%.
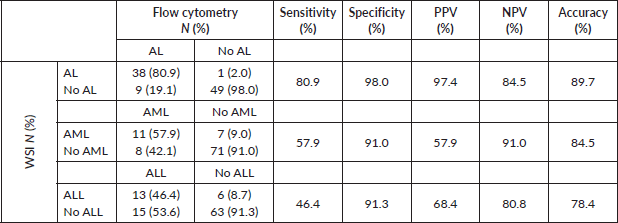
In contrast, when compared to flow cytometry results, WSI accurately diagnosed AL in 38 out of 47 patients, demonstrating a reasonable sensitivity of 80.9% (Table 2). Notably, WSI exhibited high specificity (98%) and a low FP rate of only 2%. However, WSI showed lower sensitivity in differentiating AML (57.9%) and ALL (46.4%), while maintaining a specificity of 91% for both types.
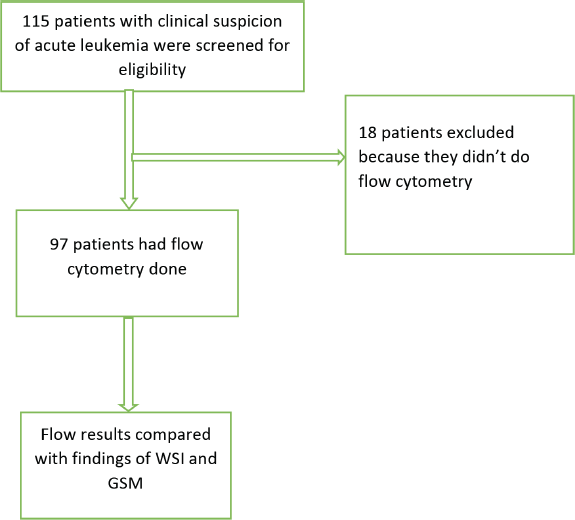
Figure 1. Flow chart of recruited patients and evaluated samples.
Table 1. Sensitivity and specificity of GSM compared to flow cytometry (gold standard) in the diagnosis of AL and its types.
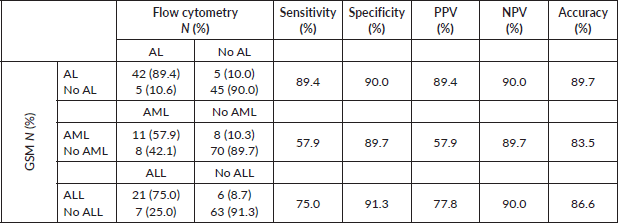
Table 2. Sensitivity and specificity of WSI compared to flow cytometry (gold standard) in the diagnosis of AL and its types.
Discussion
In this study, our aim was to assess the performances of GSM, a commonly used technique for diagnosing AL and WSI which is a potential solution for overcoming the shortage of hematologists in resource-limited settings like Tanzania. We identified 47 patients with AL through flow cytometry, including 19 with AML and 28 with ALL. Our results indicate that GSM demonstrates reasonably high sensitivity and accuracy, around 90%, in diagnosing AL. This suggests that morphology is useful for the initial diagnosis, as most bone marrow aspirates at presentation have blasts exceeding 90%, making immature cells easily detectable under a microscope. However, up to 10% of cases may be missed or misclassified as non-AL, particularly when the blast percentage is not markedly high or blasts are not remarkably immature. While this underscores the importance of combining both morphology and flow cytometry when evaluating suspected AL cases [6], it also reinforces the value of GSM as a primary diagnostic tool for AL in resource-limited settings.
Similarly, WSI performance showed acceptable sensitivity (81%) and accuracy (90%), consistent with findings from other studies validating its use in diagnosing various malignancies [7, 8]. WSI was found to be non-inferior to conventional light microscopy [8, 9], suggesting its potential for replacing GSM in regions lacking hematologists, thereby fostering institutional collaborations for early referral of the patients. Laboratory personnel from peripheral centers without access to hematology consultations could collaborate with hematologists from tertiary centers in Tanzania to accurately screen patients likely to have AL and refer them for definitive diagnosis and timely treatment, thus potentially overcoming human resource shortages and shorten the time to diagnosis, which is crucial and potentially lifesaving in patients with AL.
However, both GSM and WSI faced challenges in distinguishing between AL types, with sensitivity ranging from 50% to 60%. Morphologically, AML blasts may resemble ALL, especially in undifferentiated and minimally differentiated subtypes, where granules may be minimally present or completely absent [6]. This indicates that morphology alone may not suffice for confidently classifying AL types for treatment purposes. Therefore, in resource-limited settings, where the cost of flow cytometry is prohibitive for most, institutions should strive to at least incorporate immunocytochemistry and immunohistochemistry markers in the morphological evaluation of AL. These stains can assist in lineage assignment for difficult AL cases where simple morphological assessment of blasts proves challenging [10, 11].
While advocating for and supporting advanced treatment options like the stem cell transplant services introduced under government sponsorship in Tanzania in 2023 [12], policymakers and the government should also prioritise enhancing other advanced diagnostic services. These include the routine availability of flow cytometry, cytogenetics and molecular studies at the tertiary centers, which are essential for accurate diagnosis and prognosis of AL.
Despite our study having demonstrated the utility of WSI in diagnosing AL, there are still pertinent research gaps that must be addressed in future studies to fully realise the potential of WSI in overcoming the challenge of late diagnosis, a common problem in many low and middle income countries (LMICs). In this study, bone marrow slides were used for diagnosing AL by WSI, as they maximised the visualization of blasts. However, to achieve the ultimate goal of shortening the time to diagnosis in clinical practice, it is crucial to evaluate the performance of WSI in peripheral blood, where fewer blasts may pose a diagnostic challenge. Additionally, in this study, a single experienced hematologist interpreted both GSM and WSI, eliminating the opportunity to assess inter-observer reliability of WSI among personnel with varying levels of expertise.
In conclusion, WSI is non-inferior to GSM, the mainstay for diagnosing AL in many LMICs. Therefore, WSI is a potential solution to overcome the challenges of late diagnosis of AL, which is often due to the limited number of hematologists and pathologists in peripheral centers.
Acknowledgments
The authors would like to thank Dr. Yonazi Mbonea for his guidance in designing and conducting the study. The authors would also like to extend their sincere gratitude to all the members of the Department of Haematology and Blood Transfusion at MUHAS and MNH for their support during the conduct of the study. Last, but not least, the authors would like to extend their appreciation to the patients for their participation and contribution to this study.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
Funding for the reagents and the scanner was donated by Oxford Global Health Grant of Higher Education Funding Council for England (HEFCE).
Author contributions
HI – contributed in conception and designing of the work, data collection, drafting of the original manuscript, review and approval of the final draft; AN – contributed in data interpretation and analysis, drafting of the original manuscript, review and approval of the final draft,
AH – contributed in design of the work, data analysis, review and approval of the final draft; AS – contributed in conception and designing of the work, data interpretation, reviewing and approval of the final draft; CC – contributed in conception and designing of the work, data interpretation, reviewing and approval of the final draft.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Russell NH (1997) Biology of acute leukaemia Lancet 349 118–122 https://doi.org/10.1016/S0140-6736(96)07185-1 PMID: 8996433
3. Arber DA, Orazi A, and Hasserjian R, et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia Blood 127 2391–2405 https://doi.org/10.1182/blood-2016-03-643544 PMID: 27069254
4. Fleming K (2019) Pathology and cancer in Africa Ecancermedicalscience 13 945 https://doi.org/10.3332/ecancer.2019.945 PMID: 31552118 PMCID: 6722111
5. Makani J, Lyimo M, and Magesa P, et al (2017) Strengthening medical education in haematology and blood transfusion: postgraduate programmes in Tanzania Br J Haematol 177 838–845 https://doi.org/10.1111/bjh.14644 PMCID: 5612387
6. Ahmad N, Kumari N, and Tirkey D, et al (2024) Evaluation of acute leukaemias by flow cytometry and its correlation with diagnosis using morphological and special staining techniques Cureus 16 e54126 https://doi.org/10.7759/CUREUS.54126 PMID: 38487155 PMCID: 10939159
7. Campbell WS, Hinrichs SH, and Lele SM, et al (2014) Whole slide imaging diagnostic concordance with light microscopy for breast needle biopsies Hum Pathol 45 1713–1721 https://doi.org/10.1016/j.humpath.2014.04.007 PMID: 24913758
8. Saco A, Ramírez J, and Rakislova N, et al (2016) Validation of whole-slide imaging for histolopathogical diagnosis: current state Pathobiology 83 89–98 [https://doi.org/10.1159/000442823] PMID: 27099935
9. Thorstenson S, Molin J, and Lundström C (2014) Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: digital pathology experiences 2006-2013 J Pathol Inform 5 14 https://doi.org/10.4103/2153-3539.129452
10. Kass L and Elias JM (1988) Cytochemistry and immunocytochemistry in bone marrow examination: contemporary techniques for the diagnosis of acute leukemia and myelodysplastic syndromes: a combined approach Hematol Oncol Clin North Am 2 537–555 https://doi.org/10.1016/S0889-8588(18)30583-5 PMID: 3065317
11. Pant S and Misra RK (2020) Role of immunohistochemistry in diagnosis and subtyping of acute leukemia using selected IHC markers in a resource limited setting
12. Rwezaula S, Yonazi M, and Panchal A, et al (2024) Challenges and outcomes of the first stem cell transplant program in Tanzania, East Africa Adv Hematol 2024 1–10 https://doi.org/10.1155/2024/1937419