Pattern of care and treatment outcomes of metastatic non-clear cell kidney cancer: a single centre experience from India
Somnath Roy1, Sreejata Raychaudhuri1, Bivas Biswas1, Deepak Dabkara1, Arnab Bhattacherjee1, Sandip Ganguly1, Joydeep Ghosh1, Yesha Sandipbhai Patel1, Souhita Pal1, Jagriti Karmakar1, Anindita Mitra1 and Sujoy Gupta2
1Department of Medical Oncology, Tata Medical Center, 14 MAR (EW), New Town, Rajarhat, Kolkata 700160, West Bengal, India
2Uro-Surgery, Tata Medical Center, 14 MAR (EW), New Town, Rajarhat, Kolkata 700160, West Bengal, India
Abstract
Background: Non-clear-cell renal cell carcinoma (nccRCC) refers to a rare diverse heterogeneous group of tumours; usually treated with immune check point inhibitors and or tyrosine kinase inhibitors (TKIs). Prospective large-scale data from Asian countries is limited.
Methods: This is a retrospective study of patients with metastatic nccRCC treated at Tata Medical Centre, Kolkata, India, from 2012 to 2022. Demographic profiles, histologic subtypes, treatment details, response to therapy (by response evaluation criteria in solid tumours (RECIST v1.1)) and survival status were captured from electronic medical records (EMRs) of hospitals up till May 2023. Kaplan Meier methods were estimated to assess progression-free survival (PFS) and overall survival (OS).
Results: A total of 89 consecutive patients were screened for this study, 24 were excluded due to inadequate data in EMR. 65 patients were included in the final analysis, with a median age at diagnosis of 59 years (range 20–84) of which 81% were male. Histologic subtypes comprised of 43% papillary, 31% clear cell with mixed histology, 3% sarcomatoid and 23% others including chromophobe, mucinous-tubular, spindle cell, oncocytic, medullary, poorly differentiated and rhabdoid). The most common site of metastasis was the lung 62% (n = 40) followed by non-regional nodes 32%, bone 26% and liver 14%. 15% patients presented with haematuria and 62% underwent nephrectomy prior to systemic therapy. The majority received pazopanib 46% (n = 30), chemotherapy 20% (n = 13) including bevacizumab plus erlotinib, sunitinib 15% (n = 10) or cabozantinib 14% (n = 9). Only 3(5%) patients received nivolumab plus cabozantinib combination. Response to treatment showed complete response in 1.5%, partial response in 20%, stable disease in 51% and progressive disease in 23% as per RECIST v1.1. 17 patients required dose reduction and interruption due to adverse effects and 33% (n = 22) received second-line therapy with nivolumab 18% (n = 4), axitinib and everolimus among others. After a median follow up of 44 months, the median PFS was 13 months (95%CI 7.2–18.9) and the median OS was 17 months (95%CI 12.1–22.1) for the entire cohort.
Conclusion: The overall response and survival for metastatic nccRCC was relatively better in comparison with published data, despite the limited number of patients treated with ICIs due to cost and access barriers.
Keywords: non-clear cell kidney cancer, demographic features, treatment response, outcomes
Correspondence to: Somnath Roy
Email: sroysskm1980@gmail.com
Published: 23/09/2024
Received: 04/03/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Renal cell carcinoma (RCC) is one of the United States’ ten most common cancers. In 2023, the American Cancer Society reported that 81,800 new patients were diagnosed with cancers of the kidney and renal pelvis; it is currently one of the most frequently diagnosed cancers in men [1]. The incidence of RCC in Asia is lower, however, possibly due to underreporting [2].
There are two major histologic subtypes: approximately 75% of RCC is clear cell, and the remaining 25% is non-clear cell [3]. Non-clear cell renal cell carcinoma (nccRCC) is a heterogeneous entity that is further divided into several subtypes, such as papillary renal cell carcinoma (pRCC), chromophobe renal cell carcinoma (chRCC), collecting duct carcinoma, renal medullary carcinoma, translocation-associated renal cell carcinoma and unclassified renal cell carcinoma. Sarcomatoid differentiation can occur in both major subtypes of RCC in variable proportions [4].
At least 17 different genes that dysregulate tumour cells’ ability to respond to changes in oxygen, iron, nutrients and energy levels are associated with RCC [5]. The management of RCC has rapidly evolved with the introduction of drugs that can target these alterations in oxygen metabolism and cellular proliferation [6, 7]. Despite this, treating RCC remains particularly challenging because most RCCs are clinically silent and are diagnosed at an advanced or metastatic stage [8]. Novel therapies that focus on different downstream products of the von Hippel-Lindau/hypoxia-inducible factor pathway, such as the vascular endothelial growth factor (VEGF), transforming growth factor-α and platelet-derived growth factor-β, have demonstrated benefits in RCC treatment [9, 10]. Even certain subtypes of nccRCC like sarcomatoid differentiation usually resistant to traditional chemotherapy [11].
In recent years, promising clinical trial data has shifted the standard of care for advanced/metastatic RCC toward immune checkpoint inhibitors (ICIs), mostly with clear cell histology [12, 13]. Additionally, for nccRCC, there is limited data on the efficacy of other targeted therapies used in RCC, such as tyrosine kinase inhibitors (TKIs) and inhibitors of mammalian target of rapamycin (mTOR), so potential effects must be extrapolated from trials of ccRCC [14–16]. Currently, the National Comprehensive Cancer Network’s 2024 guidelines for managing advanced/metastatic nccRCC recommend enrollment in clinical trials or treatment with TKIs, such as cabozantinib, sunitinib or ICIs [17]. Prospective large-scale data from India on the management of nccRCC is limited [18–20]. Hence, we aimed to study the demographic profile and treatment outcomes of metastatic nccRCC patients from a tertiary care cancer center in India.
Materials and methods
Study population
This is a single-center, retrospective, non-interventional study on patients with metastatic nccRCC treated from May 2012 to December 2022 at Tata Medical Center, Kolkata, India. Consecutive patients who met the eligibility criteria were enrolled and followed up to May 2023. All patients aged ≥18 years registered in the Department of Medical Oncology with a histopathological diagnosis of RCC (stage IV, as per the American Joint Committee of Cancer Staging 6th and 7th edition) and non-clear cell histology were considered eligible for this study. Patients with measurable disease according to the response evaluation criteria in solid tumours (RECIST v1.1) were included in this study [21]. Patients with pure clear-cell histology were excluded. The survival analysis excluded patients with inadequate medical records and those who did not receive treatment after their initial evaluation. Due to the study’s retrospective nature, the investigators received a waiver of consent from the Institutional Review Board as per institutional policy (IEC Protocol Waiver no-EC/WV/TMC/09/23).
Data collection and source
All data were obtained from hospital electronic medical records (EMRs). The demographic details included age, gender, presence of hematuria, sites of metastasis and Eastern Cooperative Oncology Group (ECOG) performance status. The histologic subtypes captured included papillary, sarcomatoid, clear cell with mixed histology and other variants. Treatment details, including for surgery (nephrectomy), first-line systemic therapy (including pazopanib, sunitinib, cabozantinib, chemotherapy plus minus bevacizumab and a combination of nivolumab plus cabozantinib) and second-line systemic therapy were retrieved. The response evaluation was performed using RECIST v1.1 criteria and dose modifications data were also captured. Dose reductions and dose interruptions are done according to standard guidelines based on the severity of adverse effects.
Statistical analysis
There was no formal sample size calculation, as it was a single-institution, non-interventional, single-arm retrospective chart review. Descriptive statistics, tables and charts were used to analyze the demographic, clinical and treatment-related variables. The Kaplan–Meier method was used to estimate progression-free survival (PFS) and overall survival (OS). Patients who received at least 1 week of therapy were evaluated for survival outcomes and the data were censored on 31 May 2023. Patients who were lost to follow-up were censored at the time of last contact or follow-up. PFS was calculated from the date of initiation of systemic treatment to the date of disease progression or death from any cause. OS was calculated from the date of diagnosis to the date of death from any cause. Patients who were lost to follow-up or had abandoned therapy were included in PFS and OS analyses, and outcomes for these patients were confirmed via telephone contact. Statistical analysis was performed using STATA/SE 13.0 (StataCorp, College Station, TX).
Results
A total of 89 consecutive patients were screened for this study. However, due to incomplete medical records in the EMR, 24 were excluded; as such, 65 patients were included in the final analysis. The median age at diagnosis was 59 years (range: 20–84) and 81% were male. The histologic subtypes comprised papillary (43%, n = 28), clear cell with mixed histology (31%, n = 20), sarcomatoid (3%, n = 2) and 23% (n = 15) other histologies, including chromophobe, mucinous-tubular, spindle cell, oncocytic, medullary, poorly differentiated and rhabdoid.
At the time of diagnosis, 15% of patients presented with hematuria. The most common site of metastasis was the lungs (62%, n = 40), followed by non-regional nodes (32%, n = 21), bones (26%, n = 17) and liver (14%, n = 9). The majority of patients (79%) had a good performance status of ECOG 0–1. A total of 62% (n = 40) underwent nephrectomy (either cytoreductive nephrectomy for localized disease or removal of a kidney tumor with palliative intent in a metastatic setting) before systemic therapy. The baseline demographics are shown in Table 1.
Table 1. Demographic variables.
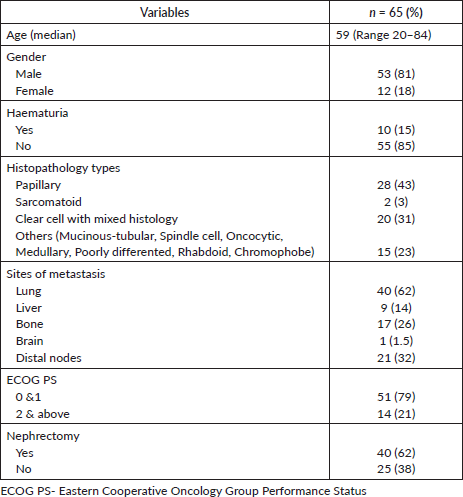
Table 2. Treatment details.
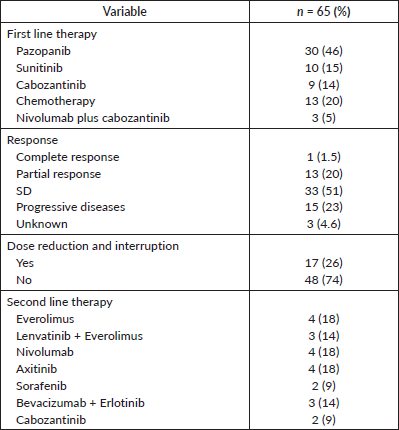
For first-line systemic therapy, the majority (46%, n = 30) received pazopanib, followed by chemotherapy (including bevacizumab) (20%, n = 13), sunitinib (15%, n = 10) and cabozantinib (14%, n = 9). Only 5% (n = 3) received nivolumab plus cabozantinib combination. For second-line therapy, 18% of patients received single-agent everolimus, nivolumab or axitinib and 14% of patients received combinations of either lenvatinib and everolimus or bevacizumab and erlotinib. Only 9% of patients were treated with either sorafenib or cabozantinib. One patient had a complete response to treatment, 20% (n = 13) had a partial response and 51% (n = 33), per RECIST v1.1 criteria, were classified as stable disease (SD). Seventeen patients (26%) required dose reduction and interruption due to adverse effects. Table 2 shows the treatment details and responses.
After a median follow-up at 44 months (95% CI, 39.2–54.3), the median PFS was 13 months (95% CI, 7.2–18.9) (Figure 1) and the median OS was 17 months (95% CI, 12.1–22.1). See Figure 2 for the entire cohort.
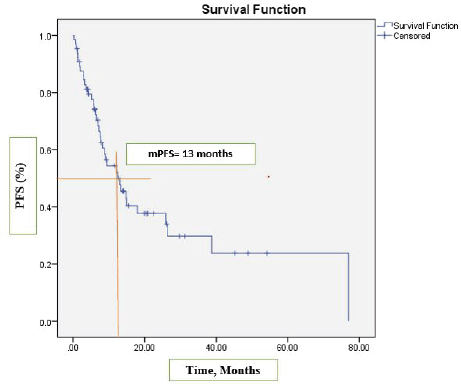
Figure 1. Kaplan-Meier curve for PFS.
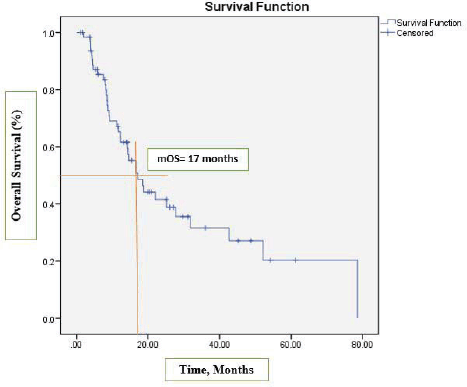
Figure 2. Kaplan-Meier curve for OS.
Discussion
Non-clear cell histology is a less common variant of kidney cancer, so the optimal systemic therapy for advanced or metastatic disease remains still unclear. Most treatment recommendations have been extrapolated from clinical trials done on clear cell subtypes. Given the paucity of data, this retrospective study explored the demographic profile and treatment outcomes of metastatic nccRCC from the Indian cohort.
The modern therapeutic landscape and success in clear cell subtypes began in the early 2000s with the development of TKIs and mTOR inhibitors. This prompted investigators to examine responses to these agents in non-clear subtypes. Sunitinib, a VEGF-receptor TKI, demonstrated promising therapeutic activity in a multicenter phase II trial of 31 nccRCC patients (71% pRCC and 10% chRCC). Sunitinib had an objective response rate (ORR) of 35%, a median PFS of 6.4 months and a 1-year OS of 65% [22]. Subsequently, two randomized phase II trials conducted by the European Society of Parenteral and Enteral Nutrition (ESPEN) and the American Society of Parenteral and Enteral Nutrition (ASPEN) compared sunitinib with everolimus, an mTOR inhibitor. The ESPEN’s study, which included 68 patients, was the first head-to-head comparison of these two drugs and did not demonstrate a statistically significant difference in median PFS (6.1 versus 4.1 months) or OS (16.2 versus 14.9 months) [23]. The ASPEN’s research, which analyzed a larger cohort of patients (n = 108), found that sunitinib significantly increased the median PFS when compared to everolimus (8.3 versus 5.6 months) but did not significantly improve OS [24]. The Central European Society for Anticancer Drug Research trial compared sunitinib (n = 10) to another mTOR inhibitor, temsirolimus (n = 12) and reported a median PFS of 13.2 and 9.3 months and an OS of 19.8 and 19.4 months for sunitinib and temsirolimus, respectively [25].
Compared to sunitinib and pazopanib, axitinib has a higher affinity with VEGFR-1, VEGFR-2 and VEGFR-3 [26]. In a phase II study of predominantly pRCC patients (66% pRCC and 10% chRCC), pazopanib demonstrated promising therapeutic value with an ORR of 28%, a median PFS of 16.5 months and a 1-year OS of 69% [27]. Another phase II study demonstrated that axitinib was better when used after failure with temsirolimus in papillary and chromophobe subtype, with an ORR of 37.5%, median PFS of 7.4 months and median OS of 12.1 months [28]. To date, no trials have prospectively compared the efficacy of pazopanib and axitinib head-to-head in nccRCC, but the similar ORR and PFS data across trials highlights the utility of next-generation VEGFR TKIs in the non-clear subtype.
ICIs have also been tested, mainly in pRCC variants with apparent clinical activity. In KEYNOTE-427, a phase II study, enrolled 165 patients with nccRCC (pRCC (71.5%), cRCC (12.7%) and unclassified (15.8%)) and each received pembrolizumab 200 mg every 3weeks for ≤24 months with an ORR was 28.8% for pRCC, 9.5% for chromophobe [29]. In another phase, IIIb/IV CheckMate-374 trial of 44 patients with predominantly pRCC (n = 24); flat dose nivolumab 240 mg showed an ORR of 13% with a median OS of 16 months [30]. In phase II; savolitinib was combined with durvalumab with a response rate of 29% and median PFS of 4.9 months with a higher response with mesenchymal epithelial transition (MET) alteration in the papillary subtype [31, 32]. In another phase II trial of 47 patients with non-clear cell histology; nivolumab and cabozantinib showed an ORR of 47% with a median duration of response of 13.6 months and a PFS rate of 52% at 1 year [33]. The results of recent prospective clinical trials (S1500, PAPMET) suggest that cabozantinib should be the current first-line treatment for untreated pRCC [34]. In Keynote-B61, lenvatinib plus pembrolizumab showed promising results in non-clear cell subtypes [35].
Prospective data on the treatment of metastatic nccRCC from India is limited. Ramaswamy et al [18] conducted a registry-based retrospective study of 212 patients with metastatic RCC. In the study, 67.9% of patients had conventional clear-cell RCC and 32% had non-clear cell variants. TKIs (sunitinib, sorafenib and pazopanib) and mTOR inhibitors (everolimus and temsirolimus) were primarily used as the first-line treatment, with a median PFS of 7.09 months and median OS of 12.87 months for the entire cohort [18]. However, the study mainly included ccRCC cases, thus emphasizing the importance of our research, which focused solely on non-clear cell variants. The authors have acknowledged that the accessibility and affordability of such expensive targeted therapies limit the treatment of patients in resource-poor settings like India. Another retrospective study from a tertiary care hospital in Eastern India analyzed demographic and survival data for 81 patients with RCC [19]. The median OS for stage III was 52.9 months as compared with 18.7 months for metastatic RCC [19]. Agnihotri et al [20] observed 617 patients with renal tumors at a tertiary care center in Northern India; 67.7% had clear-cell subtype and 4.7% had metastatic disease. Younger patients had a higher proportion of non-clear cell RCC; only 48.6% had conventional ccRCC. Patients <39 years of age had a lower survival rate compared to those >70 years of age (5-year OS was 53% versus 80%, respectively) [20].
Our study’s strengths include the fact that to our knowledge, this is the first study focusing on 65 patients with non-clear cell RCC in a real-world Indian setting. The lack of proper treatment guidelines and paucity of large-scale prospective data; represent an unmet need that we attempted to address in this study with the best available resources. The survival analyses show encouraging results, which are better than published data despite financial and access barriers. The limitations of the study include the relatively small sample size and its retrospective nature. In addition, chemotherapy was given to some patients which is not the current standard. ICIs were not being able to offer due to availability and high cost. We acknowledge that we were unable to report toxicity data also.
Conclusion
The overall response and survival of metastatic nccRCC were relatively better in this Indian cohort compared to previously published data, despite the limited number of patients treated with ICIs due to access barriers. However, gathering large-scale prospective data with proper treatment guidelines from the Indian context is an urgent need.
Conflicts of interest
SR – received a PI grant in a clinical trial from AstraZeneca which was paid to the institution.
SG – received PI grant in a clinical trial from Novartis, AstraZeneca and Jhonson and Jhonson which was paid to the institution.
JG – received PI grant in a clinical trial from AstraZeneca which was paid to the institution.
BB – received PI grant in a clinical trial from Novartis, Pfizer, Roche, AstraZeneca, Jhonson and Jhonson which was paid to the institution.
AB – received PI grant in a clinical trial from AstraZeneca, Roche which was paid to the institution.
Other authors – ‘no conflicts of interest to declare.’
Funding
No funding sources for this study.
IRB approval
Taken (IEC Protocol Waiver no- EC/WV/TMC/09/23).
References
1. Siegel RL, Miller KD, and Wagle NS, et al (2023) Cancer statistics, 2023 CA Cancer J Clin 73(1) 17–48 https://doi.org/10.3322/caac.21763 PMID: 36633525
2. Pallagani L, Choudhary GR, and Himanshu P, et al (2021) Epidemiology and clinicopathological profile of renal cell carcinoma: a review from tertiary care referral centre J Kidney Cancer VHL 8(1) 1–6 https://doi.org/10.15586/jkcvhl.v8i1.154 PMID: 33552876 PMCID: 7827726
3. Cohen HT and McGovern FJ (2005) Renal-cell carcinoma N Engl J Med 353(23) 2477–2490 https://doi.org/10.1056/NEJMra043172 PMID: 16339096
4. Lobo J, Ohashi R, and Amin MB, et al (2022) WHO 2022 landscape of papillary and chromophobe renal cell carcinoma Histopathology 81(4) 426–438 https://doi.org/10.1111/his.14700 PMID: 35596618
5. Linehan WM, Schmidt LS, and Crooks DR, et al (2019) The metabolic basis of kidney cancer Cancer Discov 9(8) 1006–1021 https://doi.org/10.1158/2159-8290.CD-18-1354 PMID: 31088840
6. Pfaffenroth EC and Linehan WM (2008) Genetic basis for kidney cancer: opportunity for disease-specific approaches to therapy Expert Opin Biol Ther 8(6) 779–790 https://doi.org/10.1517/14712598.8.6.779 PMID: 18476789 PMCID: 2768039
7. Patel HV, Srivastava A, and Srinivasan R (2021) A challenging frontier – the genomics and therapeutics of nonclear cell renal cell carcinoma Curr Opin Oncol 33(3) 212–220 https://doi.org/10.1097/CCO.0000000000000721 PMID: 33818540 PMCID: 8244822
8. Zisman A, Pantuck AJ, and Wieder J, et al (2002) Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma J Clin Oncol 20(23) 4559–4566 https://doi.org/10.1200/JCO.2002.05.111 PMID: 12454113
9. Escudier B, Eisen T, and Stadler WM, et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma N Engl J Med 356(2) 125–134 https://doi.org/10.1056/NEJMoa060655 PMID: 17215530
10. Nelson EC, Evans CP, and Lara PN Jr (2007) Renal cell carcinoma: current status and emerging therapies Cancer Treat Rev 33(3) 299–313 https://doi.org/10.1016/j.ctrv.2006.12.005 PMID: 17329029
11. Diamond E, Molina AM, and Carbonaro M, et al (2015) Cytotoxic chemotherapy in the treatment of advanced renal cell carcinoma in the era of targeted therapy Crit Rev Oncol Hematol 96(3) 518–526 https://doi.org/10.1016/j.critrevonc.2015.08.007 PMID: 26321263
12. Motzer RJ, Escudier B, and McDermott DF, et al (2015) CheckMate 025 investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma N Engl J Med 373(19) 1803–1813 https://doi.org/10.1056/NEJMoa1510665 PMID: 26406148 PMCID: 5719487
13. Motzer RJ, Tannir NM, and McDermott DF, et al (2018) CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma N Engl J Med 378(14) 1277–1290 https://doi.org/10.1056/NEJMoa1712126 PMID: 29562145 PMCID: 5972549
14. Palma Dos Reis AF, Simão D, and Odeny T, et al (2022) A systematic review of immune checkpoint inhibitors in non-clear-cell renal cancer Kidney Cancer 6(2) 115–127 https://doi.org/10.3233/KCA-210012 PMID: 36212797 PMCID: 9490428
15. Motzer RJ, Hutson TE, and Tomczak P, et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma N Engl J Med 356(2) 115–124 https://doi.org/10.1056/NEJMoa065044 PMID: 17215529
16. Hudes G, Carducci M, and Tomczak P, et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma N Engl J Med 356(22) 2271–2281 https://doi.org/10.1056/NEJMoa066838 PMID: 17538086
17. [https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf]
18. Ramaswamy A, Joshi A, and Noronha V, et al (2017) Patterns of care and clinical outcomes in patients with metastatic renal cell carcinoma-results from a tertiary cancer center in India Clin Genitourin Cancer 15(3) e345–e355 https://doi.org/10.1016/j.clgc.2016.09.006 PMID: 28077238
19. Ray RP, Mahapatra RS, and Khullar S, et al (2016) Clinical characteristics of renal cell carcinoma: five years review from a tertiary hospital in Eastern India Indian J Cancer 53(1) 114–117 https://doi.org/10.4103/0019-509X.180851 PMID: 27146757
20. Agnihotri S, Kumar J, and Jain M, et al (2014) Renal cell carcinoma in India demonstrates early age of onset & a late stage of presentation Indian J Med Res 140(5) 624–629
21. Oken MM, Creech RH, and Tormey DC, et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group Am J Clin Oncol 5(6) 649–655 https://doi.org/10.1097/00000421-198212000-00014 PMID: 7165009
22. Lee JL, Ahn JH, and Lim HY, et al (2012) Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma Ann Oncol 23(8) 2108–2114 https://doi.org/10.1093/annonc/mdr586 PMID: 22228449
23. Tannir NM, Jonasch E, and Albiges L, et al (2016) Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial Eur Urol 69(5) 866–874 https://doi.org/10.1016/j.eururo.2015.10.049 PMCID: 4879109
24. Armstrong AJ, Halabi S, and Eisen T, et al (2016) Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial Lancet Oncol 17(3) 378–388 https://doi.org/10.1016/S1470-2045(15)00515-X PMID: 26794930 PMCID: 6863151
25. Bergmann L, Grünwald V, and Maute L, et al (2020) A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society Oncol Res Treat 43(7-8) 333–339 https://doi.org/10.1159/000508450
26. Bukowski RM (2012) Third generation tyrosine kinase inhibitors and their development in advanced renal cell carcinoma Front Oncol 2 13 https://doi.org/10.3389/fonc.2012.00013 PMID: 22655261 PMCID: 3356077
27. Jung KS, Lee SJ, and Park SH, et al (2018) Pazopanib for the treatment of non-clear cell renal cell carcinoma: a single-arm, open-label, multicenter, phase II study Cancer Res Treat 50(2) 488–494 https://doi.org/10.4143/crt.2016.584 PMCID: 5912146
28. Park I, Lee SH, and Lee JL (2018) A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus Clin Genitourin Cancer 16(5) e997–e1002 https://doi.org/10.1016/j.clgc.2018.05.011 PMID: 29903415
29. McDermott DF, Lee JL, and Ziobro M, et al (2021) Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma J Clin Oncol 39(9) 1029–1039 https://doi.org/10.1200/JCO.20.02365 PMID: 33529058 PMCID: 8078262
30. Vogelzang NJ, Olsen MR, and McFarlane JJ, et al (2020) Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase IIIb/IV CheckMate 374 study Clin Genitourin Cancer 18(6) 461–468.e3 https://doi.org/10.1016/j.clgc.2020.05.006 PMID: 32718906
31. Suárez C, Larkin JMG, and Patel P, et al (2023) Phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO) J Clin Oncol 41(14) 2493–2502 https://doi.org/10.1200/JCO.22.01414 PMID: 36809050
32. Erratum: phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO) J Clin Oncol 41(23) 3961 PMID: 37315260
33. Lee CH, Voss MH, and Carlo MI, et al (2022) Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates J Clin Oncol 40(21) 2333–2341 https://doi.org/10.1200/JCO.21.01944 PMID: 35298296 PMCID: 9287282
34. Maughan BL and Sirohi D (2023) Papillary renal cell carcinoma: a review of prospective clinical trials Curr Treat Options Oncol 24(9) 1199–1212 https://doi.org/10.1007/s11864-023-01107-x PMID: 37407886
35. Albiges L, Gurney H, and Atduev V, et al (2023) Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial Lancet Oncol 24(8) 881–891 https://doi.org/10.1016/S1470-2045(23)00276-0 PMID: 37451291






