Oncologic safety of breast conservation following NACT in women with locally advanced breast cancer
Sanjit Kumar Agrawal1, Dimple Patel1, Pradyumn Shenoy1, Rosina Ahmed1, Indu Arun2 and Sanjoy Chatterjee3
1Department of Breast Oncosurgery, Tata Medical Center, Kolkata 700156, India
2Department on Oncopathology, Tata Medical Center, Kolkata 700156, India
3Department of Radiation Oncology, Tata Medical Center, Kolkata 700156, India
Abstract
Introduction: Breast conservation surgery (BCS) is the accepted standard of treatment for early breast cancer, with evidence from randomized controlled and population-based studies. The oncological outcome of BCS in locally advanced breast cancer (LABC) is mainly available from retrospective series with a small sample size and a shorter follow-up duration.
Methods: A retrospective observational study of 411 non-metastatic LABC patients who received neoadjuvant chemotherapy (NACT) followed by surgery from 2011 to 2016. We retrieved the data from a prospectively maintained database and electronic medical records. Survival data were analyzed by Kaplan–Meier curves and Cox regression using Statistical Package for the Social Sciences 25 and STATA 14.
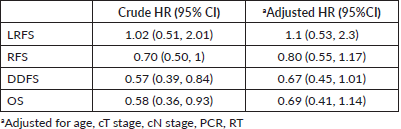
Results: 146/411 (35.5%) women had BCS with a margin positivity rate of 3.42%. With a median follow-up of 64 months (IQR 61, 66), the local relapse rate was 8.9% in BCS and 8.3% after mastectomy. The estimated 5-year locoregional recurrence-free survival (LRFS), recurrence-free survival (RFS), distant disease-free survival (DDFS) and overall survival (OS) rates of BCS were 86.9%, 63.9%, 71% and 79.3%, and 90.1%, 57.9%, 58.3% and 71.5% in the mastectomy group. On univariate analysis, BCS showed superior survival outcomes compared to mastectomy (unadjusted HR (95% CI) for RFS: 0.70 (0.50–1), DDFS: 0.57 (0.39–0.84), OS: 0.58 (0.36–0.93)). After adjusting for age, cT stage, cN stage, poorer chemotherapy response (ypT0/is, N0) and radiotherapy, BCS and mastectomy groups were found comparable in terms of LRFS (HR: 1.1, 0.53–2.3), DDFS (HR: 0.67, 0.45–1.01), RFS (HR: 0.80, 0.55–1.17) and OS (HR: 0.69, 0.41–1.14).
Conclusion: BCS is technically feasible in LABC patients. LABC patients who respond well to NACT can be offered BCS without compromising survival outcomes.
Keywords: breast conservation surgery, locally advanced breast cancer, post chemotherapy
Correspondence to: Sanjit Kumar Agrawal
Email: sanjitgrwl@gmail.com
Published: 25/05/2023
Received: 12/03/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Neoadjuvant chemotherapy (NACT) is the standard treatment for non-metastatic locally advanced breast cancer (LABC). It offers the advantage of downstaging the inoperable disease and increases the prospect of breast conservation surgery (BCS) in patients who would otherwise have been candidates for mastectomy [1–3]. NACT allows the assessment of in vivo chemosensitivity, which could guide subsequent drug selection [4]. Furthermore, NACT has also exhibited benefits in de-escalating axillary surgery, with sentinel lymph node biopsy (SLNB) now being the standard for clinically node-negative patients at presentation. The role of SLNB in initially node-positive patients is also being evaluated in the post-NACT setting [5]. Patients treated with NACT have shown to have similar recurrence-free survival (RFS) and overall survival (OS) in comparison with upfront surgery followed by adjuvant therapy, with the added advantage of breast conservation and axilla preservation as described above [6–8].
The safety of BCS in early breast cancer (EBC) has been well established [9]. Despite a mounting rise in the literature that supports the feasibility of BCS in LABC, concerns exist regarding the increased likelihood of locoregional recurrence (LRR) or ipsilateral breast tumour recurrence reported in patients who underwent BCS after NACT in LABC [10–13]. Surgical margins free of tumour have a significant impact on the outcome of BCS, and in some patients, it may be challenging to achieve a clear margin in LABC post-NACT due to a honeycomb pattern response [13].
Unlike EBC, no randomized trial has been conducted to establish the efficacy of BCS in LABC. The only data available is from small observational studies and case series. In India, approximately 29%–52% of women present at stage III [14], with peak age between 40 and 50 years [15]. To date, only one large single-centre study has been published from India addressing the safety of BCS in LABC, which is the common presentation of most patients in lower-middle-income countries. The objective of our study was to compare the survival outcomes of BCS versus mastectomy in LABC post-NACT.
Patients and methods
A retrospective review of medical records identified 619 patients who received NACT at Tata Medical Center, Kolkata, between January 2011 and December 2016 (ethics committee waiver No: EC/WV/TMC/003/19). 619 patients were analyzed, as illustrated in Figure 1. All the patients underwent core biopsy for diagnosis.
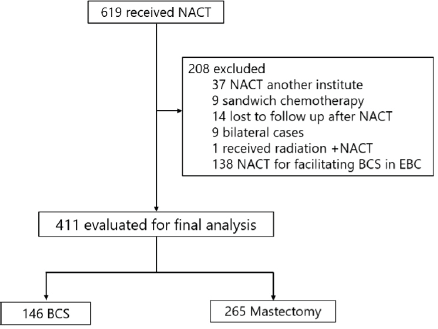
Figure 1. Patient selection. NACT: Neo-adjuvant chemotherapy; BCS: Breast conservation surgery; EBC: Early breast cancer.
The patients were staged as per clinical and radiological findings before systemic chemotherapy. The fine needle aspiration cytology of the axillary nodes was done only in the clinical normal axilla with suspicious metastatic axillary nodes in the radiological examination. All patients underwent a baseline mammogram, and those planned for BCS also underwent a post-NACT mammogram to assess the response. In addition, before initiating chemotherapy, a metastatic workup (CT thorax and whole abdomen + whole body bone scan) was done in all the patients. The inflammatory breast cancer patients were offered mastectomy only as per the institutional protocol.
The chemotherapy regimen was given as six or eight cycles at intervals of 3 weeks, with the majority receiving anthracycline followed by a taxane. The final decision for the type of surgery was based on the clinical and radiological response. Along with breast surgery, all patients also underwent level 3 axillary dissection. Patients who underwent BCS and had a positive margin on the final histopathology report were treated with cavity excision or completion mastectomy. Per institutional protocol, the surgery was followed by radiation (chest wall/breast and SCF) and adjuvant endocrine/target therapy as per the immunohistochemistry status.
Patients were followed up clinically every 3 months for the initial 2 years and 6 months thereafter, up to 5 years. Subsequently, the follow-up was yearly. Annual mammography was done in all patients.
RFS was defined as the interval between the date of diagnosis and the date of first recurrence or death. Locoregional recurrence-free survival (LRFS) was defined as the interval between the date of diagnosis and the date of local (breast/chest wall/loco-regional) recurrence or death. Distant-disease-free survival (DDFS) was calculated from the date of diagnosis to distant relapse or death. The OS was defined as the interval between the date of diagnosis and the date of death or last known follow-up time.
The data normalcy was checked by the Shapiro–Wilks test. The median (IQR) and percentage were used for summary statistics. We used the Mann–Whitney test to compare continuous variables and the chi-square/Fisher exact test as applicable for categorical variables. Survival data were analyzed by Kaplan–Meier curves and Cox regression. p < 0.05 was considered statistically significant. The analysis was done using Statistical Package for the Social Sciences 25 and STATA 14 software.
Results
411 patients with LABC who received NACT were included in the analysis. The median age was 49.2 years (IQR 43, 56), and BMI was 26 kg/m2 (IQR 23.1, 29.7). Invasive ductal carcinoma comprised 96.3% of cases. At presentation, 261 (64.1%) had T4 disease, whereas 124 (30.1%) had T3 disease. 269 (65.4%) patients were N1, and 105 (25.6%) were N2–N3. 360 (87.6%) patients received anthracycline and taxane, while 31 (7.5%) received anthracycline only. 146 (35.5%) patients were treated with BCS with axillary dissection, and 265 (64.6%) underwent modified radical mastectomy (MRM). For BCS, the margin positivity rate was 3.42% (Table 1).
Comparing the two groups (Table 2), the BCS patients were younger than those who had a mastectomy (45.1 versus 50 years). Mastectomy patients had higher T stage (T4: 70.6 versus 52.1%) and N stage and had a poorer chemotherapy response (pCR 14.3 versus 22.6%) compared to those who had BCS, as summarized in Table 2.
Follow-up and survival analysis
With a median follow-up of 64 months (IQR 61, 66), there were 7 local, 6 regional and 36 distant relapses in the BCS group, and 5 local, 17 regional and 106 distant relapses in the mastectomy group (Table 3). The estimated 5-year LRFS, RFS, DDFS and OS rates of the BCS group were 86.9%, 63.9%, 71% and 79.3%, respectively, and those of the mastectomy group were 90.1%, 57.9%, 58.3 and 71.5%, respectively (Figure 2). On univariate analysis, BCS showed superior survival outcomes compared to mastectomy (unadjusted HR (95% CI) for RFS: 0.70 (0.50–1), DDFS: 0.57 (0.39–0.84) and OS: 0.58 (0.36–0.93)). After adjusting for age, cT stage, cN stage, PCR (ypT0/is, N0) and radiotherapy, BCS and mastectomy groups were found equivalent in terms of LRFS (HR: 1.1, 0.53–2.3), DDFS (HR: 0.67, 0.45–1.01), RFS (HR:0.80, 0.55–1.17) and OS (HR:0.69, 0.41–1.14) (Table 4).
Table 1. Demographics and histopathological feature (n = 411).
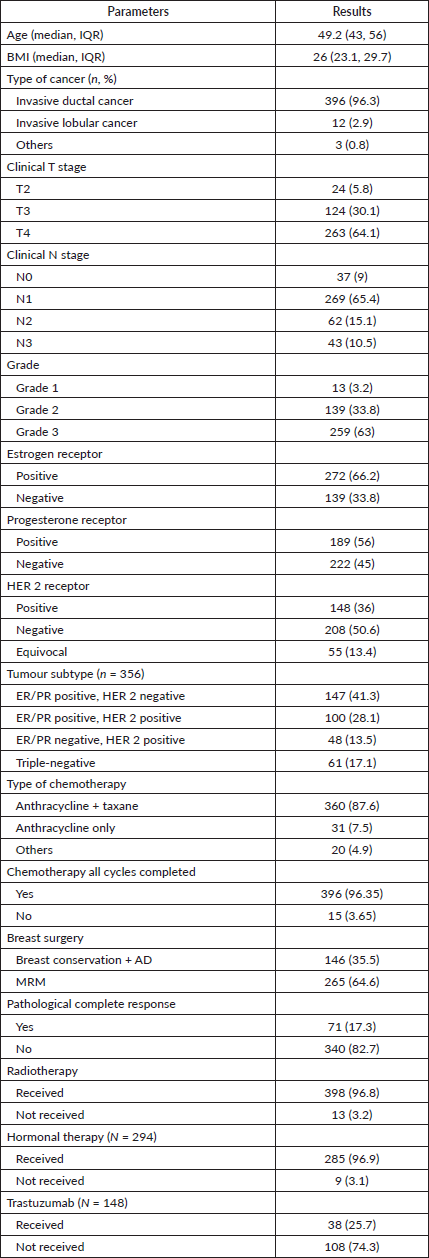
Table 2. Comparison between two groups (BCS versus mastectomy).
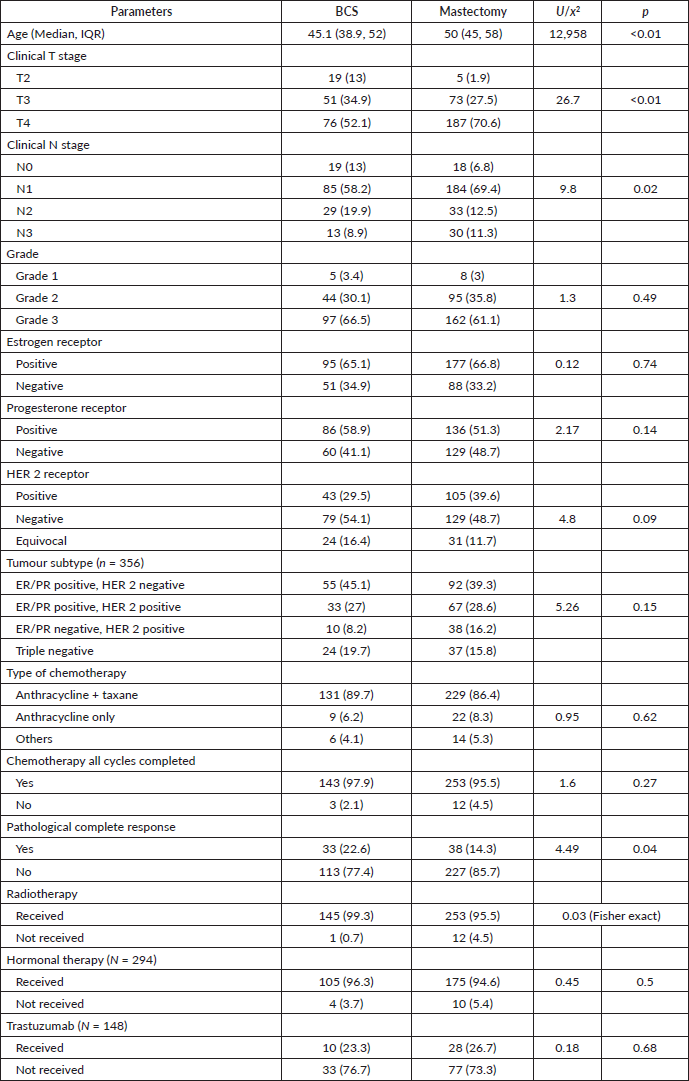
Table 3. Type of recurrence.
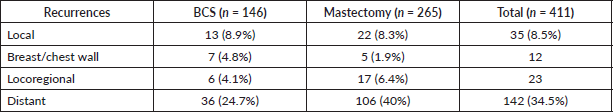
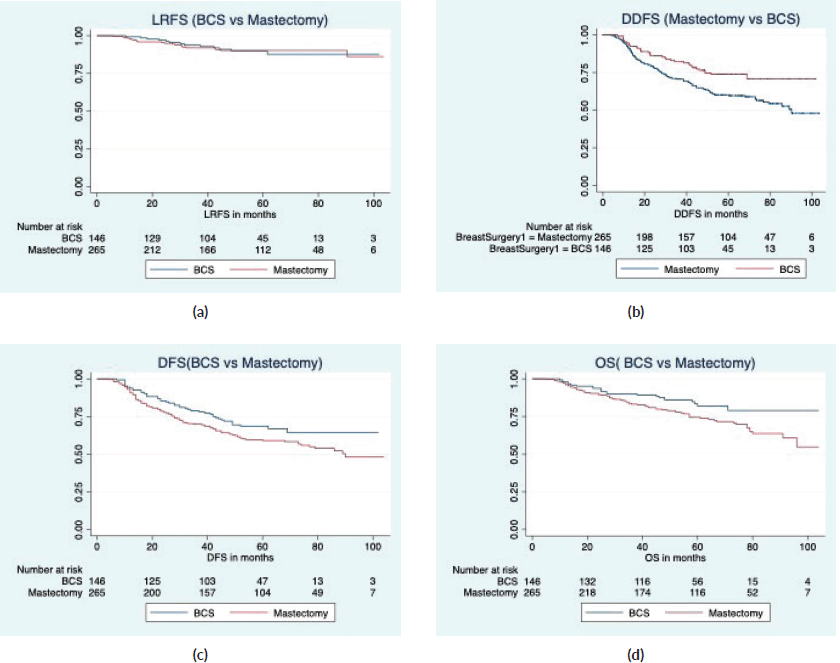
Figure 2. Survival comparison (Kaplan–Meier graph) between BCS versus mastectomy in LABC patients. (a): LRFS comparison between BCS and mastectomy. (b): DDFS comparison between BCS and mastectomy. (c): RFS comparison between BCS and mastectomy. (d): OS comparison between BCS and mastectomy.
Table 4. Cox regression survival analysis (BCS versus mastectomy).
Discussion
NACT has a proven advantage in enabling BCS in patients who were not suitable candidates during the initial presentation. Our study evaluated the local recurrence rates of BCS compared with mastectomy in patients with LABC who received NACT. The BCS in selected LABC patients has shown equivalent survival outcomes in our cohort.
The breast cancer patients subjected to NACT may show a honeycomb pattern response preventing an accurate assessment of surgical margins. Thus, concern regarding the increased likelihood of recurrence in LABC patients treated with BCS after NACT is a major deterrent in the application of BCS in advanced breast cancer. Some studies have reported higher recurrence rates; however, for a large percentage of these patients, radiation therapy was the only local-regional treatment without an attempt to resect the primary tumour site, and some included patients with inflammatory carcinoma [16–18]. In contrast, other recent studies have not found any significant difference in local recurrence rate following BCS in LABC patients [19–22].
In our study, the LR was 8.9% for BCS and 8.3% for mastectomy. The results are comparable to the study by Chou et al [23] (N = 1,047, 59.2 months follow-up), with an LRR of 8.8% in the BCS group and 10.7% in the mastectomy group. Similarly, a meta-analysis of eight trials published in 2016 also showed a local recurrence rate of 9.2% in the BCS versus 8.3% in the mastectomy (odds ratio 1.07, 95% confidence interval 0.28–1.48; p = 0.66) [20].
The EBC Trialists’ Collaborative Group meta-analysis reported that NACT was associated with more frequent local recurrence in BCS patients (15-year LRR 21.4% for NACT versus 5.9% for adjuvant chemotherapy). The authors concluded that the likely reason for this was an increase in BCS rate in women who responded well to NACT. However, patients included in the meta-analysis were treated between 1983 and 2002 and have received chemotherapy regimens that have now been abandoned. Only 18·9% had received taxane-based chemotherapy, while anti-HER2 drugs were unavailable. For some patients with complete clinical responses, surgery was omitted altogether. There was no information about imaging modalities used to assess the extent of disease, the rationale for conversion from mastectomy to breast-conserving therapy, preoperative tumour localization, axillary management, and radiotherapy or BRCA gene status [24].
The NSABP B-27 trial reported that anthracycline-based regimens with the addition of taxane were associated with higher pCR rates and better local control [25]. In our study, a total of 87.6% patients and 89.7% of those treated with BCS had received anthracyclines + taxane. The 8-year isolated breast recurrence rate was 4.8% in the BCS group. The results can be compared to the study by Fowble et al [26], who reported the 10-year results of BCS in early-stage cancer, which reported isolated breast recurrence of 6% at 5 years and 16% at 10 years.
In our study, after adjusting for age, cT stage, cN stage, PCR (ypT0/is, N0) and radiotherapy, no statistically significant difference was observed in LRFS, DDFS, RFS and OS between BCS and mastectomy groups. A single large retrospective cohort study from India with 5 years follow-up by Parmar et al [27] reported better RFS and lower recurrence in the BCS group, suggesting the feasibility of BCS in LABC in the NACT setting. Several other studies have also reported similar findings, establishing the oncologic safety of BCS in LABC (Table 5).
This study has some potential limitations. First, this was a retrospective study, subject to some inherent biases like different socioeconomic status, comorbidities and loss of follow-up among patients who had a poor outcome. Additionally, all HER2-positive patients did not receive targeted therapy, which restricts the response assessment in this group of patients. The current availability of biosimilar trastuzumab has overcome the affordability issue significantly in developing countries. However, the strength of this study lies in the single institution design, which allowed uniformity in all facets.
Table 5. Studies on the oncologic safety of BCS in LABC.

Conclusion
The result of this study demonstrates that, following NACT, rates of LRFS, DDFS, RFS and OS are comparable between BCS and mastectomy. The importance of a multidisciplinary approach with adequate chemotherapy (anthracycline and taxane) and good localisation technique should be considered before offering BCS in LABC patients. These results support BCS as a safe and effective alternative to mastectomy in a selected group of LABC patients.
Conflicts of interest
The authors declare no conflict of interest for this study.
Funding disclosure
No external funding received for the study.
References
1. Fitzal F, Mittlboeck M, and Steger G, et al (2012) Neoadjuvant chemotherapy increases the rate of breast conservation in lobular-type breast cancer patients Ann Surg Oncol 19 519–526 https://doi.org/10.1245/s10434-011-1879-9
2. Chen S, Chen CM, and Yu KD, et al (2012) A prognostic model to predict outcome of patients failing to achieve pathological complete response after anthracycline-containing neoadjuvant chemotherapy for breast cancer J Surg Oncol 105 577–585 https://doi.org/10.1002/jso.22140
3. Ishitobi M, Ohsumi S, and Inaji H, et al (2012) Ipsilateral breast tumor recurrence (IBTR) in patients with operable breast cancer who undergo breast-conserving treatment after receiving neoadjuvant chemotherapy Cancer 118 4385–4393 https://doi.org/10.1002/cncr.27377 PMID: 22252882
4. Cortazar P, Zhang L, and Untch M, et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis Lancet 384 164–172 https://doi.org/10.1016/S0140-6736(13)62422-8 PMID: 24529560
5. Wazir U and Mokbel K (2020) De-escalation of axillary surgery in the neoadjuvant chemotherapy (NACT) setting for breast cancer: is it oncologically safe? Anticancer Res 40 5351–5354 https://doi.org/10.21873/anticanres.14542 PMID: 32988853
6. Makris A, Powles TJ, and Ashley SE, et al (1998) A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer Ann Oncol 9 1179–1184 https://doi.org/10.1023/A:1008400706949 PMID: 9862047
7. Chen AM, Meric-Bernstam F, and Hunt KK, et al (2004) Breast conservation after neoadjuvant chemotherapy: the M.D. Anderson cancer center experience JCO 22 2303–2312 https://doi.org/10.1200/JCO.2004.09.062
8. Singletary SE, McNeese MD, and Hortobagyi GN (1992) Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma Cancer 69 2849–2852 https://doi.org/10.1002/1097-0142(19920601)69:11<2849::AID-CNCR2820691134>3.0.CO;2-P PMID: 1571916
9. Fisher B, Anderson S, and Bryant J, et al (2009) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer [Internet] Date accessed: 22/06/21
10. Smith IE and Lipton L (2001) Preoperative/neoadjuvant medical therapy for early breast cancer Lancet Oncol 2 561–570 https://doi.org/10.1016/S1470-2045(01)00490-9
11. Rouzier R, Extra JM, and Carton M, et al (2021) Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery J Clin Oncol [Internet] Date accessed: 06/06/21
12. Broët P, Scholl SM, and de la Rochefordière A, et al (1999) Short and long‐term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial Breast Cancer Res Treat 58 151–156 https://doi.org/10.1023/A:1006339918798
13. Fuksa L, Micuda S, and Grim J, et al (2012) Predictive biomarkers in breast cancer: their value in neoadjuvant chemotherapy Cancer Investig 30 663–678 https://doi.org/10.3109/07357907.2012.725441
14. Bhattacharyya GS, Doval DC, and Desai CJ, et al (2020) Overview of breast cancer and implications of overtreatment of early-stage breast cancer: an Indian perspective JCO Glob Oncol 6 789–798 https://doi.org/10.1200/GO.20.00033 PMID: 32511068 PMCID: 7328098
15. Alkabban FM and Ferguson T (2021) Breast Cancer [Internet] StatPearls (Treasure Island: StatPearls Publishing) Date accessed: 14/06/21
16. Jacquillat C, Weil M, and Baillet F, et al (1990) Results of neoadjuvant chemotherapy and radiation therapy in the breast-conserving treatment of 250 patients with all stages of infiltrative breast cancer Cancer 66 119–129 https://doi.org/10.1002/1097-0142(19900701)66:1<119::AID-CNCR2820660122>3.0.CO;2-3 PMID: 2112976
17. Danforth DN, Zujewski J, and O’Shaughnessy J, et al (1998) Selection of local therapy after neoadjuvant chemotherapy in patients with stage IIIA,B breast cancer Ann Surg Oncol 5 150–158 https://doi.org/10.1007/BF02303848 PMID: 9527268
18. Merajver SD, Weber BL, and Cody R, et al (1997) Breast conservation and prolonged chemotherapy for locally advanced breast cancer: the University of Michigan experience JCO 15 2873–2881 https://doi.org/10.1200/JCO.1997.15.8.2873
19. Sun Y, Liao M, and He L, et al (2017) Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after good response to neoadjuvant chemotherapy: a PRISMA-compliant systematic review and meta-analysis Medicine (Baltimore) 96 e8367 https://doi.org/10.1097/MD.0000000000008367 PMID: 29069026 PMCID: 5671859
20. Zhou X and Li Y (2016) Local recurrence after breast-conserving surgery and mastectomy following neoadjuvant chemotherapy for locally advanced breast cancer - a meta-analysis Breast Care (Basel) 11 345–351 https://doi.org/10.1159/000450626 PMID: 27920628 PMCID: 5123019
21. Sweeting RS, Klauber-Demore N, and Meyers MO, et al (2011) Young women with locally advanced breast cancer who achieve breast conservation after neoadjuvant chemotherapy have a low local recurrence rate Am Surg 77 850–855 https://doi.org/10.1177/000313481107700718 PMID: 21944346 PMCID: 4167782
22. Levy A, Borget I, and Bahri M, et al (2014) Loco-regional control after neo-adjuvant chemotherapy and conservative treatment for locally advanced breast cancer patients Breast J 20 381–387 https://doi.org/10.1111/tbj.12277 PMID: 24890310
23. Chou HH, Chung WS, and Ding RY, et al (2021) Factors affecting locoregional recurrence in breast cancer patients undergoing surgery following neoadjuvant treatment BMC Surg 21 160 https://doi.org/10.1186/s12893-021-01158-7 PMID: 33757489 PMCID: 7988904
24. Asselain B, Barlow W, and Bartlett J, et al (2018) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomized trials Lancet Oncol 19 27–39 https://doi.org/10.1016/S1470-2045(17)30777-5
25. Bear HD, Anderson S, and Smith RE, et al (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: national surgical adjuvant breast and bowel project protocol B-27 JCO 24 2019–2027 https://doi.org/10.1200/JCO.2005.04.1665
26. Fowble BL, Solin LJ, and Schultz DJ, et al (1991) Ten year results of conservative surgery and irradiation for stage I and II breast cancer Int J Radiat Oncol Biol Phys 21 269–277 https://doi.org/10.1016/0360-3016(91)90771-U PMID: 1648040
27. Parmar V, Krishnamurthy A, and Hawaldar R, et al (2006) Breast conservation treatment in women with locally advanced breast cancer - experience from a single centre Int J Surg 4 106–114 https://doi.org/10.1016/j.ijsu.2006.01.004
28. Barranger E, Antomarchi J, and Chamorey E, et al (2015) Effect of neoadjuvant chemotherapy on the surgical treatment of patients with locally advanced breast cancer requiring initial mastectomy Clin Breast Cancer 15 e231–e235 https://doi.org/10.1016/j.clbc.2015.03.001 PMID: 25887149
29. Cho JH, Park JM, and Park HS, et al (2013) Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer J Surg Oncol 108 531–536 https://doi.org/10.1002/jso.23439 PMID: 24115142






