Breast cancer burden in eastern Sudan: seven-year retrospective study
Ahmed Balla M Ahmed1, Salma Alrawa1, Ahmed A Yeddi1, Esraa S A Alfadul1, Hind Mohi Aldin Abd Allah2 and Muhannad Bushra Masaad Ahmed³
1Faculty of Medicine, University of Khartoum, Khartoum 11111, Sudan
2East Oncology Center, Ministry of Health, Gadarif 11111, Sudan
3Faculty of Medicine and Health Sciences, University of Gadarif, Gadarif 11111, Sudan
Abstract
Background: Breast cancer (BC) is prevalent in Sudan, yet data on its epidemiology in Eastern Sudan is limited. This study aims to provide insights into the demographic and clinicopathologic features of BC patients treated at the East Oncology Centre (EOC) in Gadarif State, Eastern Sudan. Furthermore, we aim to identify the factors that contribute to a late-stage diagnosis.
Methods: This cross-sectional study included patients diagnosed with BC and treated in the EOC between 2016 and 2022. Data obtained from medical records were analysed using R software, with descriptive statistics and multiple logistic regressions applied to determine determinants of advanced-stage presentation. A p‐value < 0.05 was considered statistically significant.
Results: Among the 394 patients studied, the majority were women (96%), married (66%) and from rural areas (43%). The peak years for BC diagnoses were 2018 and 2022, with a median age at diagnosis of 48 years. A family history of cancer was reported by 20% of patients. Clinical stages were distributed as follows: I (1.6%), II (17%), III (50%) and IV (32%). Twenty-five percent tested positive for human epidermal growth factor receptor 2, while 73% tested negative and 43% had triple-negative BC. Modified radical mastectomy was performed in 47% of patients, with 21% undergoing breast-conserving surgery. Treatment rates were 38% for radiotherapy, 84% for chemotherapy and 46% for hormonal therapy. Higher grade BC and lower education levels were associated with advanced-stage presentation, while a family history of cancer reduced the risk of advanced-stage disease (OR: 0.38, 95% CI: 0.18–0.78).
Conclusion: The study found that females in East Sudan often present at a young age and advanced stage, with a significant prevalence of triple-negative BC. Notably, family cancer history exhibited a protective effect against advanced-stage presentation, while grade 3 cancer was positively associated with advanced disease.
Keywords: breast cancer, burden, oestrogen receptor, Sudan
Correspondence to: Ahmed Balla M Ahmed
Email: m.salahballa@gmail.com
Published: 21/05/2024
Received: 12/01/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Globally, breast cancer (BC) emerged as the most frequently diagnosed malignancy in women, with an estimated 2.3 million new cases (11.7% of total cases) in the year 2020 [1].
BC is a complex disease with diverse clinical and pathological characteristics, showing significant variations in its presentation, histologic type, molecular biomarkers, prognosis and treatment outcomes [2]. The stage at which the disease is diagnosed strongly influences overall survival and mortality rates. Approximately 54% of women are diagnosed in stage II, while only 16% are diagnosed in stage I [3].
In Africa, BC accounts for 186,598 new cases, comprising approximately 17% of the total cancer cases in the region based on GLOBOCAN 2020 estimates [4]. Although it is less prevalent in Africa compared to Western industrialised nations, the mortality rates are alarmingly high [5]. The stage at which the disease is diagnosed strongly influences survival and mortality rates [3]. There is a concern that most African women with BC present at later stages and lack hormonal receptor expression [6].
Cancer estimates in Sudan rely mainly on data from hospital case series since there is no official population-based cancer registry in the country [6, 7]. Studies have shown that BC is the most common cancer among Sudanese women [8, 9], similar to other sub-Saharan African nations. These studies have also found that Sudanese women, like their counterparts in developed countries, tend to be diagnosed with BC at a younger age, at advanced stages, and with higher tumour grades [3, 7, 10]. A recent study from central Sudan revealed that patients' education level and duration from identification of BC symptoms to diagnosis significantly impact the stage at presentation time [10].
Little information is available about BC in the Gadarif Estate, Eastern Sudan. Therefore, the present study aimed to provide baseline information about the demographic features and tumour characteristics and investigate the associations between demographic variables and presentation stage in BC patients attending the East Oncology Centre (EOC), the main cancer treatment centre in this region.
Methods
This is a facility-based cross-sectional study implemented using medical records of patients with BC treated at the EOC between 2016 and 2022. Cases were required to have histologically confirmed BC for inclusion in the study. Reviewing patients' medical files retrieved demographic, clinical and pathologic data.
Study setting
The EOC was established in 2016 and is located in Gadarif City, the capital of Gadarif State, Eastern Sudan. The cancer treatment modalities available at the EOC include chemotherapy, hormonal therapy and palliative care. All patients with BC are reviewed at weekly BC multidisciplinary team meetings at the EOC in collaboration with the surgery department at Gadarif Teaching Hospital.
Data collection method
All patients with BC who had been treated at the EOC during the study period were identified. A structured form was used to collect data from patients' medical files. These data included sociodemographic data (e.g., age, gender, education status, residence, family history of cancer, height, weight, marital status, age at menarche, age at menopause, age at first birth and parity); clinical characteristics (presenting complaints, stage at diagnosis according to the American Joint Committee on Cancer 2009 system and background medical history); pathologic features (histopathologic subtype and grade); molecular biomarkers (expression of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status) and types of treatment.
Ethical consideration
This study’s ethical approval was obtained from the ethics committee, Ministry of Health, Gadarif State, on 1 September 2023, under approval number RC.Q3.1.9.23. All files were anonymized and assigned an ID number.
Data management and analysis
The data were transferred into an Excel spreadsheet and then imported into R software version 4.2.2. The normality of the data was checked by examining histograms. Descriptive statistics were used to analyse the frequencies and percentages for categorical variables, as well as the median and interquartile range for continuous variables. The study focused on assessing the burden of BC at EOC and examining the BC-related characteristics of female patients. Multiple logistic regression was utilised to identify the factors associated with advanced stage BC. The goodness-of-fit of the logistic model was evaluated using Hosmer and Lemeshow's test. A significance level of < 0.05 was considered for this research.
Results
Sociodemographic characteristics
The study included 394 BC patients, primarily females (96%), housewives (81%), married (66%), illiterate (30%) and residing in rural Gadarif (43%) Table 1. In 2018, there were 77 cases of BC admissions, accounting for 19.5% of the patient cohort. There was a subsequent resurgence in cases in 2022, following a decline. The highest incidences of BC compared to other malignancies were observed in 2016 (25.6%) and 2018 (18.7%) Figure 1. Among female patients, the median age at menarche, menopause, childbirth and diagnosis was 14, 45, 20 and 48 years, respectively. The parity distribution showed that 42% had 1–3 children, 39% had 4–7 children and 19% had 8 or more children. Additionally, 20% had a family history of cancer Table 2.
Clinical and pathological characteristics of BC and treatment modalities of the participants
The distribution of body mass index (BMI) showed that 12% were classified as underweight, 45% had a healthy weight, 29% were overweight and 14% were obese. There were 121 cases with missing BMI data. BC staging revealed that 1.6% were in stage I, 17% in stage II, 50% in stage III and 32% in stage IV. The tumour grade distribution showed that 8.1% were in grade one, 46% were in grade two and 46% were in grade three. Twenty-five percent were Her2 positive, while 73% were Her2 negative and 43% had triple-negative BC. Surgery types included: 47% who underwent modified radical mastectomy and 21% opted for breast conservative surgery. In terms of treatment, 38% received radiotherapy, 84% underwent chemotherapy and 46% received hormonal therapy. Palliative treatment was administered to 40% of participants Table 2.
The associations between demographic variables and clinical stage
The study found that grade three BC significantly increased the risk of advanced stage compared to grade one (OR: 3.78, 95% CI: 1.30–10.9). Secondary school (OR: 0.28, 95% CI: 0.10–0.70) reduced the risk of advanced stage compared to illiteracy. Additionally, a family history of cancer (OR: 0.38, 95% CI: 0.18–0.78) lowered the risk of advanced-stage BC Table 3.
Discussion
Sudan had only two healthcare facilities that provided cancer care until 2008, when the government started to establish other cancer centres [11]. Between 2008 and 2021, 12 cancer centres were established; among them, 2 were in Eastern Sudan, and both provided medical oncology services only. They were established in 2015 and started to function in 2016 [11]. The aim of this study was to assess sociodemographic characteristics, clinical characteristics and utilisation of cancer services in the EOC in Sudan.
Table 1. Demographic characteristics of the participants.
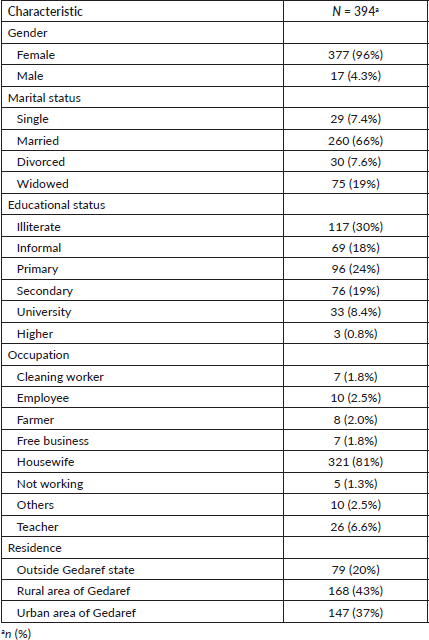
Between 2016 and 2022, the EOC served 394 BC patients. The centre started with 32 patients in 2016, and this increased to 77 patients in 2018. The number of newly diagnosed patients was the lowest in 2020 and 2021. This can be attributed to the COVID-19 infection, which discouraged patients from seeking immediate medical care, as noted in other African countries [12]. It is worth noting that 4.3% of BC cases occur in males. This is higher than the 1% commonly reported in the literature [13–15]. It is also higher than the 2.2% reported in central Sudan [10]. The cause of this higher percentage should be studied.
The median age of diagnosing BC in Eastern Sudan is less than 50 years, similar to what has been reported from previous studies in Sudan [7], Arab countries [16] and Sub-Saharan Africa [17]. In the UK and USA, the majority of BC patients are diagnosed after 50 years [18, 19]. The difference in age at diagnosis is explained by the young population structure and overall short life expectancy in low-income countries [20]. The effect of genes, environment and lifestyle on this phenomenon is yet to be investigated. The average age at first childbirth is 20 years less than 29.4 years in Europe [21] and similar to the 19 years reported in lower income countries [22]. This was a couple with high parity, as almost a fifth of the patients had eight children or more. Parity is known as a protective factor against BC [23, 24]. Increased parity reduces the risk of BC but does not eliminate it; in fact, it may be associated with a higher risk of certain subtypes of cancer, like HER-2 enriched BC, as reported by Fortner et al [25]. Almost half of the patients were obese. A previous study in Eastern Sudan showed a higher prevalence of obesity compared to the Northern State and other African countries [26]. Obesity is associated with worse outcomes in BC [27, 28]. The high prevalence of obesity among patients with BC in Eastern Sudan necessitates the inclusion of lifestyle modification in management plans to improve outcomes [29].
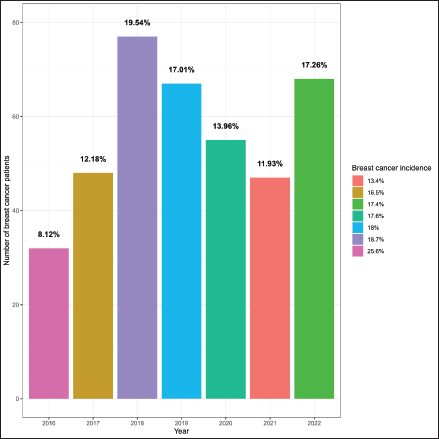
Figure 1. Burden of breast cancer at East Oncology Center from 2016–2022.
Table 2. BC-related characteristics of female patients.
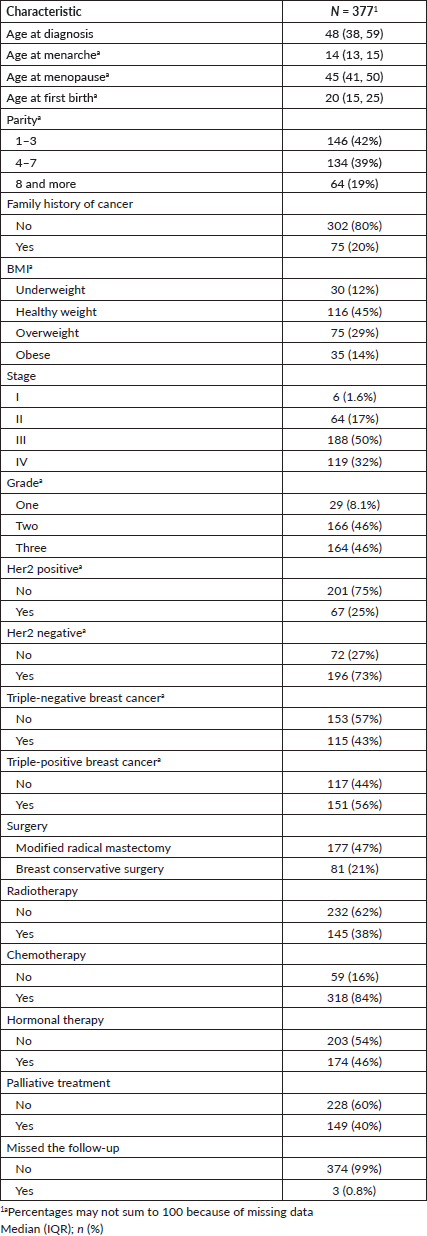
Table 3. Predictors of advanced stage of BC patients in Eastern Sudan.
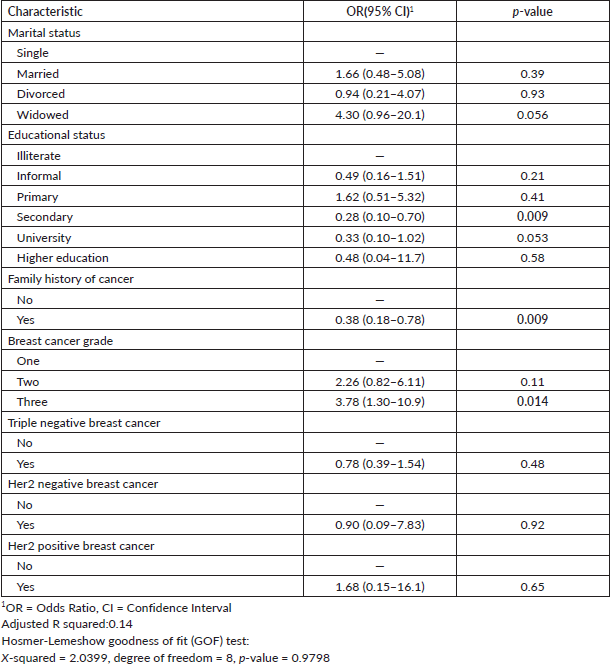
More than 80% of participants presented at stage III or IV. The high percentage of females who present at late stages is slightly higher than the 60.7% and 65.3% reported in central Sudan [7, 30]. It is similar to the 74.7% of patients who are diagnosed at late stages in Sub-Saharan Africa [31] and contradictory to high-income countries where more than 70% of patients are diagnosed at stage I or II [32]. This late stage of presentation directly contributes to the high mortality of BC in low- and middle-income countries [33, 34]. BC screening via mammography is the most effective strategy to detect it early, but it can be economically challenging for low- and middle-income countries [35]. A recent systematic review showed that accurate clinical BC examination can be used as an alternative in low- and middle-income countries, but high-quality studies are needed to support it [35]. Almost half of the participants presented with grade 3 BC. A recent study demonstrated the prognostic value of grade, which can be used as an alternative to molecular methods in low- and middle-income countries [36]. In this study, grade 3 cancer was associated with an advanced stage of BC. Almost half of the patients had triple-negative BC. Triple-negative BC is common among black females, especially those who are younger than 40 years [37]. This is concerning given the aggressive nature and lack of targeted therapy for this cancer [38]. Interestingly, triple-negative BC was not significantly associated with advanced stages in our population.
The use of modified radical mastectomy is more common than breast conservative surgery for a large number of patients with advanced stage at presentation. Palliative treatment is needed by more than a third of patients, and more than a third of patients need radiotherapy. These patients are referred to other centres that offer radiotherapy in Wad Madani, Khartoum or Merowe, as radiotherapy is not available at EOC.
In this study, 20% of the patients had a family history of cancer, aligning with findings from a study of 6,706 women suggesting that familial or genetically predisposed BCs comprise approximately 15%–20% of cases [39]. Additionally, a family history of BC was negatively associated with the advanced stage of BC. Family history is a major risk factor for BC [40] and females with family history tend to present early, probably because they are more likely to be educated about BC [41]. We should build on this positive attitude and activate BC screening, especially for high-risk females. Future studies may focus on assessing these females for BRCA mutations and studying the effect of family history on ER, PR and HER2 status. Similarly, females with secondary school education tend to present at an earlier stage, emphasising the importance of education, which is highlighted in the literature [42–44].
This study described the clinicopathological characteristics of BC among patients receiving treatment at the EOC, which serves most of the population in Eastern Sudan. It adds to the knowledge of BC profiles in low- and middle-income countries like Sudan. The main limitation of this study is the limited and missing data caused by incomplete medical records in the centre. In the absence of a national cancer registry, the medical records of oncology centres carry extra value and should receive more care.
Conclusion
The EOC covered 394 cancer patients in Eastern Sudan. Males constitute 4.3% of BC patients in the centre. Females tend to present at a young age and at an advanced stage. Almost half of the females were triple negative. A family history of BC was negatively associated with an advanced stage of BC. Grade 3 was positively associated with an advanced stage of BC.
Acknowledgments
The authors would like to express our gratitude to the Statistics Office at the EOC for their support and help in collecting data and organising files. This work would not have been possible without their contributions.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
The authors declare that this study was conducted without receiving any funding or financial support.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Am Cancer Soc 71(3) 209–249
2. World Cancer Research Fund International Breast Cancer Statistics (London: World Cancer Research Fund International) [https://www.wcrf.org/cancer-trends/breast-cancer-statistics/]
3. Sengal AT, Haj-Mukhtar NS, and Elhaj AM, et al (2017) Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; hospitals based case series BMC Cancer 17(1) 804 https://doi.org/10.1186/s12885-017-3805-4 PMID: 29191181 PMCID: 5710067
4. World Health Organization (2020) International Agency for Research on Cancer; Africa (Geneva: World Health Organization)
5. Ahmed HG, Ali AS, and Almobarak AO (2010) Frequency of breast cancer among Sudanese patients with breast palpable lumps Indian J Cancer 47(1) 23–26 https://doi.org/10.4103/0019-509X.58854 PMID: 20071785
6. Maki HAH (2022) General oncology care in Sudan Cancer in the Arab World eds HO Al-Shamsi, IH Abu-Gheida, and F Iqbal F, et al (Singapore: Springer Singapore) pp 251–264 https://doi.org/10.1007/978-981-16-7945-2_16
7. Muddather HF, Elhassan MMA, and Faggad A (2021) Survival outcome of breast cancer in Sudanese women: a hospital-based study JCO Glob Oncol 7 324–332 https://doi.org/10.1200/GO.20.00538 PMID: 33617296 PMCID: 8081542
8. Ali Elhassan MM (2018) Personal perespective: access to treatment for gynaecological malignancies in Sudan South Afr J Gynaecol Oncol 10(2) 21–23
9. Saeed IE, Weng HY, and Mohamed KH, et al (2014) Cancer incidence in Khartoum, Sudan: first results from the cancer registry, 2009-2010 Cancer Medi 3(4) 1075–1084 https://doi.org/10.1002/cam4.254
10. Eltayeb MA, Faggad A, and Abbadi OS, et al (2020) Characteristics of breast cancer at first presentation in Sudanese patients attending the National Cancer Institute-University of Gezira (NCI-UG) Arch Breast Cancer 7(3) 104–110 https://doi.org/10.32768/abc.202073104-110
11. Al-Ibraheem A, Abdlkadir AS, and Mohamedkhair A, et al (2022) Cancer diagnosis in areas of conflict Front Oncol 12 1087476 https://doi.org/10.3389/fonc.2022.1087476
12. Martei YM, Rick TJ, and Fadelu T, et al (2021) Impact of COVID-19 on cancer care delivery in Africa: a cross-sectional survey of oncology providers in Africa JCO Glob Oncol 7 368–377 https://doi.org/10.1200/GO.20.00569 PMID: 33689484 PMCID: 8081536
13. Konduri S, Singh M, and Bobustuc G, et al (2020) Epidemiology of male breast cancer Breast (Edinburgh, Scotland) 54 8–14 https://doi.org/10.1016/j.breast.2020.08.010 PMID: 32866903 PMCID: 7476060
14. Miao H, Verkooijen HM, and Chia KS, et al (2011) Incidence and outcome of male breast cancer: an international population-based study J Clin Oncol 29(33) 4381–4386 https://doi.org/10.1200/JCO.2011.36.8902 PMID: 21969512
15. Wang F, Shu X, and Meszoely I, et al (2019) Overall mortality after diagnosis of breast cancer in men vs women JAMA Oncol 5(11) 1589–1596 https://doi.org/10.1001/jamaoncol.2019.2803 PMID: 31536134 PMCID: 6753503
16. Najjar H and Easson A (2010) Age at diagnosis of breast cancer in Arab nations Int J Surg 8(6) 448–452 https://doi.org/10.1016/j.ijsu.2010.05.012 PMID: 20601253
17. Joko-Fru WY, Miranda-Filho A, and Soerjomataram I, et al (2020) Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: a population-based registry study Int J Cancer 146(5) 1208–1218 https://doi.org/10.1002/ijc.32406
18. National Cancer Registeration and Analysis service (NCRAS) Breast Cancer in the Elderly-NCIN Data Briefing (England: NCRAS) [http://www.ncin.org.uk/publications/data_briefings/breast_cancer_elderly]
19. American Cancer Society Key Statistics for Breast Cancer (Atlanta: American Cancer Society) [https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html]
20. Mariani-Costantini R, Elhassan MMA, and Aceto GM, et al (2017) Epidemiology, pathology, management and open challenges of breast cancer in Central Sudan: a prototypical limited resource African setting Breast Cancer – From Biology to Medicine ed P Van Pham (Vietnam: InTech) https://doi.org/10.5772/67175
21. eurostat (2021) Women in the EU are having their first child later [https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210224-1]
22. Negash WD and Asmamaw DB (2022) Time to first birth and its predictors among reproductive age women in high fertility countries in Sub-Saharan Africa: inverse Weibull gamma shared frailty model BMC Pregnancy Childb 22(1) 844 https://doi.org/10.1186/s12884-022-05206-9
23. Nindrea RD, Aryandono T, and Lazuardi L (2017) Breast cancer risk from modifiable and non-modifiable risk factors among women in Southeast Asia: a meta-analysis Asian Pac J Cancer Prev: APJCP 18(12) 3201–3206 PMID: 29281867 PMCID: 5980871
24. Li C, Fan Z, and Lin X, et al (2021) Parity and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis Cancer Epidemiol 75 102050 https://doi.org/10.1016/j.canep.2021.102050 PMID: 34706325
25. Fortner RT, Sisti J, and Chai B, et al (2019) Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses’ Health Studies Breast Cancer Res 21(1) 40 https://doi.org/10.1186/s13058-019-1119-y PMID: 30867002 PMCID: 6416887
26. Omar SM, Taha Z, and Hassan AA, et al (2020) Prevalence and factors associated with overweight and central obesity among adults in the Eastern Sudan PLoS One 15(4) e0232624 https://doi.org/10.1371/journal.pone.0232624 PMID: 32353069 PMCID: 7192465
27. Lee K, Kruper L, and Dieli-Conwright CM, et al (2019) The impact of obesity on breast cancer diagnosis and treatment Curr Oncol Rep 21(5) 41 https://doi.org/10.1007/s11912-019-0787-1 PMID: 30919143 PMCID: 6437123
28. Engin A (2017) Obesity-associated breast cancer: analysis of risk factors Adv Exp Med Biol 960 571–606 https://doi.org/10.1007/978-3-319-48382-5_25 PMID: 28585217
29. Hamer J and Warner E (2017) Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health CMAJ 189(7) E268–e274 https://doi.org/10.1503/cmaj.160464 PMID: 28246240 PMCID: 5318212
30. Elgaili EM, Abuidris DO, and Rahman M, et al (2010) Breast cancer burden in central Sudan Int J Women's Health 2 77–82
31. Jedy-Agba E, McCormack V, and Adebamowo C, et al (2016) Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis Lancet Glob Health 4(12) e923–e935 https://doi.org/10.1016/S2214-109X(16)30259-5 PMID: 27855871 PMCID: 5708541
32. Unger-Saldaña K (2014) Challenges to the early diagnosis and treatment of breast cancer in developing countries World J Clin Oncol 5(3) 465–77 https://doi.org/10.5306/wjco.v5.i3.465 PMID: 25114860 PMCID: 4127616
33. Alkabban FM and Ferguson T (2023) Breast Cancer [Updated 2022 Sep 26] (Treasure Island: StatPearls Publishing) [Internet] [https://www.ncbi.nlm.nih.gov/books/NBK482286/]
34. Manson EN and Achel DG (2023) Fighting breast cancer in low-and-middle-income countries – what must we do to get every woman screened on regular basis? Sci Afr 21 e01848
35. Coleman C (2017) Early detection and screening for breast cancer Semin Oncol Nurs 33(2) 141–155 https://doi.org/10.1016/j.soncn.2017.02.009 PMID: 28365057
36. Rakha EA, Reis-Filho JS, and Baehner F, et al (2010) Breast cancer prognostic classification in the molecular era: the role of histological grade Breast Cancer Res 12(4) 207 https://doi.org/10.1186/bcr2607 PMID: 20804570 PMCID: 2949637
37. American Cancer Society Triple-Negative Breast Cancer (Atlanta: American Cancer Society) [https://www.cancer.org/cancer/types/breast-cancer/about/types-of-breast-cancer/triple-negative.html]
38. Aysola K, Desai A, and Welch C, et al (2013) Triple Negative breast cancer – an overview Hereditary Genet Curr Res 2013(Suppl 2) 001
39. Haber G, Ahmed NU, and Pekovic V (2012) Family history of cancer and its association with breast cancer risk perception and repeat mammography Am J Public Health 102(12) 2322–2329 https://doi.org/10.2105/AJPH.2012.300786 PMID: 23078489 PMCID: 3519312
40. Braithwaite D, Miglioretti DL, and Zhu W (2018) Family history and breast cancer risk among older women in the breast cancer surveillance consortium cohort JAMA Intern Med 178(4) 494–501 https://doi.org/10.1001/jamainternmed.2017.8642 PMID: 29435563 PMCID: 5876845
41. Liu L, Hao X, and Song Z, et al (2021) Correlation between family history and characteristics of breast cancer Sci Rep 11(1) 6360 https://doi.org/10.1038/s41598-021-85899-8 PMID: 33737705 PMCID: 7973811
42. de Almeida RJ, de Moraes Luizaga CT, and Eluf-Neto J, et al (2022) Impact of educational level and travel burden on breast cancer stage at diagnosis in the state of Sao Paulo, Brazil Sci Rep 12(1) 8357 https://doi.org/10.1038/s41598-022-12487-9 PMID: 35589860 PMCID: 9120502
43. Liu Y, Zhang J, and Huang R, et al (2017) Influence of occupation and education level on breast cancer stage at diagnosis, and treatment options in China: a nationwide, multicenter 10-year epidemiological study Medicine 96(15) e6641 https://doi.org/10.1097/MD.0000000000006641 PMID: 28403116 PMCID: 5403113
44. Mathew A, George PS, and Ramadas K, et al (2019) Sociodemographic factors and stage of cancer at diagnosis: a population-based study in South India JCO Glob Oncol 5 1–10






