Metastatic breast cancer in Kenya: survival, prognosis and management at a tertiary referral centre
Mwongeli Matheka1, Miriam Mutebi1, Shahin Sayed1, Jasmit Shah2,3 and Asim Jamal Shaikh4
1Aga Khan University Hospital Nairobi, PO Box 30270 - 00100, Nairobi, Kenya
2Department of Medicine, Aga Khan University, PO Box 30270 - 00100, Nairobi, Kenya
3Brain and Mind Institute, Aga Khan University, PO Box 30270 - 00100, Nairobi, Kenya
4Sultan Qaboos Comprehensive Cancer Care and Research Centre, Muscat, Oman
Abstract
There has been an increase in breast cancer in Africa with up to 77% of patients diagnosed with advanced disease. However, there is little data on survival outcomes and prognostic factors affecting survival in patients with metastatic breast cancer (MBC) in Africa. The study objective was to establish the survival of patients with MBC at a single tertiary health facility, the clinical and pathological characteristics affecting survival and describe the treatment modalities used. This was a retrospective descriptive study conducted at Aga Khan University Hospital, Nairobi of patients diagnosed with MBC between 2009 and 2017. Survival data was collected on metastatic free survival, survival time between diagnosis of first metastasis and death and overall survival. Data on patient’s age, menopausal status and stage at diagnosis, tumour grade, receptor status, site of metastasis and treatment given was also collected. The Kaplan-Meier Estimator was used to estimate survival. Prognostic factors for survival outcomes were analysed using univariate analysis. Standard descriptive statistics were used to describe patient characteristics.
A total of 131 patients were included in the study. The median survival was 22 months. The 3 and 5-year survivals were 31.3% and 10.7%, respectively. On univariate analysis, the Luminal A molecular subtype was a significant positive prognostic factor hazard ratios (HR 0.652 95% confidence interval (CI) 0.473–0.899) while metastasis to the liver or brain were significant negative prognostic factors (HR 0.615 95% CI 0.413–0.915 and HR 0.566 95% CI 0.330–0.973, respectively). A large proportion (87.0%) received some treatment for metastatic disease. Our study concluded that survival rates for patients diagnosed with MBC were lower compared to studies from Western countries but higher than in studies from Sub-Saharan Africa. Luminal A molecular subtype was found to be a positive prognostic factor and metastasis to the liver or brain were found to be negative prognostic factors. Improved access to adequate treatment for MBC is required in the region.
Keywords: breast neoplasms, metastasis, survival rate
Correspondence to: Miriam Mutebi
Email: miriam.mutebi@aku.edu
Published: 27/06/2023
Received: 21/02/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Although the incidence of breast cancer in Africa is lower in comparison to developed countries, mortality from breast cancer is higher [1]. The higher mortality rate has been linked to advanced disease at diagnosis and inadequate treatment [2].
The tumours in African patients show more necrosis, greater nuclear atypia and have higher mitotic activity [3]. Triple-negative breast cancer (TNBC) and human epidermal growth factor receptor 2 (HER2)-enriched breast cancer are more common in African populations and are associated with more aggressive diseases [4].
Metastatic breast cancer (MBC) is a disease that has advanced further than the breast and the regional lymph nodes. MBC can be diagnosed at first presentation (de novo disease) or present in patients initially diagnosed with non-metastatic disease (relapsed or recurrent disease) [5]. Recent studies in Kenya have found that 25% of patients diagnosed with breast cancer are diagnosed with metastatic disease at initial diagnosis and more than 50% of patients go on to develop MBC [6].
Several clinical and biochemical factors which affect the outcomes in patients with MBC have been identified. These factors include age, performance status, metastatic site and number of lesions, hazard ratios (HR) status, HER2 receptor status, disease-free interval (DFI) and prior chemotherapy [7].
Five years of survival for MBC in the West ranges from 16% to 28% [8, 9]. There is little data on survival outcomes of breast cancer in Africa and even less on patients with MBC. Studies have reported widely variable 5-year survival rates of MBC of between 8% and 39% [10–13]. These studies were limited by high rates of loss of follow-up and inadequate treatment of metastatic disease.
Due to the higher occurrence, advanced disease and unique tumour biology in the African population, it is important to know the survival outcomes of patients with MBC in our setting [2, 3].
We have a cohort of patients who are on regular follow-up at the breast service and receive chemotherapy and radiotherapy as needed. This addresses the limitations of inadequate treatment and loss to follow-up in most studies from Sub-Saharan Africa.
The purpose of this study was to establish 3 and 5-year survival outcomes of patients with MBC, the clinical and pathological characteristics affecting survival and to describe the treatment modalities used.
Materials and methods
Patients
This was a retrospective study conducted at Aga Khan University Hospital Nairobi (AKUHN). This is a private tertiary hospital in Nairobi, Kenya. Most of the patient population are middle and upper-income earners with a tertiary education and majority pay through private insurance. Patients have access to multi-disciplinary care with chemotherapy, radiotherapy and surgical services.
Patients diagnosed and treated for MBC at AKUHN from 1st Jan 2009 to 31st Dec 2017 with complete records were included in the study. Patients with incomplete records or a previous history of another cancer were excluded.
Data collection
Patient records were reviewed retrospectively.
Survival data collected was:
I. Metastasis free survival (MFS): time between first diagnosis of breast cancer and diagnosis of distant metastasis in patients presenting with non-metastatic disease
II. Survival (S): time between diagnosis of first metastasis and death
III Overall survival (OS): time between first diagnosis of breast cancer and death
Data on patients’ demographic and clinical characteristics was collected including age, menopausal status, stage at diagnosis, molecular subtype, metastatic site and treatment given.
Data analysis
The demographic and pathologic characteristics were presented using summary statistics. Continuous variables were shown as medians and ranges and categorical variables shown as percentages and frequencies. Differences between categorical variables and continuous variables were analysed using chi-squared test or Fishers Exact test for categorical data and Student’s t-test or Mann–Whitney U test for continuous data.
Survival was estimated using the Kaplan–Meier method and compared between the different subgroups of patients using log-rank test. The survival and OS rates were presented as percentages. Median survival time was also calculated.
HRs and 95% confidence intervals (CIs) were calculated by univariable Cox proportional hazard models to assess the relative contribution of age, menopausal status, tumour grade, molecular subtype, metastatic site and treatment type to survival after diagnosis of MBC.
The statistical analysis was carried out using SPSS software (SPSS Inc).
Results
During the period from 2009 to 2017, 1,210 patients were diagnosed with breast cancer. Out of these, 146 patients were diagnosed with MBC. Among those with MBC, 15 patients were excluded due to incomplete records. A total of 131 patients were included.
Patient characteristics
The baseline patient characteristics are summarised in Table 1. The median age at diagnosis of breast cancer was 47.00 years (IQR 18). Overall, 30.5% (40) of patients were diagnosed with de novo MBC while 69.5% (91) had progression of disease from an earlier stage.
The most common single metastatic site was bone 58% (76) followed by lung 56.6% (74). Overall, 58.8% (77) of the patients had metastasis to multiple sites with lung plus bone metastasis being the most common sites 11.5% (15) (Table 1).
Treatment
Out of the 131 patients included in the study, 87.0% (114) received treatment for metastatic disease. Out of these, 63.3% (83) patients had chemotherapy, 57.3% (75) had hormonal therapy and 47.7% (56) had radiotherapy. In those who received treatment for metastatic disease, 53.4% (70) had both chemotherapy and hormonal therapy. Only three of the patients had surgery for metastatic disease; two patients had a ‘toilette’ mastectomy done and one patient had intramedullary nailing for an impending fracture femur (Table 2).
Out of the 91 patients with recurrent disease, 61.5% (56) received systemic chemotherapy for metastatic disease. Among these patients, 15.3% (14) presented with a visceral crisis. Only 46.2% (42) had received prior chemotherapy. In the group receiving chemotherapy for recurrence, 67.9% (38) received taxane-based therapy for metastatic disease while 32.1% (18) received non taxane-based therapy. Capcetabine was the most common single agent used (n = 16), (Table 3).
There were seven patients with HER2 enriched disease among the patients with recurrent disease. Of these, only one received herceptin in the adjuvant setting and two patients received herceptin for metastatic disease, (Table 3).
Survival
The median follow up period was 35 months (range 1–133 months). The survival rate was 31.3% at 3 years and 10.7% at 5 years (Figure 1). The median survival was 22 months (95% CI 14.98–29.01). The OS rate from primary diagnosis of breast cancer was 49.6% at 3 years and 24.4% at 5 years. The median MFS was 17 months (Range 2–105 months).
Table 1. Patient characteristics.
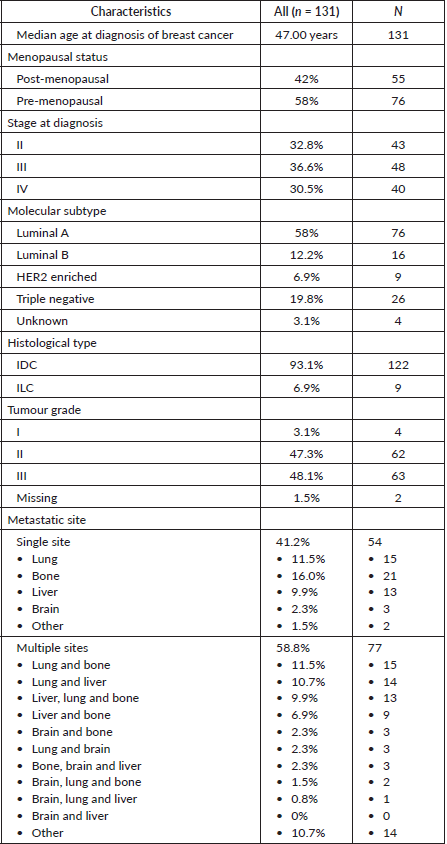
Table 2. Treatment of metastatic disease.
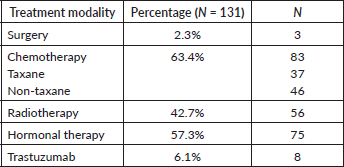
Table 3. Treatment of recurrent disease.
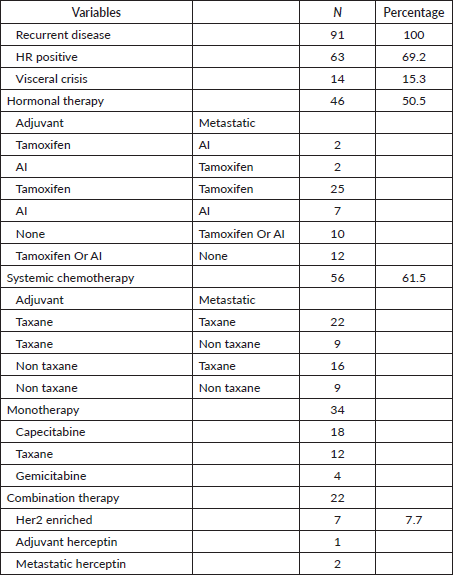
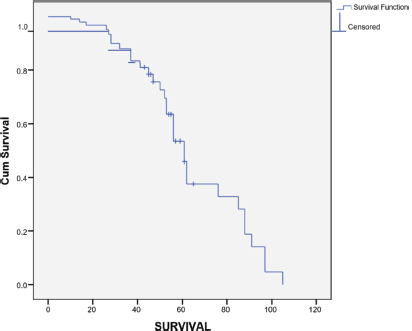
Figure 1. Survival from time from diagnosis of MBC.
Survival after diagnosis of MBC was compared for different molecular subtypes. Luminal A molecular subtype was associated with the longest median survival time at 30 months (95% CI 20.8–39.2) while HER2 enriched disease had the shortest survival at 15 months (95% CI 3.3–26.7). Survival for MBC was also compared for the stage at initial diagnosis using the Kaplan–Meier curve. Those diagnosed at stage IV had the longest median survival at 28 months (95% CI 16.8–39.2) while those initially diagnosed at stage III had the poorest median survival at 19 months (95% CI 13.5–24.5) (Figure 2).
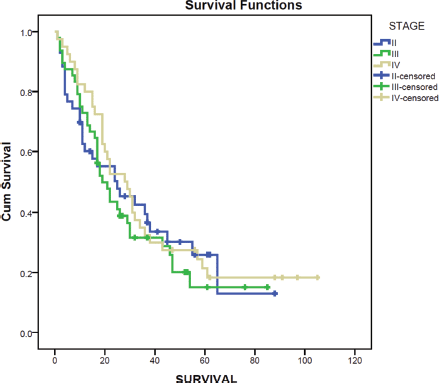
Figure 2. Survival by stage at diagnosis.
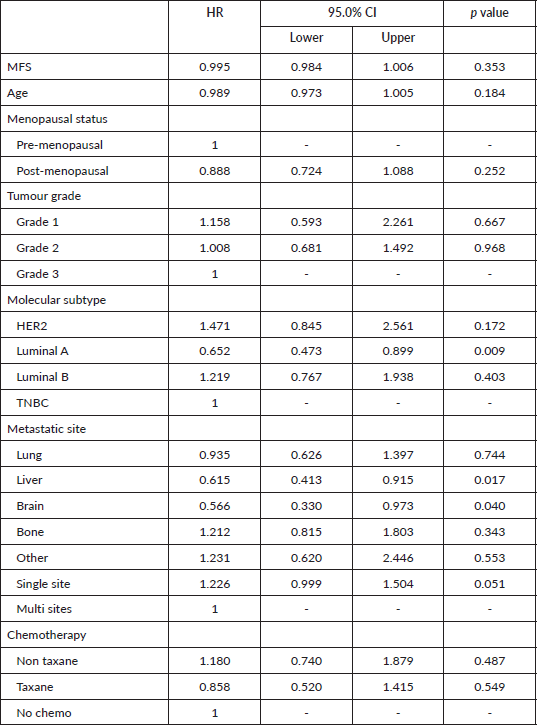
Using the Cox regression model, several potential prognostic factors were evaluated (Table 4). Disease factors which showed a significant positive effect on survival time were Luminal A molecular subtype (HR 0.652 CI 0.473–0.899). Metastasis to the liver or brain was significant negative prognostic factors (HR 0.615 CI 0.413–0.915 and HR 0.566 CI 0.330–0.973, respectively). MFS, age at diagnosis, menopausal status, tumour grade and type of chemotherapy given did not show any statistically significant effect on survival after the diagnosis of MBC on univariate analysis.
Table 4. Prognostic factors.
Discussion
Although breast cancer outcomes have improved over the last few decades, MBC is still the predominant cause of breast cancer-specific mortality [14]. Our results showed median survival after diagnosis of MBC of 22 months and 3 and 5-year survival of 31.3% and 10.7%, respectively. This is higher than that reported by Adisa et al [12] in Nigeria where they found a survival rate of 27.1% for patients with MBC at 1 year and 8% at 2 years. However, our findings are lower than studies from Uganda which found a 5-year survival rate of 39% for patients with advanced breast cancer (Stage III and IV) between 1996 and 2000 and 37.89% between 2004 and 2012 [10, 11]. Both studies from Uganda had a very large proportion of censored patients due to poor follow up and could have overestimated the cumulative survival. In our study, we included patients who were on regular follow up at our institution and therefore minimizing the bias due to loss of follow up. Similar results to ours were found in a study at a tertiary institution in Tunisia with an OS of 63% at 1 year and 12% at 5 years between the year 2000 and 2007 [13]. In that study, 94.6% of the patients received either chemotherapy, hormonal therapy or both treatments for metastatic disease. In our study, 87% of the patients received treatment for metastatic disease. Our findings are lower than 5-year survival rates for MBC in the West which range from 16% to 28% [8, 9]. This may be due to limitations in access to treatment such as anti-HER2 therapy due to high cost. Higher rates of TNBC and high grade disease may also account for the poorer outcome observed in our study. Our findings are comparable to those of African-American women in a surveillance, epidemiology and end results (SEER) database analysis from 2001 to 2011 which found a median survival of 22 months and a 5-year survival of 11% for African-American women with MBC [15]. This may be explained by similar tumour biology in our patients and African-American women.
The median age at diagnosis of BC was 47 years. This is more than a decade lower than that of Western populations where the median age at diagnosis is 62 years [16]. Advanced age at diagnosis of MBC is associated with poor outcomes. Population-based studies in Europe and America have found that patients over 70 years had higher mortality than those 50–69 years and below 50 years [5, 17–19]. In our analysis, we did not find age at diagnosis to be a significant prognostic factor. This may be due to a small sample size.
In our study, 58% (76) of our patients had bone metastasis. Patients with bone metastasis have up to twice longer survival than those with visceral metastasis [20–22]. Our study did not find bone metastasis to affect prognosis (HR 1.212 CI 0.815–1.803 p = 0.343). However, metastasis to the liver and brain was a significant negative prognostic factor (HR 0.615 CI 0.413–0.915 and HR 0.566 CI 0.330–0.973, respectively). This is in keeping with a SEER database analysis of 7,575 patients between 2010 and 2013 which found liver and brain metastasis to be a negative prognostic factor whereas lung and bone metastasis had no significant effect on survival [23]. Patients with bone metastasis may benefit from treatments such as radiotherapy and bisphosphonates whereas there is no specific therapy for brain and liver metastasis. This may account for the differences in outcomes.
Hormone receptor status is a significant prognostic factor in MBC [24]. In our study, we only found the Luminal A molecular subtype to be positively associated with survival (HR 0.652, 95% CI 0.473–0.899). Estrogen receptor (ER)/progesterone receptor (ER) positive tumours have been shown to have lower proliferation and thus lower grade [25]. They are amenable to hormonal therapy and this may explain the better survival outcomes in these patients [26]. Even though HR-positive and HER2-positive disease has been associated with a more favourable outcome, our study found the lowest median survival in patients with Luminal B and HER2 enriched molecular subtypes (median survival 17 and 15 months, respectively). This may be explained by inadequate treatment with trastuzumab. Out of 25 patients with either Luminal B or HER2 enriched molecular subtypes, only eight patients received trastuzumab therapy. This was due to the high cost of trastuzumab.
MFS has been found to be a prognostic factor in MBC. In a study examining the survival of 3,524 patients with MBC, Dawood et al [5] found that those with MBC at initial diagnosis had better survival outcomes than those with relapsed disease (27.2 versus 39.2 months, p < 0.0001). However, patients with relapse who had a DFI of more than 5 years had better survival than those with MBC at initial diagnosis [5]. In our patients, those with MBC at initial diagnosis had a better survival of 28 months (95% CI 16.8–39.2) than those initially diagnosed with stage II or III disease (25 months, 95% CI 7.2–42.8 and 19 months, 95% CI 13.5–24.5, respectively). The theory of clonal evolution due to adjuvant chemotherapy in those with recurrent disease may explain these findings. Studies have suggested that the use of chemotherapy eliminates sensitive clones and recurrent disease consists of resistant cells [27]. In a study of 815 patients with MBC, Lobbezoo et al [28] found no significant difference in survival between patients with MBC at initial diagnosis and those with relapse with a DFI >24 months. However, those with DFI <24 months had a poorer survival than those with de novo MBC. Patients with a longer progression free survival are likely to have less aggressive tumours and therefore a better prognosis [29].
With earlier detection of breast cancer and use of adjuvant chemotherapy, most patients with MBC will have been exposed to prior systemic chemotherapy. Taxanes have been shown to be effective in patients previously treated with anthracyclin-based chemotherapy [30]. A systematic review of taxane-based therapy for MBC found that taxane-based therapy improved OS in patients with MBC compared to non-taxane-based therapy [31]. However, there was significant heterogeneity among the trials due to the varying comparator regimens. Although we found a better survival in patients who received taxane-based therapy compared to non-taxane based therapy, the difference was not statistically significant. This may be due to a small sample size.
Limitations
The limitations of our study were small sample size and the retrospective study design. Some records such as tumour grade and chemotherapy regimen in some of the charts were missing. During the period of this study, cyclin-dependent kinase 4/6 inhibitor therapy was not standard of care and was not given to the patients. Molecular tests such as phosphoinositide 3-kinase mutations, breast cancer susceptibility gene 1 or 2 mutations (BRCA1 OR BRCA2) and PD-L1 expression were not done and the relevant targeted therapy was not given.
Conclusion
Our study found survival rates for patients diagnosed with MBC to be lower compared to studies from Western countries but higher than studies from Sub-Saharan Africa. Survival rates were similar to those of African American women. Luminal A molecular subtype was a significant positive prognostic factor and metastasis to the liver or brain were found to be significant negative prognostic factors. Improved access to adequate treatment for MBC is required in the region.
Recommendation statement
Further studies are needed to look into the relationship between the diagnoses of de novo versus relapsed MBC and its impact on the survival outcome. Larger prospective studies on associations of risk factors and breast cancer subtypes with survival outcomes are also recommended.
Ethics statement
The Aga Khan University Research Ethics Committee gave ethical clearance (Reference number 2020/IERC-46 (v2)).
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Contributorship statement
All authors contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Conflicts of interest
There are no competing interests for any author.
Funding
There is no funding to report for this submission.
References
1. Observatory TGC (2020) New Global Cancer Data [https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf] Date accessed: 07/01/21
2. Jedy-Agba E, McCormack V, and Adebamowo C, et al (2016) Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis Lancet Glob Health 4(12) e923–e935 https://doi.org/10.1016/S2214-109X(16)30259-5 PMID: 27855871 PMCID: 5708541
3. Ikpatt OF, Kuopio T, and Collan Y (2002) Proliferation in African breast cancer: biology and prognostication in Nigerian breast cancer material Mod Pathol 15(8) 783–789 https://doi.org/10.1097/01.MP.0000021764.03552.BD PMID: 12181262
4. Sørlie T, Perou CM, and Tibshirani R, et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications Proc Nat Acad Sci 98(19) 10869–10874 https://doi.org/10.1073/pnas.191367098 PMID: 11553815 PMCID: 58566
5. Dawood S, Broglio K, and Ensor J, et al (2010) Survival differences among women with de novo stage IV and relapsed breast cancer Ann Oncol 21(11) 2169–2174 https://doi.org/10.1093/annonc/mdq220 PMID: 20427349 PMCID: 2962259
6. Wambua MD, Degu A, and Tegegne GT (2022) Treatment outcomes and its associated factors among breast cancer patients at Kitui Referral Hospital SAGE Open Med 10 20503121211067857 https://doi.org/10.1177/20503121211067857 PMID: 35024144 PMCID: 8744162
7. Beslija S, Bonneterre J, and Burstein HJ, et al (2009) Third consensus on medical treatment of metastatic breast cancer Ann Oncol 20(11) 1771–1785 https://doi.org/10.1093/annonc/mdp261 PMID: 19608616
8. Rapiti E, Verkooijen HM, and Vlastos G, et al (2006) Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis J Clin Oncol 24(18) 2743–2749 https://doi.org/10.1200/JCO.2005.04.2226 PMID: 16702580
9. Andre F, Slimane K, and Bachelot T, et al (2004) Breast cancer with synchronous metastases: trends in survival during a 14-year period J Clin Oncol 22(16) 3302–3308 https://doi.org/10.1200/JCO.2004.08.095 PMID: 15310773
10. Galukande M, Wabinga H, and Mirembe F (2015) Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study World J Surg Oncol 13(1) 220 https://doi.org/10.1186/s12957-015-0632-4 PMID: 26187151 PMCID: 4506617
11. Gakwaya A, Kigula-Mugambe JB, and Kavuma A, et al (2008) Cancer of the breast: 5-year survival in a tertiary hospital in Uganda Br J Cancer 99 63 https://doi.org/10.1038/sj.bjc.6604435 PMID: 18577991 PMCID: 2453032
12. Adisa AO, Arowolo OA, and Akinkuolie AA, et al (2011) Metastatic breast cancer in a Nigerian tertiary hospital Afr Health Sci 11(2) PMID: 21857862 PMCID: 3158525
13. Khanfir A, Lahiani F, and Bouzguenda R, et al (2013) Prognostic factors and survival in metastatic breast cancer: a single institution experience Rep Pract Oncol Radiother 18(3) 127–132 https://doi.org/10.1016/j.rpor.2013.01.001 PMCID: 3863182
14. Gobbini E, Ezzalfani M, and Dieras V, et al (2018) Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort Eur J Cancer 96 17–24 https://doi.org/10.1016/j.ejca.2018.03.015 PMID: 29660596
15. Wieder R, Shafiq B, and Adam N (2020) Greater survival improvement in African American vs. caucasian women with hormone negative breast cancer J Cancer 11(10) 2808–2820 https://doi.org/10.7150/jca.39091 PMID: 32226499 PMCID: 7086262
16. Howlader N, Noone AM, and Krapcho M et al (2017) SEER Cancer Statistics Rreview, 1975-2014 (Bethesda: National Cancer Institute) p 1–12
17. Ernst MF, van de Poll-Franse LV, and Roukema JA, et al (2007) Trends in the prognosis of patients with primary metastatic breast cancer diagnosed between 1975 and 2002 Breast 16(4) 344–351 https://doi.org/10.1016/j.breast.2007.01.001 PMID: 17303426
18. Largillier R, Ferrero JM, and Doyen J, et al (2008) Prognostic factors in 1038 women with metastatic breast cancer Ann Oncol 19(12) 2012–2019 https://doi.org/10.1093/annonc/mdn424 PMID: 18641006 PMCID: 2733115
19. Chen MT, Sun HF, and Zhao Y, et al (2017) Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis Sci Rep 7(1) 1–8
20. Solomayer EF, Diel IJ, and Meyberg GC, et al (2000) Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis Breast Cancer Res Treat 59(3) 271–278 https://doi.org/10.1023/A:1006308619659 PMID: 10832597
21. Eichbaum MHR, Kaltwasser M, and Bruckner T, et al (2005) Prognostic factors for patients with liver metastases from breast cancer Breast Cancer Res Treat 96(1) 53 https://doi.org/10.1007/s10549-005-9039-1 PMID: 16319993
22. Chang J, Clark GM, and Allred DC, et al (2003) Survival of patients with metastatic breast carcinoma Cancer 97(3) 545–553 https://doi.org/10.1002/cncr.11083 PMID: 12548595
23. Wu SG, Li H, and Tang LY, et al (2017) The effect of distant metastases sites on survival in de novo stage-IV breast cancer: a SEER database analysis Tumor Biol 39(6) 1010428317705082 https://doi.org/10.1177/1010428317705082
24. Idirisinghe PKA, Thike AA, and Cheok PY, et al (2010) Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer: pathologic correlations and clinical significance Am J Clin Pathol 133(3) 416–429 https://doi.org/10.1309/AJCPJ57FLLJRXKPV PMID: 20154280
25. Loi S, Haibe-Kains B, and Desmedt C, et al (2007) Definition of clinically distinct molecular subtypes in estrogen receptor–positive breast carcinomas through genomic grade J Clin Oncol 25(10) 1239–1246 https://doi.org/10.1200/JCO.2006.07.1522 PMID: 17401012
26. Lobbezoo DJ, van Kampen RJW, and Voogd AC, et al (2013) Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome Breast Cancer Res Treat 141(3) 507–514 https://doi.org/10.1007/s10549-013-2711-y PMID: 24104881
27. Aparicio S and Caldas C (2013) The implications of clonal genome evolution for cancer medicine N Engl J Med 368(9) 842–851 https://doi.org/10.1056/NEJMra1204892 PMID: 23445095
28. Lobbezoo DJA, van Kampen RJW, and Voogd AC, et al (2015) Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer 112(9) 1445–1451 https://doi.org/10.1038/bjc.2015.127 PMID: 25880008 PMCID: 4453676
29. den Brok WD, Speers CH, and Gondara L, et al (2017) Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed Breast Cancer Res Treat 161(3) 549–556 https://doi.org/10.1007/s10549-016-4080-9
30. Figgitt DP and Wiseman LR (2000) Docetaxel Drugs 59(3) 621–651 https://doi.org/10.2165/00003495-200059030-00015
31. Ghersi D, Wilcken N, and Simes R (2005) A systematic review of taxane-containing regimens for metastatic breast cancer Br J Cancer 93(3) 293–301 https://doi.org/10.1038/sj.bjc.6602680 PMID: 16052223 PMCID: 2361568






