Outcomes and toxicity of oral Fosfestrol in metastatic castration-resistant prostate cancer—a real-world experience
Nandini Devi R, Praveen Kumar Shenoy VP, Irshad Ismail and Manuprasad Avaronnan
Department of Clinical Hematology and Medical Oncology, Malabar Cancer Centre, Thalassery, Kannur 670103, India
Abstract
Introduction: Although there are multiple drugs approved for the treatment of metastatic castration-resistant prostate cancer (CRPC), the cost can be a limiting factor in using them in a resource-limited setting. Therefore, less expensive alternatives are the need of the hour. We have been using Fosfestrol which is a cheap and orally administered oestrogen analogue in metastatic CRPC. We carried out a retrospective study to analyse its efficacy and toxicity.
Results: A total of 65 patients received Fosfestrol during 2015–2020. The median age was 65 years (range 50–83 years). Thirty-four patients (53%) had other medical comorbidities. Skeletal-only metastasis was the commonest pattern of metastasis (n = 41, 64%) followed by skeletal with nodal metastasis (n = 15, 23%). The majority of the patients had undergone upfront surgical castration (n = 60, 93%). All the patients had adenocarcinoma and 38 patients (58%) had a high Gleason’s score. Forty-one patients (63%) had a prostate-specific antigen (PSA) response (decrease of ≥50% in the PSA concentration from the pre-treatment baseline PSA value) and 54 patients (83%) had a symptomatic response. At the end of a median follow-up of 16 months, the median progression-free survival (PFS) was 8.3 months (CI 4.7–11.8) and the median overall survival (OS) was 27.5 months (CI 25.4–29.5). PSA response and prior treatment with abiraterone acetate were found to have a significant association with survival outcomes. Patients with PSA response had better median PFS and OS; while patients who have received prior abiraterone acetate therapy had worse survival outcomes. Twenty-nine patients (45%) received some form of subsequent treatment after stopping Fosfestrol. The most common oxicity observed was thrombosis (n = 9, 13%) followed by gynecomastia (n = 4, 6%).
Conclusion: We conclude that oral Fosfestrol is a cheap and effective agent in the armamentarium against metastatic CRPC and warrants further studies in a clinical trial setting.
Keywords: castration-resistant prostate cancer, Fosfestrol, hormonal therapy, LMIC
Correspondence to: Manuprasad Avaronnan
Email: drmanuprasad@gmail.com
Published: 14/08/2023
Received: 23/04/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Carcinoma prostate is the second most common cancer in males with around 1,414,259 (7.3%) new cases and 375,304 (3.8%) deaths annually [1]. As the majority of patients with prostate cancer present in the early stage, 5-year survival rates are as high as 97% in developed countries [2]. But all patients who present with advanced disease progress to the castration-resistant stage, treatment of which is challenging. The therapeutic landscape of castration-resistant prostate cancer (CRPC) has dramatically changed over the last few years with the approval of many novel agents including androgen receptor antagonists like enzalutamide or darolutamide, immunotherapy like Sipuleucel-t or Pembrolizumab, and targeted agents like poly(ADP-ribose) polymerase (PARP) inhibitors [3]. But the exact sequencing of these agents to achieve maximum survival rates remains debatable. Also, most of these drugs are expensive and the majority of patients in developing countries do not have access to them [4].
As prostate cancer is an androgen-dependent cancer, androgen deprivation therapy (ADT) was the standard first-line therapy until the role of Docetaxel in hormone naïve metastatic prostate cancer was established in 2014 [5]. Second-line hormonal agents approved in CRPC also act on the androgen synthesis pathways or receptors [6, 7]. Another hormonal strategy found to be effective in prostate cancer is the use of exogenous oestrogens. Exogenous oestrogens may exert negative feedback inhibition on the pituitary gland with a resultant reduction in luteinising hormone and subsequent fall in testosterone levels. But later on, these agents fell out of favour due to adverse events related to high-dose oestrogens, lack of proven survival benefit and the emergence of newer forms of hormonal therapies. Diethylstilbestrol (DES) and its analogues were the most common oestrogen derivatives that were studied. DES increases the level of sex hormone binding globulin which also reduces the free testosterone levels. DES is also known to have a direct cytotoxic effect on prostatic cancer cells by transforming growth factor beta (TGF-β) upregulation and apoptosis [8]. But cardiovascular and thrombotic events were a major concern in many of the studies using DES [9].
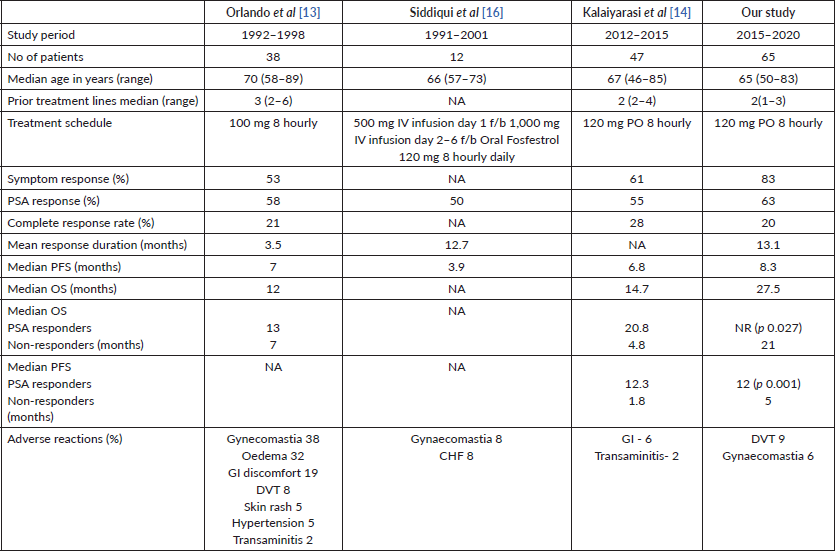
Fosfestrol tetrasodium – DES diphosphate – is a synthetic oestrogen derivative which is available in parenteral as well as oral formulations [10]. Oral Fosfestrol is advantageous compared to DES, due to the following reasons (a) Fosfestrol is a non-toxic prodrug of DES (b) It can be administered safely/easily (c) It selectively accumulates in prostatic cancer cells and (d) exerts its cytotoxic effect only after conversion to active metabolite DES. These active metabolites are said to inhibit the electron flow from Ubiquinone to Cytochrome C1 in mitochondria [11, 12]. There are only very few studies examining the efficacy of oral Fosfestrol. In a study of 38 patients with CRPC, Orlando et al [13] reported an improvement in pain score in 53% of patients and a median progression-free survival (PFS) of 7 months. This study, which used low-dose Fosfestrol (100 mg three times a day), also reported significant toxicities including peripheral oedema, worsening of gynaecomastia and deep vein thrombosis. Another study from India reported the use of oral Fosfestrol (120 mg three times a day) in 47 patients with CRPC. In this study, 55% of the patients had PSA response >50% and had a median survival of 14 months after initiation of Fosfestrol. But unlike the initial study thrombotic complications were not observed in this group and the most common side effect was gastrointestinal side effects (6%) [14]. In order to circumvent the first pass hepatic metabolism of oestrogen analogues and thereby prevent thrombotic complications, transdermal oestrogen patches are being studied in the management of CRPC in the UK PATCH study and results are awaited. Once available the transdermal oestrogen patch might be a patient-friendly and safer therapeutic approach [15]. In a low-middle-income country like India, where the newer agents cannot be afforded by a majority of patients, Fosfestrol can be an important drug. Also, as the optimal sequencing of all available agents is not yet defined, Fosfestrol may have its own place in the armamentarium against CRPC. Here we report our experience with oral Fosfestrol in metastatic CRPC.
Materials and methods
This is a retrospective study conducted at the Medical Oncology Department of a tertiary referral oncology centre in North Kerala. The study was approved by the Institution Review Board. The primary objective of the study was to find out the response rates, PFS, and toxicities with Fosfestrol in CRPC. The secondary objective was to estimate the overall survival (OS) with Fosfestrol. All patients with CRPC who received Fosfestrol at least for 1 month, during the period of 2015–2020, were included. Patients with incomplete data and patients initiated on Fosfestrol from outside were excluded. Details about baseline characteristics, prior treatment, toxicities observed and outcomes of Fosfestrol therapy were collected from the case records. The following operational definitions were used:
1. CRPC was defined as two consecutive increases in the PSA concentration (over a reference value) or radiographic evidence of disease progression with a serum testosterone level of 50 ng per decilitre or less (≤2.0 nmol per litre) in patients with metastatic prostate cancer. Patients who underwent bilateral orchidectomy were included even if serum testosterone levels were unavailable.
2. PSA response was defined as a decrease of ≥50% in the PSA concentration from the pre-treatment PSA value, which was confirmed after ≥4 weeks by one more value.
3. PFS was calculated from the date of the start of Fosfestrol to the date of progression. Progression is considered when there is a progression in at least 2 of 3 domains – biochemical, radiological or symptomatic progression.
4. OS was calculated from the date of the start of Fosfestrol till the date of the last follow-up or death
5. Biochemical progression was defined as a serial rise in S.PSA on 3 consecutive tests at least 4 weeks apart
All the patients were started on oral Fosfestrol at a dose of 120 mg three times a day. They underwent 1–3 monthly clinical examinations and S.PSA estimations. All patients with biochemical progression or symptomatic progression undergo radiologic examination. Fosfestrol was continued till progression or till grade 3/4 toxicity.
Statistical methods
Being a retrospective study, sample size calculation was not performed for this study. Statistical analysis was done using the Statistical Package for the Social Sciences version 20 (IBM Corp., Armonk, NY, USA). Descriptive analysis was used for frequency and percentages. Prognostic factors for survival were identified by univariate analysis using the log-rank test. Also, prognostic factors were tested using the Cox regression for the multivariate analysis. p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
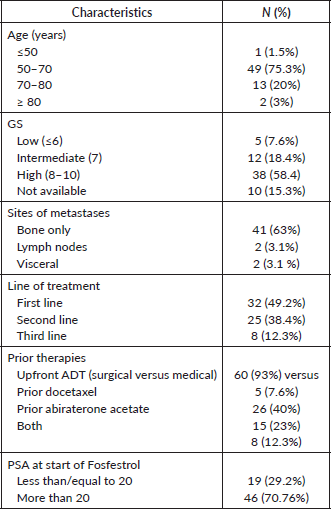
A total of 65 patients received Fosfestrol during the study period. The median age was 65 years (50–83 years). The baseline Eastern Cooperative Oncology Group (ECOG) performance status was PS-1 in 7 (10.8%) patients, PS-2 in 44 (67.7%) and PS-3 in 5(7.7%) patients. Thirty-four patients (53%) had other medical comorbidities. The most common co-morbidity was systemic hypertension in 15 (23.1%) patients and type 2 diabetes mellitus in 11 (16.9%) patients. All the patients had prostatic adenocarcinoma. Skeletal-only metastasis was the commonest pattern of metastasis (n = 41, 64%) followed by skeletal with nodal metastasis (n = 15, 23%). The majority of the patients had upfront surgical castration (n = 60, 93%). The majority (n = 50, 76.9%) had Gleason’s score (GS) more than 6. Fifteen patients had received prior Abiraterone, 26 patients received prior Docetaxel and 8 patients received both Docetaxel and Abiraterone. Other baseline characteristics are shown in Table 1.
Outcomes
All patients received oral Fosfestrol at the dose of 120 mg thrice daily. Fifty-four patients (83%) had a symptomatic response. Forty-one patients (63%) had a PSA response, of which 13 (20%) patients had normalisation of PSA (complete response). At the end of a median follow-up of 16 months, median PFS was 8.3 months (CI 4.7–11.8) and the median OS was 27.5 months (CI 25.4–29.5). On univariate analysis, PSA response and prior exposure to abiraterone acetate were predictive of survival. The median OS was significantly longer for patients who received Fosfestrol prior to abiraterone acetate (28.7 versus 15 months, p-value < 0.001). Also, patients who achieved PSA response had better OS compared to those who did not (NR versus 21 months, p-value 0.027). The use of Fosfestrol prior to abiraterone acetate and PSA response were found to be predictors of longer survival in the multivariate analysis also (Figures 1 and 2). The PFS was better in patients with PSA response (12 versus 5 months, p-value 0.001). In patients who received Fosfestrol prior to abiraterone, PFS was numerically longer but there was no statistically significant difference (11 versus 7 months, p-value 0.060). The ECOG performance status at the time of initiation of Fosfestrol was associated with better PFS in univariate analysis, but there was no difference in OS. Also, there was no statistically significant difference in OS or PFS according to prior use of docetaxel, and the site of metastasis. The univariate analysis results are summarised in Table 2. Twenty-nine patients received some form of subsequent treatment. The subsequent therapies were abiraterone acetate in 25 patients, Docetaxel in 4 patients and enzalutamide in 3 patients. One patient each received Cabazitaxel and Ketoconazole.
Table 1. Baseline characteristics.
Toxicity
Sixteen (24%) patients had adverse reactions, and in three patients, it led to treatment discontinuation. Deep vein thrombosis was seen in 9 (13.8%) patients and gynecomastia in 4 (6%) patients. All the cases of deep vein thrombosis involved the lower limbs. None of our patients had pulmonary thromboembolism. The cause for treatment discontinuation was disease progression in 46 patients, thrombosis in 3 patients and not available in rest. No deaths occurred due to thrombotic complications.
Discussion
In our study of 65 patients, oral Fosfestrol showed favourable outcomes in metastatic CRPC with a manageable toxicity profile. The majority of our patients underwent surgical castration as upfront ADT and had bone metastasis. About half of the patients received Fosfestrol as the first-line treatment for CRPC. More than 80% of our patients had a symptomatic response and 60% had a PSA response. Median PFS was 8.3 months and OS was 27.5 months. Survival was better in patients who did not have prior abiraterone exposure and those who had a PSA response. Only a minority of our patients had significant toxicities and thrombotic events were an important adverse event. Our study showed that this cheap and widely available oral drug can be an important agent in the management of CRPC, especially in a resource-limited setting.
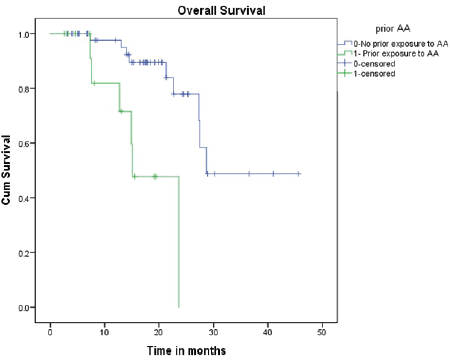
Figure 1. OS based on prior abiraterone acetate therapy.
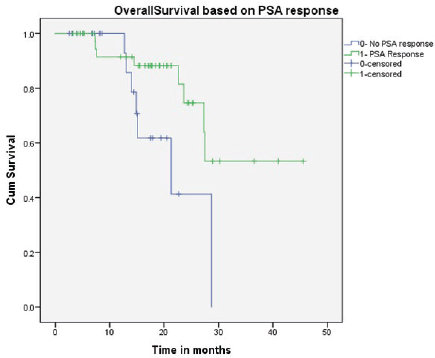
Figure 2. OS based on PSA response.
Table 2. Univariate analysis of PFS and OS.
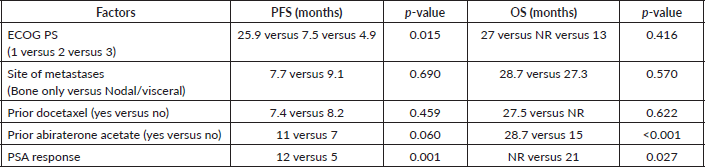
Orlando et al [13] reported an overall response rate of 79% and symptom response of 53% with low-dose oral Fosfestrol. Siddiqui et al [16] described a 50% PSA response with a median response duration of 12.7 months. In an Indian study, oral Fosfestrol demonstrated symptomatic response in 61% and PSA response in 55% of patients [14]. In our study, 83% of patients had symptomatic response and 63% of patients had PSA response, both of which are superior compared to the aforementioned studies. One reason for the better response rates in our study may be related to its use in earlier lines of treatment. Also, the response rates with Fosfestrol are comparable to other agents approved in CRPC like abiraterone acetate, enzalutamide, and taxane chemotherapy [6, 17–19]. Table 3 shows the comparison of our study with others.
Table 3. Comparison of our study with similar studies.
In our study, patients with PSA response had better OS as well as PFS, which was consistent with previous literature. The median OS described by Orlando et al [13] was 13 months in PSA responders compared to 7 months in non-responders. Similarly, Kalaiyarasi et al [14] reported a median OS of 20.8 months in responders (versus 4.8 m in non-responders). An interesting observation was the negative impact of prior abiraterone exposure on survival. Patients who had received abiraterone prior had inferior OS of 15.1 months (versus 28.7 m in Abiraterone naive) which means prior abiraterone therapy might be conferring cross resistance to Fosfestrol therapy. There is ample retrospective data and a prospective trial proving cross-resistance between abiraterone and enzalutamide [20]. We think similar mechanisms of cross-resistance might be responsible for the inferior survival in our study post abiraterone therapy. Hence, we think that Fosfestrol at earlier lines might be an efficient and cost-effective therapeutic choice for CRPC. Omlin et al [21] have demonstrated a favourable PSA response with abiraterone therapy in DES-treated patients. This is in keeping with our observation that Fosfestrol might be beneficial at an earlier line and any further disease progression can be effectively salvaged with other hormonal agents like abiraterone. The cross-resistance between various hormonal therapies needs to be explored further as this is important for proper sequencing of therapies in CRPC. Although ECOG performance status was found to be associated with PFS, the number of patients with a performance status of one or three was too small to draw any definite conclusions.
The toxicities observed were slightly different among all these studies. The most commonly reported adverse effect was gynaecomastia and gastrointestinal disturbances, including diarrhoea and transaminitis. The incidence of thrombotic complications varied the most. In the study by Siddiqui et al [16], no thrombotic events were observed. But in this study, anti-thrombotic prophylaxis with warfarin and aspirin was given, which might have prevented the thrombotic events. Kalaiyarasi et al [14] also reported no thrombotic complications and they had suggested genetic variation between Caucasian and Indian people as a possible explanation for the lesser thrombotic complications. In our study, around 9 (13.8%) patients had deep vein thrombosis and in 3 patients it led to treatment discontinuation confirming the thrombotic risk of oestrogens in our population. But it cannot be ruled out if the thrombotic risk is mainly related to the prostatic malignancy or other factors like co-morbidities.
Our study is the largest report proving the efficacy of oral Fosfestrol in CRPC. The use of Fosfestrol in the earlier lines of treatment clearly showed impressive response rates and survival comparable to other novel agents. Also, it was noted that using Fosfestrol prior to abiraterone has led to improved survival which can be important information in the sequencing of drugs in CRPC, especially in a resource-limited setting. The pharmacoeconomic implications of Fosfestrol are particularly appealing. Fosfestrol is a cost-effective choice among the hormonal therapies of CRPC. The cost for abiraterone acetate/Prednisolone is 28,800 INR/year, for Fosfestrol is 27,600 INR/year and for enzalutamide 1.92 lakhs INR/year. The median PFS for Fosfestrol in our study was 12.3 months, so when comparing Fosfestrol versus abiraterone, an additional 1,230 INR will be saved per person per year. This is particularly important when these therapies are partly/fully supported by government funds. Not to mention additional costs involved in the monitoring of dyselectrolytemia and hyperglycaemia and their subsequent treatment while on abiraterone therapy [22, 23]. Although thrombotic events are reported in the study, many of the patients with multiple comorbidities including coronary artery disease tolerated the drug well. The limitation of our study includes a small sample size and retrospective design leading to inadequate capture of minor adverse events. Though clinical and biochemical response was assessed regularly, the radiological assessment was done only when clinically indicated.
Conclusion
Our study shows that Fosfestrol is an effective drug in metastatic CRPC with a manageable toxicity profile and it has an important role in the sequencing of drugs in CRPC. In view of the low cost and wide availability, it must be studied in a randomised clinical trial setting to confirm its efficacy.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
There were no external sources of funding for this project.
References
1. Cancer today [Internet] [http://gco.iarc.fr/today/home] Date accessed: 26/02/23
2. SEER [Internet] Cancer of the prostate – cancer stat facts [https://seer.cancer.gov/statfacts/html/prost.html] Date accessed: 24/02/23
3. Crawford ED, Higano CS, and Shore ND, et al (2015) Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies J Urol 194(6) 1537–1547 https://doi.org/10.1016/j.juro.2015.06.106 PMID: 26196735
4. Rawla P (2019) Epidemiology of prostate cancer World J Oncol 10(2) 63–89 https://doi.org/10.14740/wjon1191 PMID: 31068988 PMCID: 6497009
5. Sweeney CJ, Chen YH, and Carducci M, et al (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer N Engl J Med 373(8) 737–746 https://doi.org/10.1056/NEJMoa1503747 PMID: 26244877 PMCID: 4562797
6. de Bono JS, Logothetis CJ, and Molina A, et al (2011) Abiraterone and increased survival in metastatic prostate cancer N Engl J Med 364(21) 1995–2005 https://doi.org/10.1056/NEJMoa1014618 PMID: 21612468 PMCID: 3471149
7. Beer TM, Armstrong AJ, and Rathkopf DE, et al (2014) Enzalutamide in metastatic prostate cancer before chemotherapy N Engl J Med 371(5) 424–433 https://doi.org/10.1056/NEJMoa1405095 PMID: 24881730 PMCID: 4418931
8. Bosland MC (2005) The role of estrogens in prostate carcinogenesis: a rationale for chemoprevention Rev Urol 7(Suppl 3) S4–S10
9. Kent JR, Bischoff AJ, and Arduino LJ, et al (1973) Estrogen dosage and suppression of testosterone levels in patients with prostatic carcinoma J Urol 109(5) 858–860 https://doi.org/10.1016/S0022-5347(17)60564-0 PMID: 4699685
10. Abramson FP and Miller HC (1982) Bioavailability, distribution and pharmacokinetics of diethystilbestrol produced from stilphostrol J Urol 128(6) 1336–1339 https://doi.org/10.1016/S0022-5347(17)53502-8 PMID: 7154205
11. Droz JP, Kattan J, and Keuppens F, et al (1994) Phase I trial of high-dose fosfestrol in hormone-refractory adenocarcinoma of the prostate Prostate 24(2) 62 https://doi.org/10.1002/pros.2990240203 PMID: 7508621
12. Schulz P, Link TA, and Chaudhuri L, et al (1990) Role of the mitochondrial bc1-complex in the cytotoxic action of diethylstilbestrol-diphosphate toward prostatic carcinoma cells Cancer Res 50(16) 5008–5012 PMID: 2165852
13. Orlando M, Chacón M, and Salum G, et al (2000) Low-dose continuous oral fosfestrol is highly active in “hormone-refractory” prostate cancer Ann Oncol 11(2) 177–181 https://doi.org/10.1023/A:1008360118617 PMID: 10761752
14. Kalaiyarasi JP, Radhakrishnan V, and Ganesan TS, et al (2019) Experience with using fosfestrol for treating metastatic castrate‑resistant prostate cancer in resource‑limited setting Indian J Med Paediatr Oncol 40 79–84 https://doi.org/10.4103/ijmpo.ijmpo_259_17
15. Langley RE, Gilbert DC, and Duong T, et al (2021) Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised prostate adenocarcinoma transcutaneous hormone (PATCH) trial programme Lancet 397(10274) 581–591 https://doi.org/10.1016/S0140-6736(21)00100-8 PMID: 33581820 PMCID: 7614681
16. JPMA – J Pak Med Assoc [Internet] [https://www.jpma.org.pk/article-details/475] Date accessed: 20/02/23
17. Scher HI, Fizazi K, and Saad F, et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy N Engl J Med 367(13) 1187–1197 https://doi.org/10.1056/NEJMoa1207506 PMID: 22894553
18. Berthold DR, Pond GR, and Soban F, et al (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study JCO 26(2) 242–245 https://doi.org/10.1200/JCO.2007.12.4008
19. de Wit R, de Bono J, and Sternberg CN, et al (2019) Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer N Engl J Med 381(26) 2506–2518 https://doi.org/10.1056/NEJMoa1911206 PMID: 31566937
20. Buck SAJ, Koolen SLW, and Mathijssen RHJ, et al (2021) Cross-resistance and drug sequence in prostate cancer Drug Resist Updat 56 100761 https://doi.org/10.1016/j.drup.2021.100761 PMID: 33799049
21. Omlin A, Pezaro CJ, and Zaidi S, et al (2013) Antitumour activity of abiraterone and diethylstilboestrol when administered sequentially to men with castration-resistant prostate cancer Br J Cancer 109(5) 1079–1084 Date accessed: 02/04/23 https://doi.org/10.1038/bjc.2013.446 PMID: 23928659 PMCID: 3778298
22. Szmulewitz RZ, Peer CJ, and Ibraheem A, et al (2018) Prospective international randomized phase II study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer JCO 36(14) 1389–1395 https://doi.org/10.1200/JCO.2017.76.4381
23. Patel A, Tannock IF, and Srivastava P, et al (2020) Low-dose abiraterone in metastatic prostate cancer: is it practice changing? Facts and facets JCO Glob Oncol 6 382–386 https://doi.org/10.1200/JGO.19.00341 PMID: 32125899 PMCID: 7113122