Real-world data on triple-negative breast cancer in Latin America and the Caribbean
Katsuki Arima Tiscoski1,a, Juliana Giacomazzi2,b, Matheus Soares Rocha2,c, Gustavo Gössling2,d and Gustavo Werutsky2,e
1Santa Casa de Misericórdia de Porto Alegre, Rua Professor Annes Dias, Porto Alegre 90020-090, Brazil
2Latin American Cooperative Oncology Group (LACOG), Av Ipiranga, Porto Alegre 90619-900, Brazil
ahttps://orcid.org/0000-0003-0074-4272
bhttps://orcid.org/0000-0001-5811-5140
chttps://orcid.org/0000-0001-8972-7449
dhttps://orcid.org/0000-0002-4361-2889
ehttps://orcid.org/0000-0001-6271-105X
Abstract
Breast cancer (BC) is the most prevalent cancer in women in Latin America and the Caribbean. We compiled real-world data (RWD) on the epidemiology, diagnosis, treatment, and patient outcomes of triple-negative breast cancer (TNBC), addressing the main barriers to optimal care in Latin America. The prevalence of TNBC varies between 11% and 38.5% of all BC cases diagnosed in the region, and TNBC primarily affects young patients. Delays in BC diagnosis, with consequent advanced disease stages and barriers to access efficient therapies, particularly due to high costs, negatively impact patient outcomes. Cancer clinical trials are an opportunity to access standard and novel therapies for patients with this aggressive BC subtype and thus must be prioritised. Finally, generating RWD and cost-effectiveness studies in a region with limited resources is critical for decision-makers to define the incorporation of new technologies for the treatment of BC.
Keywords: breast neoplasms, Latin America, Caribbean Region, triple negative breast neoplasms
Correspondence to: Gustavo Werutsky
Email: gustavo.werutsky@lacogcancerresearch.org
Published: 21/11/2023
Received: 28/04/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer (BC) is the most common cancer among women worldwide, and according to GLOBOCAN, in 2020, the incidence was 2,261,419 new cases and 684,886 deaths [1]. In Latin America and the Caribbean (LAC), BC was responsible for 14% (210,000 new cases) of all cancer cases in 2020, with 57,984 deaths, and it’s estimated that BC will increase to approximately 314,000 new cases per year and 94,600 deaths by 2040 [2].
Inequities in access to cancer care in LAC countries translate into unequal outcomes [3]. However, it is difficult to measure because of the absence of cancer registries and low data quality [4–6]. In Latin America, cancer registries cover only 7%–8% of the population, while the equivalent coverage is 83% in North America and 32% in Europe [7, 8]. Epidemiological and clinical data on BC subtypes are available in only a few observational studies in the region; thus, it is a challenge to evaluate a particular BC subtype, such as triple-negative breast cancer (TNBC).
Among all invasive BC, 10%–17% are classified as TNBC, an aggressive disease subtype that affects a considerable number of Latin American women. TNBC generally occurs in young patients, particularly in those under 40 years of age, is frequently diagnosed in locally advanced stages, and is associated with BRCA mutations. TNBC has a poor prognosis with approximately 30%–40% disease recurrence, often involving visceral organs. Patients with metastatic disease have a survival time of approximately 15 months [9–14]. Although new drugs that significantly impact TNBC outcomes in early and metastatic stages have been approved in recent years, limited access exists for most patients in LAC countries. Therefore, we aimed to compile real-world data (RWD) on TNBC to describe its epidemiology, diagnosis, treatment access, and patient outcomes in LAC.
Methods
To explore the RWD about TNBC in LAC, an advanced literature search was performed through the PubMed database using the following strategy: ((((((((((triple negative) OR (triple-negative)) OR (triple negative[MeSH Terms])) OR (breast cancer[MeSH Terms]))) AND (((breast cancer) OR (breast cancer[MeSH Terms]))))) AND (((((latin america) OR (latin america[MeSH Terms]))) AND (((caribbean) OR (caribbean[MeSH Terms]))))))) AND (((((((((((((((((((((Brazil) OR (argentina)) OR (mexico)) OR (chile)) OR (peru)) OR (colombia)) OR (guatemala)) OR (panama)) OR (costa rica)) OR (venezuela)) OR (cuba)) OR (ecuador)) OR (uruguay)) OR (el salvador)) OR (honduras)) OR (dominican republic)) OR (bolivia)) OR (nicaragua)) OR (paraguay)) OR (haiti)))). Gray literature was also accessed to search for scientific publications on TNBC in LAC. Sixty-five manuscripts have been revised.
Additionally, a survey was administered to oncologists from countries affiliated with the LACOG to demonstrate the availability of novel therapies for treating TNBC from public and private coverage in LAC countries.
A search in the National Library of Medicine/Clinicaltrials.gov was performed to detail the ongoing clinical research in LAC, and the following keywords and criteria were used: ‘oncology’ in the ‘condition or disease’ field; ‘triple-negative’ in the ‘other terms’ field. It was selected in the status field – ‘recruiting’, ‘not yet recruiting’, ‘active’, ‘not recruiting’, ‘completed’, ‘enrolling by invitation’,; ‘study type’ field – ‘interventional studies’; and ‘phase’ field – ‘early phase 1’, ‘phase 1’, ‘phase 2’, ‘phase 3’, ‘phase 4’. The final date in the ‘study start’ field was February 23, 2023.
Epidemiology of TNBC in LAC
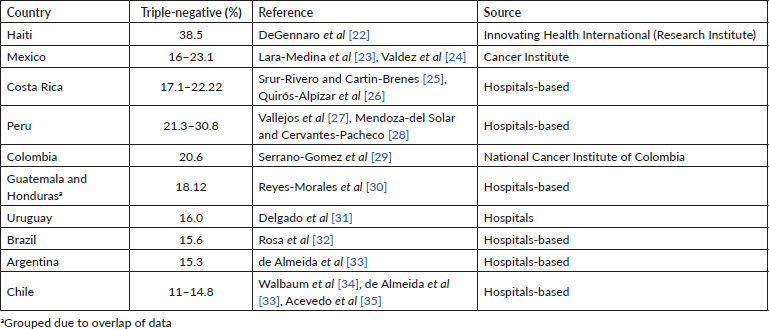
Several epidemiological studies have reported a higher prevalence of TNBC in Latin American women than in non-Hispanic women [15–18]. Table 1 shows the prevalence of TNBC reported in studies conducted in 11 LAC countries. The frequency of TNBC ranges between 11% and 38.5%, with the highest rates reported in Peru and Haiti.
Studies have shown that TNBC is more prevalent in young women. In a prospective cohort study from Brazil with more than 3,000 BC patients, those aged <40 years were more frequently diagnosed with TNBC.
Furthermore, 20% of deaths due to BC at a younger age are caused by this subgroup, which differs from that in developed countries, where the deaths correspond to less than 12% and 10%, respectively [19, 20].
A study conducted in Peru including 1,582 adolescents and young adult females with BC demonstrated that although adolescents and young adult females have more aggressive clinical features at diagnosis, survival outcomes were comparable with those of middle-aged and older women with TNBC (5-year overall survival/event-free survival for adolescents and young adults was 55%/53%, similar to middle-aged (54%/49%) and older females (56%/51%). This suggests that age is not a risk factor for worse survival outcomes if treatment is administered according to cancer stage [21].
Table 1. Prevalence of TNBC in LAC countries.
Pathology and genetic testing
Molecular testing and drug access also vary significantly across LATAM countries. Few studies have investigated the level of discordance and the quality aspects of ER/PgR and Human Epidermal Growth Factor Receptor 2 (HER2) immunohistochemistry tests in LAC. For example, a study examined the concordance in the results of HER immunohistochemistry assays performed on 500 invasive breast carcinomas between a reference laboratory and 149 local laboratories from all geographic regions of Brazil. The results showed an overall poor concordance of 34.2% regarding HER2 results between local and reference laboratories [36]. fluorescence in situ hybridisation or chromogenic in situ hybridisation techniques are only available in highly specialised laboratories and institutes, and the outsourcing of this service increases the delay in the diagnosis of BC [37]. Analysis of cancer-specific markers, such as PD-L1, required for the administration of immunotherapy in advanced disease, when available, is offered only for patients with private insurance or through programs provided by the pharmaceutical industry. However, limited access to tests of cancer-specific markers remains in countries without a commercial supplier [38, 39].
The capacity for and development of cancer genomics in Latin America was described in a recent study. It identified 221 next-generation platforms currently available in the region. Mexico, Brazil, Chile, Argentina, and Colombia are the leading countries in installed facilities, cancer genetics research groups, educational programs in genomics, and medium-impact publications in the field. Meanwhile, countries in Central America were shown to be underrepresented in all areas of ongoing cancer genomic development and implementation [40]. These disparities impact genomic testing and analysis in different clinical scenarios, such as cancer prevention (identification of high-risk cancer genes), tumour genomic profiling for diagnosis and prognosis, and personalised treatment [40–44].
In Brazil, in a subanalysis of the AMAZONA observational study including 2,950 patients, 1,094 (37%) had at least one criterion for hereditary breast and ovarian cancer syndrome. Of all patients, only 45 (6.9%) underwent BRCA testing, and of those tested, 18 (40%) had an identified pathogenic mutation [45]. Among Latin American cancer patients, the frequency of pathogenic variants in the BRCA gene has been reported to be between 1.2% and 15.6% [46–49] and between 15% and 28% of breast and ovarian cancer patients unselected for family history of BC in Mexico [50].
For BRCA1/2 testing, LAC laboratories use state-of-the-art platforms with similar quality control metrics and variant classification protocols as laboratories in Europe and other areas of the world [51]. Quality standards for pathology tests (e.g., immunohistochemistry) are still a matter of concern in LAC, and access to genetic testing is far from ideal because of its high cost and lack of insurance coverage for supportive healthcare policies [40, 44, 52].
Treatment and outcomes of TNBC
The treatment of TNBC has improved in recent years with the incorporation of new therapies for both early and advanced disease. However, the delay in diagnosis and initiating adjuvant systemic treatment is commonly described in countries from LAC, and it's associated with worsening clinical outcomes [22, 32, 53–64]. For example, Morante et al [65] showed that the 10-year-overall survival of patients with TNBC who started chemotherapy ≤ 30 days after surgery was 82% versus 65.1% for those patients who started treatment after ≥ 91 days [65].
Novel agents approved for treating metastatic TNBC, such as Poly ADP-ribose polymerase (PARP) inhibitors, immunotherapy, and antibody-drug conjugates, are not widely accessible to patients in LAC, and thus most patients from the public health system in LAC are still exposed to conventional chemotherapy only. The scenario of access to TNBC therapies by public and private health systems in eight LAC countries was evaluated through a survey conducted by oncologists working in these countries (Table 2).
At the time of our survey (May 2023), pembrolizumab in the neoadjuvant setting was not available in any country in the public health system. In the metastatic setting, pembrolizumab was available in the public health system in only two countries (Argentina and Colombia). PARP inhibitor was available in Argentina, Colombia, and Costa Rica in the public health system. In the private health system, pembrolizumab in the neoadjuvant setting is available in all countries except Colombia and Uruguay, the latter also being the only country where pembrolizumab is not available in the metastatic setting. Sacituzumab is available only in Brazil, and PARP inhibitors for metastatic disease are available in all countries except Uruguay (Table 2).
Clinical trials in TNBC
In the last 5 years, 19 (5.8%) of 323 TNBC clinical trials involving nine LAC countries were registered in the National Library of Medicine (www.clinicaltrials.gov) [66]. Among them, 16 (84.2%) were not recruiting or active. Of the total trials in LAC, 3 (15.8%) were phase I trials, 3 (15.8%) were phase 2 trials, and 13 (68.4%) were phase 3 trials (Table 3).
Table 2. Access to novel therapies for the treatment of BC in LAC.
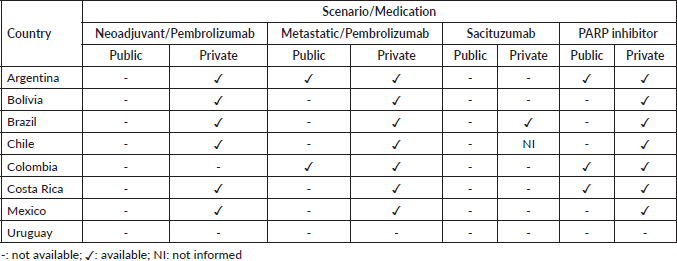
Table 3. Clinical trials in TNBC were conducted in each country of LAC.
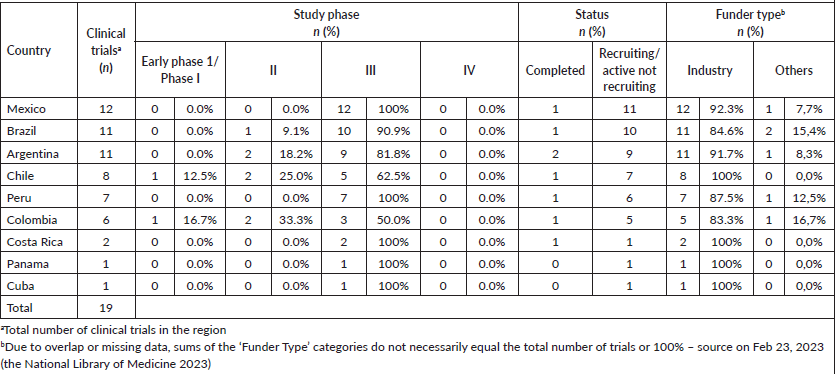
The countries most cited as participating sites in clinical trials were Mexico (12 trials; 20.3%), Brazil (11 trials; 18.6%), Argentina (11 trials; 18.6%), Chile (8 trials; 13.6%), and Peru (7 trials, 11.9%). Colombia, Costa Rica, Panama, and Cuba represented less than 15% of the trials in the LAC. No clinical trials have been registered in Guatemala, Venezuela, Ecuador, Uruguay, El Salvador, Honduras, Dominican Republic, Bolívia, Nicaragua, Paraguay, or Haiti. The industry sponsors an average of 85.7% of the TNBC clinical trials (Table 3). The drug categories evaluated in these studies were inhibitor checkpoint (n = 8; 42%), conjugated antibodies (n = 4; 21%), inhibitor AKT Kinase (n = 3; 16%), vaccine (n = 2; 11%), PARP inhibitors (n = 1; 5%), and inhibitor PIK3CA (n = 1; 5%) (Table 3).
Conclusion
The prevalence of TNBC in LAC varies between 11% and 38.5% of all BC cases diagnosed in the region and affects mostly young patients. Delays in BC diagnosis, barriers to pathology, and genetic testing affect patient outcomes.
Novel drugs that significantly affect survival have been incorporated into the private health system in the majority of LAC countries. Nonetheless, as more than 80% of the LAC population is covered by the public health system, chemotherapy is the only systemic treatment available. Generating RWD and cost-effectiveness studies on LAC is critical for deciding the incorporation of new technologies considering the country's limited resources.
Acknowledgments
We thank the LACOG-affiliated investigators Gonzalo Gomez-Abuin (Argentina), Maria Tereza Nieto Coronel (Bolívia), Bettina Müller (Chile), Sandra Ximena Franco (Colombia), Luis Corrales (Costa Rica), Cynthia Villarreal Garza (Mexico), Isabel Alonso (Uruguay) for answering to the therapies for treating TNBC survey.
Conflicts of interest
JG reports grants from Roche. GW reports grants or contracts from Novartis, Roche/Genentech, AstraZeneca/MedImmune, Lilly, GlaxoSmithKline, Novartis, Pfizer, Bristol-Myers Squibb Brazil, MSD, Merck, Bayer, Janssen, BMS, Astellas, Libbs, Takeda, Celgene, GSK; consulting fees from Merck; payment or honoraria for lectures from Pfizer, AstraZeneca/MedImmune, Libbs, and Merck. The other authors declare no conflict of interest.
Funding
This study received no funding.
Ethical statement
No ethical approval was required for this review paper, as it does not involve primary research on human subjects, animal experimentation, or the collection of personally identifiable information. We adhered to ethical guidelines for proper citation, referencing, and avoidance of plagiarism, ensuring the appropriate attribution of sources, and upholding ethical standards in our research and publication practices.
Author contributions
In accordance with the guidelines set forth by the International Committee of Medical Journal Editors (ICMJE), all authors of this paper made substantial contributions to the conception, design, execution, and interpretation of the research study. All authors have read and approved the final version of the manuscript and take full responsibility for its content.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Piñeros M, Laversanne M, and Barrios E, et al (2022) An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean Lancet Reg Health Am 13 PMID: 36189115 PMCID: 9483035
3. Cazap E (2018) Breast cancer in Latin America: a map of the disease in the region Am Soc Clin Oncol Educ Book 38 451–456 https://doi.org/10.1200/EDBK_201315 PMID: 30231404
4. Goss PE, Lee BL, and Badovinac-Crnjevic T, et al (2013) Planning cancer control in Latin America and the Caribbean Lancet Oncol [Internet] 14(5) 391–436 [https://linkinghub.elsevier.com/retrieve/pii/S1470204513700482] https://doi.org/10.1016/S1470-2045(13)70048-2 PMID: 23628188
5. Hiatt RA and Brody JG (2018) Environmental determinants of breast cancer Annu Rev Public Health [Internet] 39(1) 113–133 [https://www.annualreviews.org/doi/10.1146/annurev-publhealth-040617-014101] PMID: 29328875
6. Bidoli E, Virdone S, and Hamdi-Cherif M, et al (2019) Worldwide age at onset of female breast cancer: a 25-year population-based cancer registry study Sci Rep 9(1) 14111 https://doi.org/10.1038/s41598-019-50680-5 PMID: 31575963 PMCID: 6773713
7. Forman D, Bray F, and Brewster DH, et al eds (2014) Cancer Incidence in Five Continents vol X [Electronic Version] IARC Scientific Publication No. 164 (Lyon: International Agency for Research on Cancer) [http://ci5.iarc.fr/Default.aspx] Date accessed: 29/04/15
8. Bray F, Mery L, and Piñeros M, et al eds (2017) Cancer Incidence in Five Continents vol XI (Lyon, IARC) [https:// https://ci5.iarc.fr/Default.aspx] Date accessed: 01/10/20
9. Perou CM, Sørlie T, and Eisen MB, et al (2000) Molecular portraits of human breast tumours Nature 406(6797) 747–752 https://doi.org/10.1038/35021093 PMID: 10963602
10. Dent R, Trudeau M, and Pritchard KI, et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence Clin Cancer Res 13(15 Pt 1) 4429–4434 https://doi.org/10.1158/1078-0432.CCR-06-3045 PMID: 17671126
11. Lin NU, Claus E, and Sohl J, et al (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases Cancer 113(10) 2638–2645 https://doi.org/10.1002/cncr.23930 PMID: 18833576 PMCID: 2835546
12. Zhang L, Fang C, and Xu X, et al (2015) Androgen receptor, EGFR, and BRCA1 as biomarkers in triple-negative breast cancer: a meta-analysis Biomed Res Int 2015 357485 PMID: 25695063 PMCID: 4324735
13. Bardia A, Hurvitz SA, and Tolaney SM, et al (2021) Sacituzumab govitecan in metastatic triple-negative breast cancer N Engl J Med 384(16) 1529–1541 https://doi.org/10.1056/NEJMoa2028485 PMID: 33882206
14. Zagami P and Carey LA (2022) Triple-negative breast cancer: pitfalls and progress NPJ Breast Cancer 8(1) 95 https://doi.org/10.1038/s41523-022-00468-0 PMID: 35987766 PMCID: 9392735
15. Howlader N, Altekruse SF, and Li CI, et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status J Natl Cancer Inst 106(5) dju055 https://doi.org/10.1093/jnci/dju055 PMID: 24777111 PMCID: 4580552
16. Chen L and Li CI (2015) Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status Cancer Epidemiol Biomarkers Prev [Internet] 24(11) 1666–1672 [https://aacrjournals.org/cebp/article/24/11/1666/70365/Racial-Disparities-in-Breast-Cancer-Diagnosis-and] https://doi.org/10.1158/1055-9965.EPI-15-0293 PMID: 26464428 PMCID: 4633380
17. Serrano-Gómez SJ, Fejerman L, and Zabaleta J (2018) Breast cancer in Latinas: a focus on intrinsic subtypes distribution Cancer Epidemiol Biomarkers Prev [Internet] 27(1) 3–10 [http://www.ncbi.nlm.nih.gov/pubmed/] https://doi.org/10.1158/1055-9965.EPI-17-0420 PMID: 29054978 PMCID: 5760276
18. Zevallos A, Bravo L, and Bretel D, et al (2020) The hispanic landscape of triple negative breast cancer Crit Rev Oncol Hematol 155 103094 https://doi.org/10.1016/j.critrevonc.2020.103094 PMID: 33027724
19. Knaul F, Bustreo F, and Ha E, et al (2009) Breast cancer: why link early detection to reproductive health interventions in developing countries? Salud Publica Mex [Internet] 51 s220–s227 [http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0036-36342009000800012&lng=en&nrm=iso&tlng=em; https://doi.org/10.1590/S0036-36342009000800012]
20. Villarreal-Garza C, Aguila C, and Magallanes-Hoyos MC, et al (2013) Breast cancer in young women in Latin America: an unmet, growing burden Oncologist [Internet] 18(12) 1298–1306 [https://academic.oup.com/oncolo/article/18/12/1298/6399160] https://doi.org/10.1634/theoncologist.2013-0321 PMID: 24277771 PMCID: 3868424
21. Valcarcel B, Torres-Roman JS, and Enriquez-Vera D, et al (2023) Clinical features and outcomes of triple-negative breast cancer among Latin American adolescents and young adults compared to middle-aged and elder females: a cohort analysis over 15 years J Adolesc Young Adult Oncol 12 625–633 https://doi.org/10.1089/jayao.2022.0075 PMID: 36791318
22. DeGennaro V, Jiwani F, and Patberg E, et al (2018) Epidemiological, clinical, and histopathological features of breast cancer in Haiti J Glob Oncol 4 1–9 PMID: 30241242 PMCID: 6223428
23. Lara-Medina F, Pérez-Sánchez V, and Saavedra-Pérez D, et al (2011) Triple-negative breast cancer in Hispanic patients Cancer [Internet] 117(16) 3658–3669 https://doi.org/10.1002/cncr.25961 PMID: 21387260
24. Valdez OE, Rangel-Escareño C, and Matus Santos JA, et al (2022) Characterization of triple-negative breast cancer gene expression profiles in Mexican patients Mol Clin Oncol 18(1) 5 https://doi.org/10.3892/mco.2022.2601
25. Srur-Rivero N and Cartin-Brenes M (2014) Breast cancer characteristics and survival in a Hispanic population of Costa Rica Breast Cancer (Auckl) 8 103–108 PMID: 25125980 PMCID: 4125366
26. Quirós-Alpízar JL, Jiménez-Rodríguez Y, and Jiménez-Montero E, et al (2010) Carcinomas invasores triples negativos de la glándula mamaria: incidencia y características clínico-patológicas Acta Méd Costarric 52(2) 90–95
27. Vallejos CS, Gómez HL, and Cruz WR, et al (2010) Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a Peruvian Hospital Database Clin Breast Cancer [Internet] 10(4) 294–300 [https://linkinghub.elsevier.com/retrieve/pii/S1526820911700416] https://doi.org/10.3816/CBC.2010.n.038 PMID: 20705562
28. Mendoza-del Solar G and Cervantes-Pacheco F (2019) Cáncer de mama triple negativo Rev Soc Peruana Med Intern 27(2) 75–78
29. Serrano-Gomez SJ, Sanabria-Salas MC, and Hernández-Suarez G, et al (2016) High prevalence of luminal B breast cancer intrinsic subtype in Colombian women Carcinogenesis [Internet] 37(7) 669–676 [https://academic.oup.com/carcin/article-lookup/doi/10.1093/carcin/bgw043] https://doi.org/10.1093/carcin/bgw043 PMID: 27207651 PMCID: 4936382
30. Reyes-Morales A, Alvarado-Muñoz JF, and Bejarano S, et al (2022) Abstract P3-12-23: characterization of triple-negative breast cancer in two Central American countries Cancer Res 82(4_Supplement) P3-12-23 https://doi.org/10.1158/1538-7445.SABCS21-P3-12-23
31. Delgado LB, Fresco R, and Santander G, et al (2009) HER-2, hormone receptors, and clinicopathologic characteristics in Uruguayan breast cancer patients J Clin Oncol 27(15_suppl) https://doi.org/10.1200/jco.2009.27.15_suppl.e22202
32. Rosa DD, Bines J, and Werutsky G, et al (2020) The impact of sociodemographic factors and health insurance coverage in the diagnosis and clinicopathological characteristics of breast cancer in Brazil: AMAZONA III study (GBECAM 0115) Breast Cancer Res Treat [Internet] 183(3) 749–757 [https://link.springer.com/10.1007/s10549-020-05831-y] https://doi.org/10.1007/s10549-020-05831-y PMID: 32728860
33. de Almeida LM, Cortés S, and Vilensky M, et al (2022) Socioeconomic, clinical, and molecular features of breast cancer influence overall survival of Latin American women Front Oncol 12 845527 https://doi.org/10.3389/fonc.2022.845527 PMID: 35530311 PMCID: 9071365
34. Walbaum B, Acevedo F, and Medina L, et al (2021) Pathological complete response to neoadjuvant chemotherapy, but not the addition of carboplatin, is associated with improved survival in Chilean triple negative breast cancer patients: a report of real world data Ecancermedicalscience 15 1178 https://doi.org/10.3332/ecancer.2021.1178 PMID: 33777171 PMCID: 7987491
35. Acevedo F, Walbaum B, and Medina L, et al (2023) Clinical characteristics, risk factors, and outcomes in Chilean triple negative breast cancer patients: a real-world study Breast Cancer Res Treat 197(2) 449–459 https://doi.org/10.1007/s10549-022-06814-x
36. Wludarski SC, Lopes LF, and Berto E Silva TR, et al (2011) HER2 testing in breast carcinoma: very low concordance rate between reference and local laboratories in Brazil Appl Immunohistochem Mol Morphol 19(2) 112–118 https://doi.org/10.1097/PAI.0b013e3181f0b044
37. Pinto JA, Pinillos L, and Villarreal-Garza C, et al (2019) Barriers in Latin America for the management of locally advanced breast cancer Ecancermedicalscience 13 1–14
38. Schmid P, Cortes J, and Dent R, et al (2022) Event-free survival with pembrolizumab in early triple-negative breast cancer N Engl J Med [Internet] 386(6) 556–567 [http://www.nejm.org/doi/10.1056/NEJMoa2112651] https://doi.org/10.1056/NEJMoa2112651 PMID: 35139274
39. de Moura Leite L, Cesca MG, and Tavares MC, et al (2021) HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer Breast Cancer Res Treat 190(1) 155–163 https://doi.org/10.1007/s10549-021-06365-7 PMID: 34409551
40. Torres A, Oliver J, and Frecha C, et al (2017) Cancer genomic resources and present needs in the Latin American Region Public Health Genomics 20 194–201 https://doi.org/10.1159/000479291 PMID: 28848219
41. Luo HY and Xu RH (2014) Predictive and prognostic biomarkers with therapeutic targets in advanced colorectal cancer World J Gastroenterol 20 3858–3874 https://doi.org/10.3748/wjg.v20.i14.3858 PMID: 24744578 PMCID: 3983442
42. Sierra MS, Soerjmataram I, and Antoni S, et al (2016) Cancer patterns and trends in Central and South America Cancer Epidemiol 44(suppl 1) S23–S42 https://doi.org/10.1016/j.canep.2016.07.013 PMID: 27678320
43. Ossa CA and Torres D (2016) Founder and recurrent mutations in BRCA1 and BRCA2 genes in Latin American countries: state of the art and literature review Oncologist 21 832–839 https://doi.org/10.1634/theoncologist.2015-0416 PMID: 27286788 PMCID: 4943386
44. Achatz MI, Caleffi M, and Guindalini R, et al (2020) Recommendations for advancing the diagnosis and management of hereditary breast and ovarian cancer in Brazil JCO Glob Oncol 6 439–452 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7113069/pdf/JGO.19.00170.pdf] https://doi.org/10.1200/JGO.19.00170 PMID: 32155091 PMCID: 7113069
45. de Souza AB, Rosa D, and Frasson AL, et al (2020) Abstract P2-09-11: prevalence of patients with indication of genetic evaluation for hereditary breast and ovarian syndrome in the Brazilian cohort study-AMAZONA III Cancer Res 80(4_Supplement) P2-09 https://doi.org/10.1158/1538-7445.SABCS19-P2-09-11
46. Hernández JE, Llacuachaqui M, and Palacio GV, et al (2014) Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Medellin, Colombia Hereditary Cancer Clin Pract 12 11 https://doi.org/10.1186/1897-4287-12-11
47. Abugattas J, Llacuachaqui M, and Allende YS, et al (2015) Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru Clin Genet 88 371–375 https://doi.org/10.1111/cge.12505 PMCID: 4374018
48. Rodríguez AO, Llacuachaqui M, and Pardo GG, et al (2012) BRCA1 and BRCA2 mutations among ovarian cancer patients from Colombia Gynecol Oncol 124 236–243 https://doi.org/10.1016/j.ygyno.2011.10.027
49. Dutil J, Golubeva VA, and Pacheco-Torres AL, et al (2015) The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: a clinical perspective Breast Cancer Res Treat 154 441–453 https://doi.org/10.1007/s10549-015-3629-3 PMID: 26564481 PMCID: 4661195
50. Villarreal-Garza C, Weitzel JN, and Llacuachaqui M, et al (2015) The prevalence of BRCA1 and BRCA2 mutations among young Mexican women with triple-negative breast cancer Breast Cancer Res Treat 150 389–394 https://doi.org/10.1007/s10549-015-3312-8 PMID: 25716084 PMCID: 4532439
51. Solano AR, Palmero EI, and Delgado L, et al (2020) Sequencing technology status of BRCA1/2 testing in Latin American Countries NPJ Genom Med 5 22 https://doi.org/10.1038/s41525-020-0126-3 PMID: 32550004 PMCID: 7265546
52. Chavarri-Guerra Y, Blazer KR, and Weitzel JN (2017) Genetic cancer risk assessment for breast cancer in Latin America Rev Investig Clin [Internet] 69(2) [https://www.clinicalandtranslationalinvestigation.com/frame_esp.php?id=125; doi:10.24875/ric.17002195]
53. Yoffe de Quiroz I (2005) Retardo en el diagnóstico de los pacientes con cáncer Anal Facultad Ciencias Méd 38 22–28 [http://scielo.iics.una.py/scielo.php?pid=S1816-89492005000100003&script=sci_arttext]
54. Muñoz FD, Cálix ES, and Santos R (2011) Caracterización Epidemiológica de Pacientes con Cáncer de Mama, Admitidas en el Centro de Cáncer ‘Emma Romero De Callejas’ 1999 a 2009 Rev Facultad Ciencias Méd 8 32–44
55. Viera-Hernández RV, Amaro-Areas E, and Barro-Blanco A, et al (2011) Caracterización del cáncer de mama. Isla de la Juventud. 2000-2010 Rev Med Isla Juventud 12 74–87
56. Prieto MM (2011) Epidemiología del cáncer de mama en chile Rev Méd Clín Condes 22 428–435
57. Rebolledo VE, Ferri N, and Reigosa A, et al (2012) Perfil inmunohistoquímico y la caracterización molecular del carcinoma de mama en una población venezolana Rev Venezolana Oncol 24 42–51
58. Díaz -Vélez C (2013) Informe del Registro Hospitalario de Cancer 2007-2012 (Red Asistencial Lambayeque)
59. Camejo N, Castillo C, and Richter L, et al (2015) Evaluación de la calidad de la asistencia en la Unidad Docente Asistencial de Mastología del Hospital de Clínicas Rev Méd Uruguay 31 165–171
60. Grippo NM, Raineri E, and Yapur R, et al (2015) Análisis de las variables clinicopatológicas e inmunohistoquímicas del cáncer de mama Rev Argentina Mastol 34 14–26
61. Martinez ME, Wertheim BC, and Natarajan L, et al (2013) Reproductive factors, heterogeneity, and breast tumor subtypes in women of Mexican Descent Cancer Epidemiol Biomarkers Prev [Internet] 22(10) 1853–1861 [https://aacrjournals.org/cebp/article/22/10/1853/69375/Reproductive-Factors-Heterogeneity-and-Breast] https://doi.org/10.1158/1055-9965.EPI-13-0560 PMID: 23950213 PMCID: 3799795
62. Reynoso-Noverón N, Villarreal-Garza C, and Soto-Perez-de-Celis E, et al (2017) Clinical and epidemiological profile of breast cancer in Mexico: results of the seguro popular J Glob Oncol 3(6) 757–764 Epub 2017 Feb 8 https://doi.org/10.1200/JGO.2016.007377 PMID: 29244990 PMCID: 5735969
63. Diaz Casas S, Lancheros García E, and Sanchéz Campo A, et al (2019) Clinical behavior of triple negative breast cancer in a Cohort of Latin American women Cureus 11(6) e4963 PMID: 31453035 PMCID: 6701886
64. Duarte C, Salazar A, and Strasser-Weippl K, et al (2021) Breast cancer in Colombia: a growing challenge for the healthcare system Breast Cancer Res Treat [Internet] 186(1) 15–24 [http://link.springer.com/10.1007/s10549-020-06091-6] https://doi.org/10.1007/s10549-020-06091-6 PMID: 33611666
65. Morante Z, Ruiz R, and Araujo JM, et al (2021) Impact of the delayed initiation of adjuvant chemotherapy in the outcome of triple negative breast cancer Clin Breast Cancer [Internet] 21(3) 239-246.e4 [https://linkinghub.elsevier.com/retrieve/pii/S1526820920302354] https://doi.org/10.1016/j.clbc.2020.09.008
66. The National Library of Medicine [www.clinicaltrials.gov]






