Haematologic biomarkers and survival in gallbladder cancer: a systematic review and meta-analysis
Rogelio N Velasco, Jr1,2, Harold Nathan C Tan3 and Michael D San Juan4
1Clinical Trial and Research Division, Philippine Heart Center, Quezon City 0850, Philippines
2Lung Center of the Philippines, Quezon City 1101, Philippines
3Section of Medical Oncology, Makati Medical Center, Makati City 1229, Philippines
4Division of Medical Oncology, Philippine General Hospital, Manila 1000, Philippines
Abstract
Background: Gallbladder cancer is a rare malignancy characterised by poor survival with lack of durable response to treatment. Thus, novel biomarkers are needed to prognosticate patients. This systematic review and meta-analysis sought to examine the role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet count (PC) and serum immune inflammation index in predicting the survival of patients with gallbladder cancer.
Materials and methods: A systematic search was done using PubMed, Cochrane, ClinicalTrials.gov and Google Scholar for articles published from inception until 8 February 2022. Hazard ratios (HR) with 95% confidence intervals (CI) were pooled and subgroup analyses were conducted according to treatment, region and cut-offs. The primary outcome of interest was overall survival (OS). Data were summarised using RevMan version 5.4.
Results: Twenty studies comprising 5,183 patients were included in the analysis. High neutrophil-lymphocyte ratio (HR 1.72, 95% CI 1.47–2.02), platelet-lymphocyte ratio (HR 1.51, 95% CI 1.33–1.72), monocyte-lymphocyte ratio (HR 1.96, 95% CI 1.46–1.64), PC (HR 1.20, 95% CI 1.02–1.40) and serum inflammation index (HR 1.73, 95% CI 1.36–2.18) were all associated with worse survival. The association was consistent across most subgroups on race and cut-offs with a trend towards poor survival for PC above 252.5.
Conclusion: High neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, monocyte-lymphocyte ratio, PC and SII are associated with worse OS in gallbladder cancer and are potential biomarkers for prognostication. Prospective studies are recommended to further evaluate their use.
Keywords: gallbladder neoplasms, inflammation, survival
Correspondence to: Rogelio N Velasco
Email: rogervelascojr@gmail.com and revlasco@up.edu.ph
Published: 30/01/2024
Received: 25/09/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Gallbladder cancer is the sixth most common malignancy of the gastrointestinal tract and the most common biliary tract malignancy accounting for almost 80% of all biliary tract cancers [1]. It is characterised by a dismal survival outcome with poor response to current treatment options such as surgery and systemic treatment [2, 3]. The majority of the patients are diagnosed at an advanced stage or with distant metastasis upon presentation due to the lack of symptoms. The 5-year survival rate for gallbladder cancer patients with distant metastasis is dismal at only 2.7% [4]. Thus, new and effective clinical biomarkers are needed to predict outcomes to optimise treatment outcomes.
Inflammation is a known hallmark of cancer development and progression. Within the tumour microenvironment, cytokines, chemokines and other molecules from both malignant and host cells facilitate invasion, angiogenesis and spread. Systemic inflammation likewise involves cytokines, inflammatory proteins and immune cells. Both the local and systemic inflammation involve cross signalling and plays a crucial role in cancer biology. Agents have thus been developed targeting the inflammatory process such as anti-angiogenic agents such as bevacizumab, anti-CTL4A antibodies such as ipilimumab and antiinterleukins [5].
Tumour-derived proteins have the capacity to increase myelopoieses. This increase myelopoieses can result in tumour angiogenesis, invasion and distant spread. Neutrophils and its precursors–myelocytes and promyelocytes–may also be increased in cancer-related bone marrow dysfunction. Monocytes which reside in tissue have been shown to be associated with increased tumour stage among colon cancer patients. The neutrophil-to-lymphocyte ratio (NLR) has been utilised as a negative prognostic marker in various types of malignancies such as prostate cancer and colon cancer [7, 8]. The platelet-to-lymphocyte (PLR) ratio has also been utilised in prognosticating malignancies such as non-small cell lung cancer [9]. The monocyte-lymphocyte-ratio (MLR) has been explored as a marker of poor prognosis on cervical and colorectal cancer [10, 11].
Platelets produce platelet-derived endothelial cell growth factor, which may contribute to the induction of mitosis and angiogenesis [14]. An elevated platelet count (PC) has also been shown to be a poor prognostic factor among patients with pancreatic cancer, colorectal cancer and gallbladder cancer [12–14]. The systemic immune-inflammation index (SII), an index of platelets, neutrophils and lymphocytes, has been reported to be prognostic in several cancers such as prostate cancer, colorectal cancer and pancreatic cancer [15–17].
NLR, PLR and MLR as prognosticators in biliary tract cancer have been explored in various studies, albeit with conflicting results [4, 18–20]. The prognostic utility of PC and SII likewise have not been explored in a meta-analysis. Due to the conflicting results among different studies and the absence of a consensus on their prognostic role, we performed a systematic review and meta-analysis to examine the predictive role of NLR, PLR, MLR, PC and SII on the overall survival (OS) of patients with gallbladder cancer. These indices can be used in predicting high-risk gallbladder cancer for which more aggressive treatment and monitoring may be considered.
Methods
Search strategies
A systematic literature search was independently conducted by two investigators (Harold Tan and Rogelio Velasco) using the PubMed, Web of Science, Google Scholar and Cochrane Library databases from inception until 8 February 2022, to obtain relevant articles. Studies were retrieved using the following search terms: (‘neutrophil-to-lymphocyte ratio’ OR ‘neutrophil-lymphocyte ratio’ OR ‘NLR’ OR ‘platelet-to-lymphocyte ratio’ OR ‘platelet-lymphocyte ratio’ OR ‘PLR’ OR ‘monocyte-to-lymphocyte ratio’ OR ‘monocyte-lymphocyte ratio’ OR ‘MLR’ OR ‘platelet count’ OR ‘PC’ OR ‘systemic immune inflammation index’ OR ‘SII’) AND (‘gallbladder cancer’ OR ‘gallbladder carcinoma’). The references of each candidate article were also searched to identify other studies that can be included in the analysis. The full search strategy is shown in Figure 1.
Selection criteria
Two independent authors screened the possible articles for inclusion if they met the following criteria: (1) full-text journal articles written in English involving human subjects with histopathologically confirmed gallbladder cancer; (2) articles with data on NLR, PLR, LMR, MLR, PC or SII with corresponding cut-off values; (3) studies with reported associations between the haematologic biomarkers and prognosis expressed as hazard ratios (HR) and 95% confidence intervals (95% CI) as measures of association. Articles were excluded if they fulfilled any of the following: (1) studies with incomplete data to calculate HRs and 95% CI; and (2) case reports, review articles, conference abstracts, expert opinions and commentaries. For articles with multiple publications, only the latest and most comprehensive publication was considered. Furthermore, authors of articles with incomplete data were contacted by the investigators.
Data extraction and quality assessment
The risk of bias for each study was assessed using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (NOS) [21] by two independent authors (Rogelio Velasco and Harold Tan) and all disagreements were settled in consensus with a third independent author (Michael San Juan). Briefly, the NOS includes eight items, classified into three domains: selection of study participants, comparability of cohorts and ascertainment of outcome. Scores were defined as high quality (>7), moderate quality [5–7] or low quality (<5). The following were obtained from each study: first author, geographic region, year of publication, total number of patients, study design, tumour stage, treatment given, cut-off used, follow-up data and the outcome of Cox regression analysis using HRs and 95% CI obtained from univariate or multivariate analysis, the latter of which was preferred. NLR, PLR and MLR values were defined as the ratio of the absolute neutrophil count and the absolute lymphocyte count (NLR), the ratio of the absolute PC and the absolute lymphocyte count (PLR), and the ratio of the absolute monocyte count and the absolute lymphocyte count (MLR) in the peripheral blood. SII was defined as the absolute PC multiplied by the NLR. The primary outcome assessed was OS, characterised as the time from histopathologic diagnosis of gallbladder cancer to death from any cause.
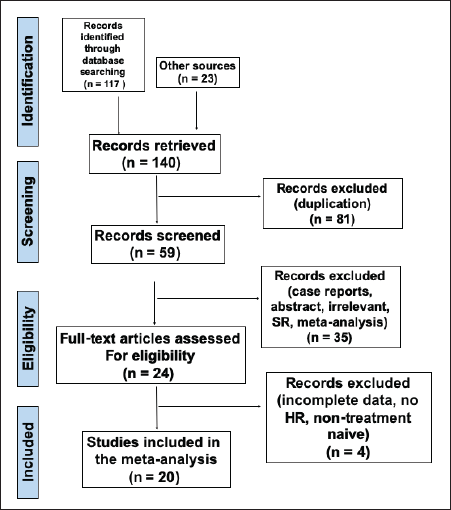
Figure 1. PRISMA flow diagram of the included studies in the meta-analysis.
Statistical analysis
HR and 95% CI were extracted from the included studies and combined using the generic inverse variance method using Review Manager 5.4. A HR of more than one indicated worse OS above the biomarker cut-off, while a HR of less than one denoted improved survival below the biomarker cut-off. Heterogeneity was determined using the Higgins Ι2 statistic and Cochran’s Q. A fixed-effects model was used to determine pooled HR when I2 is less than 50% or p is more than 0.10. Otherwise, we employed the random-effects model [22]. When I2 was more than or equal to 50%, subgroup analyses were analysed to determine possible sources of heterogeneity. Subgroup analyses were performed according to the median cut-off used and geographical region (Asia versus other regions). For analyses that included ten or more studies, publication bias was evaluated using a funnel plot.
The present systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions.
Results
A total of 140 articles were gathered from electronic databases based on the specified search strategy. Upon removal of articles not related to gallbladder cancer, a total of 24 articles were obtained. Records unrelated to prognostication and those with incomplete data were likewise excluded. Full-text articles were reviewed based on the aforementioned inclusion and exclusion criteria. A total of 20 articles were then included in the final analysis (Figure 1).
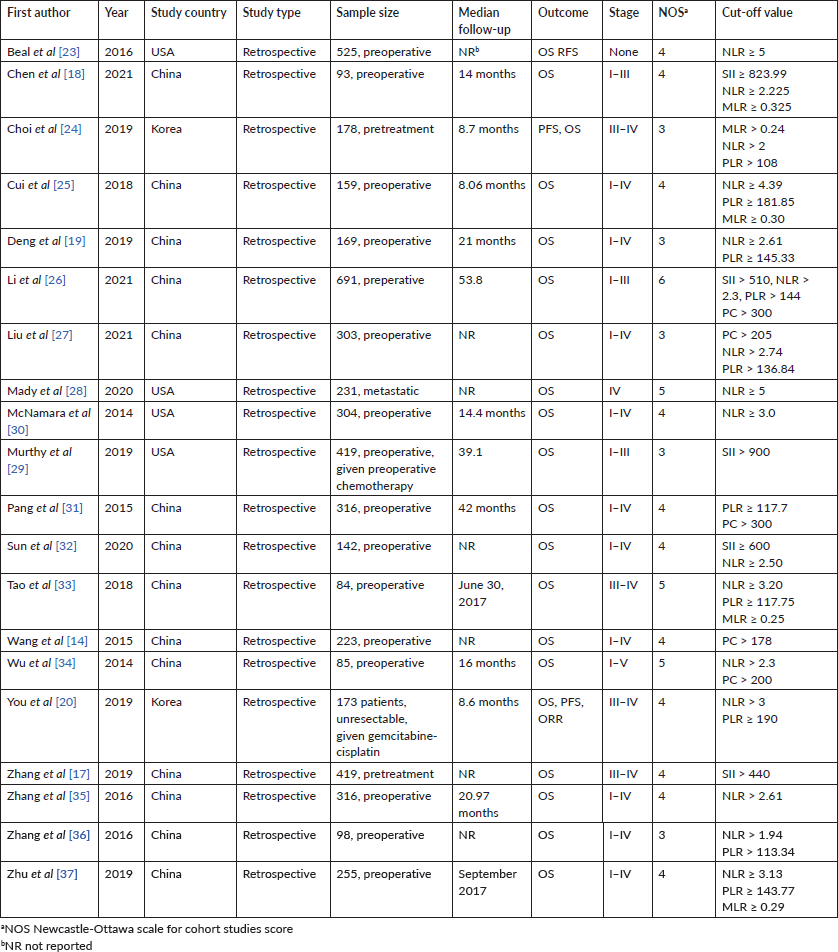
The included studies comprised 5,183 patients, with sample sizes ranging from 93 to 691 (Table 1). Studies were published from 2014 to 2021 and were conducted predominantly in China (n = 14) with the rest of the studies from Korea (n = 2), and the USA (n = 4). Treatment received varied between studies with diverse cut-offs, and treatment rendered (i.e., surgery only, chemotherapy only or a combination approach). Based on the Newcastle-Ottawa Scale for Cohort Studies, the majority of the studies were of low to moderate quality (Table 1).
Haematologic biomarkers and OS
Neutrophil-lymphocyte ratio
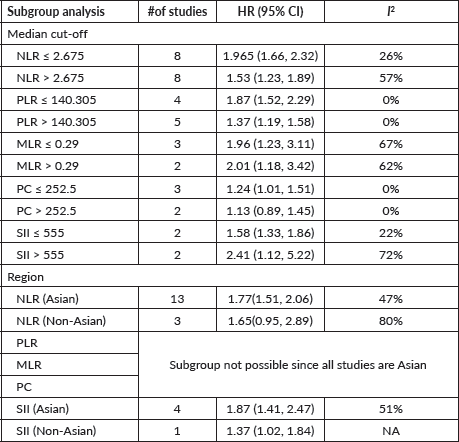
Sixteen studies were included encompassing 3,806 patients were included in the analysis. Figure 2 shows the association of elevated NLR with worse OS (HR 1.72, 95% CI 1.47–2.02, p < 0.00001) with high heterogeneity (I2 = 59%). Subgroup analyses performed according to the geographic region still showed worse OS on both the Asian (HR 1.77, 95% CI 1.51–2.06) and non-Asian subset (HR 1.65, 95% CI 0.95–2.89). Using the median cut-off value of 2.675, there was worse OS on both subgroups (NLR ≤ 2.675: HR 1.965, 95% CI 1.66, 2.32; NLR > 2.675: HR 1.53, 95% CI 1.23, 1.89). The heterogeneity was lower on the cut-off value less than the median, indicating cut-offs as a cause of heterogeneity. A sensitivity analysis excluding all studies with poor quality still confirmed its prognostic use (Supplementary Table 1 and Supplementary Figure 1).
Table 1. Characteristics of the included studies in the meta-analysis.
To account for publication bias, a funnel plot was constructed showing no evidence for publication bias in the relationship between NLR and OS (Figure 3).
Platelet-lymphocyte ratio
Nine studies with a total of 2,171 patients were included in the analysis. Elevated PLR was associated with poor OS (HR 1.51, 95% CI 1.33–1.72, p < 0.00001) (Figure 4). A subgroup analysis using the median cut-off value of 140.305 still showed poorer OS with a higher PLR (PLR ≤ 140.305: HR 1.87, 95% CI 1.52, 2.29; PLR > 140.305: 1.37, 95% CI 1.19, 1.58). Notably, there was no heterogeneity among studies using the cut-off below and above the median. A sensitivity analysis excluding all studies with poor quality still confirmed its prognostic use (Supplementary Table 1 and Supplementary Figure 2).
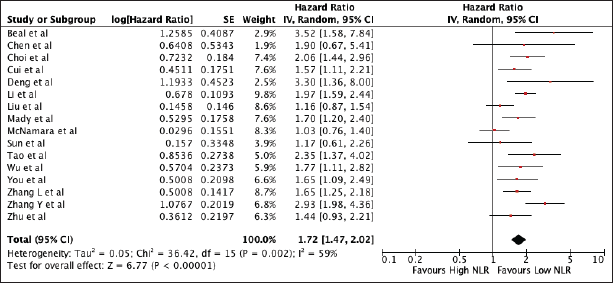
Figure 2. Forest plot of studies exploring the relationship between NLR and OS.
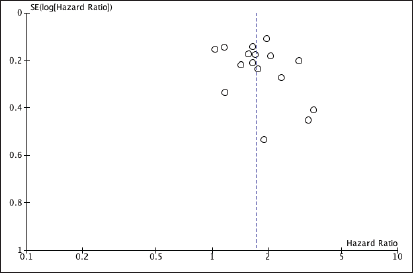
Figure 3. Funnel plot investigating publication bias in studies involving NLR.
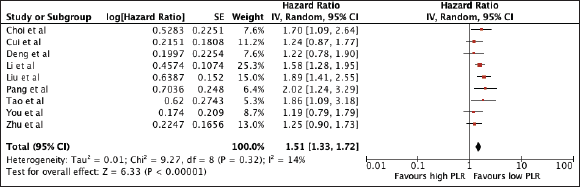
Figure 4. Forest plot of studies exploring the relationship between PLR and OS.
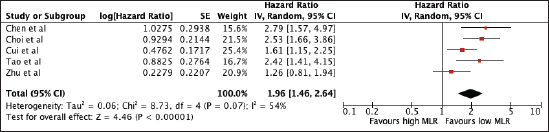
Figure 5. Forest plot of studies exploring the relationship between MLR and OS.
Monocyte-lymphocyte ratio
769 patients from five studies were included in the analysis of MLR and OS. A high NLR was associated with poor OS (HR 1.96, 95% CI 1.46–2.64, p < 0.00001) with significant heterogeneity (I2 = 54%) (Figure 5). The prognostic utility of MLR was consistent among studies using the median cut-off of 0.29 (MLR ≤ 0.29: 1.96, 95% CI 1.23, 3.11; MLR > 0.29: 2.01, 95% CI 1.18, 3.42). Subgroup analysis was not possible since all studies are Asian. A sensitivity analysis excluding all studies with poor quality still confirmed its prognostic use (Supplementary Table 1 and Supplementary Figure 3).
Platelet count
1,618 patients from five studies were included in the analysis of MLR and OS. Figure 6 shows the association of elevated NLR with worse OS (HR 1.20, 95% CI 1.02–1.40, p = 0.02) with no heterogeneity. The prognostic significance of increased PC and poorer OS was consistent among studies utilising a cut-off below the median 252.5 × 109 (PC ≤ 252.5 × 109: 1.24, 95% CI 1.01, 1.51) and a trend towards poor survival among studies with cut-offs >252.5 × 109 (HR 1.13, 95% CI 0.89, 1.45). A sensitivity analysis excluding all studies with poor quality still confirmed its prognostic use (Supplementary Table 1 and Supplementary Figure 4).
Serum immune-inflammation index
1,764 patients from four studies were included in the analysis of MLR and OS. The pooled analysis showed the association between elevated NLR with worse OS (HR 1.73, 95% CI 1.36–2.18, p < 0.00001) (Figure 7). A subgroup analysis among Asian studies showed consistent prognostic use of SII and a trend towards poor survival among non-Asian studies (Asian: 1.77, 95% CI 1.51, 2.06; non-Asian: 1.65, 95% CI 0.95, 2.89). Using the median cut-off value of 555, there was poor survival with increased SII regardless of the cut-off value (SII ≤ 555: 1.58, 95% CI 1.33, 1.86; SII > 555: 2.41, 95% CI 1.12, 5.22). A sensitivity analysis excluding all studies with poor quality still confirmed its prognostic use (Supplementary Table 1 and Supplementary Figure 5).
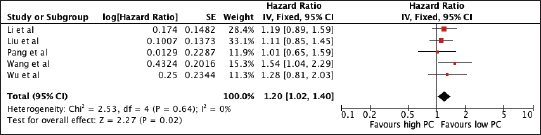
Figure 6. Forest plot of studies exploring the relationship between PC and OS.
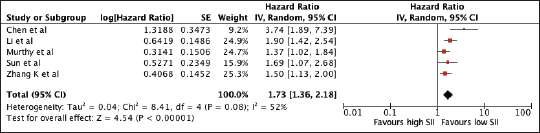
Figure 7. Forest plot of studies exploring the relationship between SII and OS.
Discussion
This systematic review meta-analysis investigated the prognostic significance of haematologic indices in gallbladder cancer. Our results show that among the 20 studies included in the analysis, NLR, PLR, MLR, PC and SII are all associated with poor OS and can potentially be used as prognostic indices in gallbladder cancer. To our knowledge, this is the first meta-analysis evaluating the prognostic significance of pretreatment PC and SII on gallbladder cancer. In addition, we updated the meta-analysis by Xu et al [38] on NLR, PLR and MLR.
The process of inflammation elicits both pro- and anti-inflammatory responses through the release of mediators. Neutrophils, key sources of cytokines, are associated with tumour progression [39, 40]. Platelets have also been shown to be potent sources of cytokines that can bind various growth factors such as vascular endothelial growth factor and fibroblast growth, both of which are key players in tumour angiogenesis, proliferation and metastasis [41–43]. Monocytes have been shown to secrete various pro-inflammatory cytokines which have been shown to adversely affect prognosis in cancer [12, 44]. Lymphocytes, most notably tumour-infiltrating lymphocytes play a crucial role in the antitumoural response of the host. Thus, these indices provided by these haematologic components may shed light on the host-tumour response [45–47].
Our results are consistent with the previous meta-analysis showing the prognostic value of NLR on resected gallbladder cancer by Saqib et al [49] and the prognostic role of NLR, PLR and MLR among gallbladder cancer patients in the meta-analysis conducted by Xu et al [38]. We obtained lower I2 values when Asian studies were analysed separately, which can partly explain the ethnicity and cut-off values as sources of heterogeneity. Worldwide, there is variation in the mortality rates for gallbladder cancer, with Asian countries such as Japan, Korea and Thailand among the top countries with high mortality rates [50, 51]. We included four studies conducted in the USA, which has 2–3 times lower mortality rates compared to other countries [52]. In addition to the differences in tumour biology based on ethnicity, there are differences in mean NLR among different countries [53, 54].
Differences in cut-off values also contributed to the heterogeneity among the pooled results. Thus, we utilised the median cut-off values in our subgroups and performed subgroup analyses on all biomarkers in contrast to the meta-analysis by Xu et al [38]. Using the median cut-offs for NLR and PLR decreased the heterogeneity. Subgroup analyses among studies with SII cut-off below 555 decreased the heterogeneity as well. Since the majority of the studies enrolled patients across stages I to IV, it was not possible to perform a subgroup analysis based on stage. This may also have affected the heterogeneity observed in the analysis of NLR, MLR and SII.
We here present the inherent limitations of our study. One of the major limitations and source of heterogeneity was the difference in cut-off values and different assays used in the determination of the peripheral blood counts. Due to the rarity of this disease, few studies were retrieved for inclusion in the study. In addition, it must be noted that most studies retrieved were derived from the Asian population limiting the generalisability of the results. The potential effect of differences between the populations studied such as age, sex and disease stage were also not investigated due to the majority of the studies investigating mixed populations. This meta-analysis primarily reviewed observational studies; hence, reporting bias which may have affected the results. The majority of the studies included did not enrol a control arm.
The results of the present study show the association of NLR, PLR and MLR with worse survival. These markers derived from the peripheral blood count are widely accessible, objective and with minimal cost. Moreover, these are promising prognosticators in gallbladder cancer, a disease characterised by poor prognosis, which may further guide treatment management. Since these tests have not been incorporated into routine practice, further prospective studies may validate their use in prognostication and treatment.
Conclusion
NLR, PLR, MLR, PC and SII are promising haematologic biomarkers for worse survival in gallbladder cancer which can be used in prognostication and treatment guidance. Prospective studies are recommended to further evaluate their use.
List of abbreviations
MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PC, platelet count; PLR, platelet-to-lymphocyte ratio; SII, serum immune-inflammation index.
Conflicts of interest
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Funding
No funding was received for this study.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contributions
All the authors contributed equally to the research project and the final approved manuscript.
RNV: Conceptualisation, methodology, validation, original draft, writing – review and editing and final approval of manuscript.
HNT: Conceptualisation, methodology, validation, original draft, writing – review and editing and final approval of manuscript.
MSJ: Conceptualisation, methodology, validation, original draft, writing – review and editing and final approval of manuscript.
References
1. Hundal R and Shaffer EA (2014) Gallbladder cancer: epidemiology and outcome Clin Epidemiol 6 99–109 PMID: 24634588 PMCID: 3952897
2. Won HS, Lee MA, and Chung ES, et al (2010) Comparison of thymidine phosphorylase expression and prognostic factors in gallbladder and bile duct cancer BMC Cancer [Internet] 10(1) 564 Date accessed: 25/02/22 https://doi.org/10.1186/1471-2407-10-564 PMID: 20955617 PMCID: 2974735
3. Hezel AF, Deshpande V, and Zhu AX (2010) Genetics of biliary tract cancers and emerging targeted therapies J Clin Oncol 28(21) 3531–3540 https://doi.org/10.1200/JCO.2009.27.4787 PMID: 20547994 PMCID: 2982782
4. Zhu X, Zhang X, and Hu X, et al (2020) Survival analysis of patients with primary gallbladder cancer from 2010 to 2015: a retrospective study based on SEER data Medicine (Baltimore) 99(40) e22292 https://doi.org/10.1097/MD.0000000000022292 PMID: 33019404 PMCID: 7535694
5. Diakos CI, Charles KA, and McMillan DC, et al (2014) Cancer-related inflammation and treatment effectiveness Lancet Oncol 15(11) e493–e503 https://doi.org/10.1016/S1470-2045(14)70263-3 PMID: 25281468
6. Stone RL, Nick AM, and McNeish IA, et al (2012) Paraneoplastic thrombocytosis in ovarian cancer N Engl J Med 366(7) 610–618 https://doi.org/10.1056/NEJMoa1110352 PMID: 22335738 PMCID: 3296780
7. Uemura K, Kawahara T, and Yamashita D, et al (2017) Neutrophil-to-lymphocyte ratio predicts prognosis in castration-resistant prostate cancer patients who received cabazitaxel chemotherapy BioMed Res Int 2017 7538647 https://doi.org/10.1155/2017/7538647 PMID: 28948170 PMCID: 5602619
8. Mazaki J, Katsumata K, and Kasahara K, et al (2020) Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis BMC Cancer [Internet] 20(1) 922 Date accessed: 26/02/22 https://doi.org/10.1186/s12885-020-07429-5 PMID: 32977767 PMCID: 7519490
9. Diem S, Schmid S, and Krapf M, et al (2017) Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab Lung Cancer Amst Neth 111 176–181 https://doi.org/10.1016/j.lungcan.2017.07.024
10. Wang L, Si H, and Wang J, et al (2020) Blood cell parameters as prognostic predictors of disease development for patients with advanced non-small cell lung cancer Oncol Lett 20(2) 1101–1110 https://doi.org/10.3892/ol.2020.11655 PMID: 32724349 PMCID: 7377095
11. Li Y-X, Chang J-Y, and He M-Y, et al (2021) Neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) predict clinical outcome in patients with stage IIB cervical cancer J Oncol 2021 2939162 https://doi.org/10.1155/2021/2939162 PMID: 34539781 PMCID: 8443385
12. Brown KM, Domin C, and Aranha GV, et al (2005) Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas Am J Surg 189(3) 278–282 https://doi.org/10.1016/j.amjsurg.2004.11.014 PMID: 15792750
13. Sasaki K, Kawai K, and Tsuno NH, et al (2012) Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer World J Surg 36(1) 192–200 https://doi.org/10.1007/s00268-011-1329-7
14. Wang R-T, Zhang L-Q, and Mu Y-P, et al (2015) Prognostic significance of preoperative platelet count in patients with gallbladder cancer World J Gastroenterol [Internet] 21(17) 5303–5310 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4419071/] Date accessed: 26/02/22 https://doi.org/10.3748/wjg.v21.i17.5303 PMID: 25954104 PMCID: 4419071
15. Lolli C, Caffo O, and Scarpi E, et al (2016) Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone Front Pharmacol [Internet] 7 376 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5062111/] Date accessed: 26/02/22 https://doi.org/10.3389/fphar.2016.00376 PMID: 27790145 PMCID: 5062111
16. Chen J-H, Zhai E-T, and Yuan Y-J, et al (2017) Systemic immune-inflammation index for predicting prognosis of colorectal cancer World J Gastroenterol [Internet] 23(34) 6261–6272 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5603492/] Date accessed: 26/02/22 https://doi.org/10.3748/wjg.v23.i34.6261 PMID: 28974892 PMCID: 5603492
17. Zhang K, Hua Y-Q, and Wang D, et al (2019) Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer J Transl Med 17(1) 30 https://doi.org/10.1186/s12967-019-1782-x PMID: 30658662 PMCID: 6339361
18. Chen H, Huang Z, and Sun B, et al (2021) The predictive value of systemic immune inflammation index for postoperative survival of gallbladder carcinoma patients J Surg Oncol 124(1) 59–66 https://doi.org/10.1002/jso.26470 PMID: 33765331
19. Deng Y, Xu M-F, and Zhang F, et al (2020) Prognostic value of preoperative lymphocyte-to-monocyte ratio in gallbladder carcinoma patients and the establishment of a prognostic nomogram Medicine (Baltimore) 99(31) e21021 https://doi.org/10.1097/MD.0000000000021021 PMID: 32756087 PMCID: 7402783
20. You MS, Ryu JK, and Choi YH, et al (2019) Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin BMC Cancer 19(1) 10 https://doi.org/10.1186/s12885-018-5211-y PMID: 30611225 PMCID: 6321682
21. Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses Eur J Epidemiol 25(9) 603–605 https://doi.org/10.1007/s10654-010-9491-z PMID: 20652370
22. Tufanaru C, Munn Z, and Stephenson M, et al (2015) Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness Int J Evid Based Healthcare 13(3) 196–207 PMID: 26355603 https://doi.org/10.1097/XEB.0000000000000065
23. Beal EW, Wei L, and Ethun CG, et al (2016) Elevated NLR in gallbladder cancer and cholangiocarcinoma - making bad cancers even worse: results from the US Extrahepatic Biliary Malignancy Consortium HPB 18(11) 950–957 https://doi.org/10.1016/j.hpb.2016.08.006 PMID: 27683047 PMCID: 5094484
24. Choi YH, Lee JW, and Lee SH, et al (2019) A high monocyte-to-lymphocyte ratio predicts poor prognosis in patients with advanced gallbladder cancer receiving chemotherapy Cancer Epidemiol Biomark Prev 28(6) 1045–1051 https://doi.org/10.1158/1055-9965.EPI-18-1066
25. Cui X, Zhu S, and Tao Z, et al (2018) Long-term outcomes and prognostic markers in gallbladder cancer Medicine (Baltimore) 97(28) e11396 https://doi.org/10.1097/MD.0000000000011396 PMID: 29995783 PMCID: 6076111
26. Li L, Ren T, and Liu K, et al (2021) Development and validation of a prognostic nomogram based on the systemic immune-inflammation index for resectable gallbladder cancer to predict survival and chemotherapy benefit Front Oncol 11 692647 https://doi.org/10.3389/fonc.2021.692647 PMID: 34268122 PMCID: 8276054
27. Liu F, Hu HJ, and Ma WJ, et al (2019) Prognostic significance of neutrophil-lymphocyte ratio and carbohydrate antigen 19-9 in patients with gallbladder carcinoma Medicine (Baltimore) 98(8) e14550 https://doi.org/10.1097/MD.0000000000014550 PMID: 30813165 PMCID: 6407978
28. Mady M, Prasai K, and Tella SH, et al (2020) Neutrophil to lymphocyte ratio as a prognostic marker in metastatic gallbladder cancer HPB 22(10) 1490–1495 https://doi.org/10.1016/j.hpb.2020.02.002 PMID: 32122786
29. Murthy P, Zenati MS, and Al Abbas AI, et al (2020) Prognostic value of the systemic immune-inflammation index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer Ann Surg Oncol 27(3) 898–906 https://doi.org/10.1245/s10434-019-08094-0
30. McNamara MG, Templeton AJ, and Maganti M, et al (2014) Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer Eur J Cancer Oxf Engl 1990 50(9) 1581–1589
31. Pang Q, Zhang L-Q, and Wang R-T, et al (2015) Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma World J Gastroenterol 21(21) 6675–6683 https://doi.org/10.3748/wjg.v21.i21.6675 PMID: 26074706 PMCID: 4458778
32. Sun L, Jin Y, and Hu W, et al (2020) The impacts of systemic immune-inflammation index on clinical outcomes in gallbladder carcinoma Front Oncol 10 554521 https://doi.org/10.3389/fonc.2020.554521 PMID: 33194617 PMCID: 7645045
33. Tao Z, Li SX, and Cui X, et al (2018) The prognostic value of preoperative inflammatory indexes in gallbladder carcinoma with hepatic involvement Cancer Biomark 22(3) 551–557 https://doi.org/10.3233/CBM-181230 PMID: 29865040
34. Wu X-S, Shi L-B, and Li M-L, et al (2014) Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma Ann Surg Oncol 21(2) 449–457 https://doi.org/10.1245/s10434-013-3292-z
35. Zhang L, Wang R, and Chen W, et al (2016) Prognostic significance of neutrophil to lymphocyte ratio in patients with gallbladder carcinoma HPB [Internet] 18(7) 600–607 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4925805/] Date accessed: 26/02/22 https://doi.org/10.1016/j.hpb.2016.03.608 PMID: 27346141 PMCID: 4925805
36. Zhang Y, Ma C, and Wang M, et al (2017) Prognostic significance of immune cells in the tumor microenvironment and peripheral blood of gallbladder carcinoma patients Clin Transl Oncol 19(4) 477–488 https://doi.org/10.1007/s12094-016-1553-6
37. Zhu S, Yang J, and Cui X, et al (2019) Preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as predictors of clinical outcome in patients with gallbladder cancer Sci Rep 9(1) 1823 https://doi.org/10.1038/s41598-018-38396-4 PMID: 30755649 PMCID: 6372648
38. Xu B, Chen Z, and Zhang J, et al (2021) Prognostic value of peripheral whole blood cell counts derived indexes in gallbladder carcinoma: a systematic review and meta-analysis Front Oncol 11 707742 https://doi.org/10.3389/fonc.2021.707742 PMID: 34262875 PMCID: 8273513
39. Fridlender ZG, Albelda SM, and Granot Z, et al (2015) Promoting metastasis: neutrophils and T cells join forces Cell Res 25(7) 765–766 https://doi.org/10.1038/cr.2015.62 PMID: 26138787 PMCID: 4493274
40. Anand M, Chodda SK, and Parikh PM, et al (1998) Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis Hematol Oncol 16(4) 143–154 https://doi. org/10.1002/(SICI)1099-1069(199812)16:4<143::AID-HON628>3.0.CO;2-U
41. Gay LJ and Felding-Habermann B (2011) Contribution of platelets to tumour metastasis Nat Rev Cancer 11(2) 123–134 https://doi.org/10.1038/nrc3004 PMID: 21258396 PMCID: 6894505
42. Banks RE, Forbes MA, and Kinsey SE, et al (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology Br J Cancer 77(6) 956–964 https://doi.org/10.1038/bjc.1998.158 PMID: 9528841 PMCID: 2150108
43. Dunn GP, Old LJ, and Schreiber RD, et al (2004) The immunobiology of cancer immunosurveillance and immunoediting Immunity 21(2) 137–148 https://doi.org/10.1016/j.immuni.2004.07.017 PMID: 15308095
44. Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis Nat Rev Cancer 4(1) 71–78 https://doi.org/10.1038/nrc1256 PMID: 14708027
45. Man Y-G, Stojadinovic A, and Mason J, et al (2013) Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories J Cancer 4(1) 84–95 https://doi.org/10.7150/jca.5482 PMID: 23386907 PMCID: 3564249
46. Zikos TA, Donnenberg AD, and Landreneau RJ, et al (2011) Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer Cancer Immunol Immunother CII 60(6) 819–827 https://doi.org/10.1007/s00262-011-0996-4 PMID: 21373990 PMCID: 4154502
47. Rosenberg SA (2001) Progress in human tumour immunology and immunotherapy Nature 411(6835) 380–384 https://doi.org/10.1038/35077246 PMID: 11357146
48. Coffelt SB, Kersten K, and Doornebal CW, et al (2015) IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis Nature 522(7556) 345–348 https://doi.org/10.1038/nature14282 PMID: 25822788 PMCID: 4475637
49. Saqib R, Pathak S, and Smart N, et al (2018) Prognostic significance of pre-operative inflammatory markers in resected gallbladder cancer: a systematic review ANZ J Surg 88(6) 554–559 https://doi.org/10.1111/ans.14300
50. Cancer Today [Internet] [https://gco.iarc.fr/today/home] Date accessed: 26/02/22
51. Torre LA, Siegel RL, and Islami F, et al (2018) Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers Clin Gastroenterol Hepatol 16(3) 427–437 https://doi.org/10.1016/j.cgh.2017.08.017
52. Henley SJ, Weir HK, and Jim MA, et al (2015) Gallbladder cancer incidence and mortality, United States 1999-2011 Cancer Epidemiol Biomark Prev 24(9) 1319–1326 https://doi.org/10.1158/1055-9965.EPI-15-0199
53. Azab B, Camacho-Rivera M, and Taioli E, et al (2014) Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects PLoS One 9(11) e112361 https://doi.org/10.1371/journal.pone.0112361 PMID: 25375150 PMCID: 4223021
54. Lee JS, Kim NY, and Na SH, et al (2018) Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea Medicine (Baltimore) 97(26) e11138 https://doi.org/10.1097/MD.0000000000011138 PMID: 29952958 PMCID: 6039688
Supplementary materials
Supplementary Table 1. Subgroup analysis according to median cut-off and region.
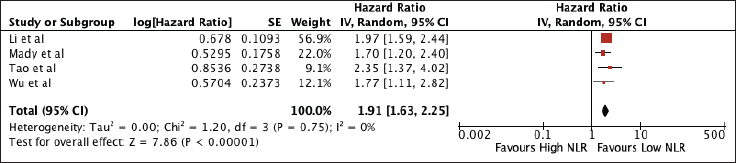
Supplementary Figure 1. Sensitivity analysis for NLR excluding studies with poor quality.

Supplementary Figure 2. Sensitivity analysis for PLR excluding studies with poor quality.

Supplementary Figure 3. Sensitivity analysis for MLR excluding studies with poor quality.

Supplementary Figure 4. Sensitivity analysis for PC excluding studies with poor quality.
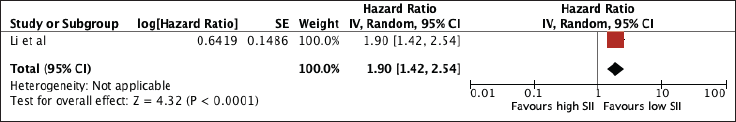
Supplementary Figure 5. Sensitivity analysis for SII excluding studies with poor quality.






