Virtual reality and surgical oncology
Peng Yun Ng1,2, Eric G Bing3, Anthony Cuevas4, Ajay Aggarwal1,2,5, Benjamin Chi6, Sudha Sundar7,8, Mulindi Mwanahamuntu9, Miriam Mutebi10, Richard Sullivan11, Groesbeck P Parham10
1King’s College London, London WC2R 2LS, UK
2Guy’s and St Thomas’ Trust, London SE1 9R, UK
3Institute for Leadership Impact, Southern Methodist University, Dallas, TX 75205, USA
4Department of Teaching and Learning, Technology-Enhanced Immersive Learning Cluster, Annette Simmons School of Education and Human Development, Southern Methodist University, Dallas, TX 75205, USA
5London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK
6Icahn School of Medicine, New York, NY 10029-6574, USA
7Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham B152TT, UK
8Pan Birmingham Gynaecological Cancer Centre, City Hospital, Birmingham, B187QH, UK
9Women and Newborn Hospital, University Teaching Hospital, Lusaka 10101, Zambia
10Department of Surgery, Aga Khan University Hospital, Nairobi 30270-00100, Kenya
11Conflict & Health Research Group, King’s College London, London WC2R 2LS, UK
Abstract
More than 80% of people diagnosed with cancer will require surgery. However, less than 5% have access to safe, affordable and timely surgery in low- and middle-income countries (LMICs) settings mostly due to the lack of trained workforce. Since its creation, virtual reality (VR) has been heralded as a viable adjunct to surgical training, but its adoption in surgical oncology to date is poorly understood. We undertook a systematic review to determine the application of VR across different surgical specialties, modalities and cancer pathway globally between January 2011 and 2021. We reviewed their characteristics and respective methods of validation of 24 articles. The results revealed gaps in application and accessibility of VR with a proclivity for high-income countries and high-risk, complex oncological surgeries. There is a lack of standardisation of clinical evaluation of VR, both in terms of clinical trials and implementation science. While all VR illustrated face and content validity, only around two-third exhibited construct validity and predictive validity was lacking overall. In conclusion, the asynchrony between VR development and actual global cancer surgery demand means the technology is not effectively, efficiently and equitably utilised to realise its surgical capacity-building potential. Future research should prioritise cost-effective VR technologies with predictive validity for high demand, open cancer surgeries required in LMICs.
Keywords: virtual reality, augmented reality, surgical oncology, global oncology, surgery, cancer, implementation science, technology, innovation
Correspondence to: Peng Yun Ng
Email: peng.ng3@nhs.net
Published: 23/03/2023
Received: 30/12/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Of 15.2 million new patients diagnosed with cancer each year, more than 80% require surgery at least once during the course of their disease [1–3]. However, less than 5% of patients from low-income countries and around 20% of patients from middle-income countries (LMICs) have access to basic cancer surgery due to lack of human resource and inadequate healthcare infrastructure [2]. Developing novel tools as public goods to help build capacity and capability in surgical oncology across very different resource settings has become one of the most pressing global needs.
Virtual reality (VR) is defined as a computer-generated simulation of an environment, where it interacts with users in a realistic way. In the past decades, the technology has evolved from a non-immersive, two-dimensional screen-based interaction into an immersive three-dimensional experience. In an immersive VR experience, one can interact with the virtual setting in a realistic manner through digital transposition of real objects and settings. With recent technology advancement in VR which improved user orientation and user sensory experience through haptic feedback, its application gained traction not only in surgical oncology where it is heralded as an effective adjunct to surgical training, but also in different fields of cancer management from radiotherapy through to rehabilitation.
The advantages of VR simulation in surgical oncology training lie in its ability to allow trainees to learn, practice and master technical skills, clinical judgement, and the in-and-out of surgical procedures in a safe and controlled environment. This has become more important nowadays, as trainees face steeper learning curve to master increasingly complex surgical procedures with more restricted training hours and higher patient expectation. There is growing evidence behind how VR can be utilised to build surgical capacity and capability through reducing the time required of trainees to develop surgical proficiency in the operating room. Besides surgical training, VR also plays an increasingly important role in optimising operative planning through better illustration of individual anatomical structure, particularly in high-risk and complex procedures.
In non-surgical oncology, VR has also grown into a new avenue to engage with cancer patients for patient education, rehabilitation and psychosocial support. The immersive experience provides a new avenue for patients to learn the complexity of their cancer treatment, such as radiotherapy, before they consent to the treatment and experience it in reality. During and after cancer treatment, patients also engage with immersive VR experience to rehabilitate physically and to process physical and psychological stress of the treatment itself.
Given the burgeoning adoption of VR in various fields of oncology and promising early data in improving patient outcome especially for surgical oncology, an up-to-date, overarching analysis of VR technology and its application in surgical oncology will shed light on the strengths and opportunities. The primary rationale of this review is to understand how VR is being utilised in surgical oncology, situated within the wider context of VR in oncology. We identify trends across geographies, intervention, tumour type and cancer care pathway and to highlight gaps. This review also provides an in-depth analysis to identify ideal characteristics (development, validity and clinical impact) of VR surgical simulation for clinical training and to inform method validation of VR-assisted surgical tools.
Materials and methods
We carried out a systematic review using the Web of Science following PRISMA guidance. We included papers (articles and reviews only) on VR and cancer. This process included papers published for 10 years between January 2011 and January 2021 and was done with a bibliometric filter in two parts. The first part involved the application of a pre-developed filter to identify papers on cancer research. The papers were then subsequently selected based on the application of VR if they had the words ‘virtual reality’ or ‘augmented intelligence’, or simply ‘VR’, in their titles. The full search strategy used is available in the supplementary data (Appendix A).
Inclusion criteria
Published full-text articles describing oncology and VR were considered for inclusion. Articles must have been published in English in a peer reviewed journal between the dates specified above.
Exclusion criteria
Any papers not related to cancer and VR were excluded (Figure 1). For example, publications describing ‘VR’ as an abbreviation of chemotherapy instead of VR were excluded. Review articles, laboratory research, case reports, conference proceedings and repeated studies were also excluded.
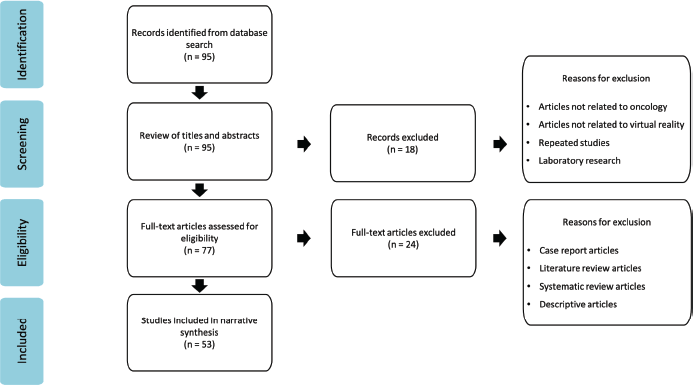
Figure 1. PRISMA flow chart of identification for articles for inclusion.
Data selection
Richard Sullivan (RS) carried out the initial search. Following which, Peng Yun Ng (PN) and RS selected articles meeting inclusion criteria from titles and abstracts for full text review. PN then reviewed full text articles to consider whether inclusion and/or exclusion criteria were met. Disagreement was discussed with Ajay Aggarwal (AA) to reach a consensus.
Data extraction
PN extracted data from included studies, with consultation from RS and AA. Data extracted from all studies included:
• location of study
• characteristics of study (funding, setting, research design and sample size)
• part of cancer care pathway where VR was applied
• purpose of VR
• modality of VR
• clinical speciality
• tumour site
• user of VR
The studies were categorised into surgical and non-surgical oncology. Studies related to surgical oncology were further compartmentalised based on the purpose of the VR, be it either for clinical training or operative planning.
For studies focusing on the use of VR for clinical training, specific data extracted included:
• characteristics of VR-assisted training tool
• method validation of VR-assisted training
The characteristics of the VR-assisted surgical training tool were set out as follows. Fidelity is defined as the extent to which the simulator describes the realism or learning experience that the simulator provides. Generally, low fidelity refers to basic psychomotor tasks while high fidelity refers to full-length operations. User feasibility is more commonly known as user acceptance rate, a measure of whether something is capable of being done or carried out. Face validity is the extent to which the examination resembles real life situations. Content validity is the extent to which the domain that is being measured is measured by the assessment tool. Construct validity is the extent to which a test measures the trait it is designed to measure; sometimes inferred as the extent to which a test discriminates between various levels of expertise. Lastly, predictive validity is the ability of examination to predict future performance of the clinician.
On the other hand, studies related to operative planning provided information on:
• pre/intra-operative use of VR-assisted tool
• surgical complication rate
• duration of procedure
• benefit of VR-assisted tool
Studies related to non-surgical oncology were divided based on the modality of the VR into radiotherapy, rehabilitation and neuropsychiatric intervention. Data were extracted for cancer speciality, purpose of intervention and outcome.
Results
The initial search identified 95 papers. After a review of titles and abstracts, 77 articles were chosen for further review. Of these, 53 full-text articles were selected for data analysis. The study selection process followed the PRISMA guidance and is illustrated in Figure 1.
A summary of the characteristics of all [53] included studies, describing the purpose and modality of VR in the cancer care pathway is available as Appendix B.
Along the stages of the cancer care pathway, VR was applied predominantly in the delivery of cancer treatment (n = 34, 64.2%) with surgical oncology dominating (n = 24) [4–36]. Other care pathway domains where VR has been studied include post-cancer treatment supportive care (n = 7, 13.2%) [37–43], palliative and end-of-life care (n = 7, 13.2%) [44–50], and prehabilitation (n = 5, 9.4%) [26, 33, 44, 51–53] (Figure 2).
In terms of purpose of VR adoption, 23 studies (42.%) studied the use of VR in providing clinical training [6–12, 14–22, 24, 26–29, 32, 54]; 15 (28.3%), supportive and psychosocial care related to cancer [4, 23, 31, 34–36, 44–50, 55, 56]; 7 (13.2%), rehabilitation [37–43]; 5 (9.4%), operative planning [5, 13, 25, 30, 33], and 3 (5.7%), patient education [51–53].
The majority of VR studies in oncology were from North America continent (n = 20, 37.8%) [4, 6, 8, 10–12, 16, 17, 25, 28, 33, 34, 37, 50, 51, 53–56]. Asia (n = 16, 30.2%) is the second most prolific continent [5, 7, 9, 13, 18, 20, 21, 29, 35, 36, 38, 39, 41, 43, 44, 47], followed by Europe (n = 11, 20.8%) [15, 19, 22, 24, 31, 32, 45, 46, 48]. Fewer articles were published from centres based in Australia (n = 3, 5.7%), [27, 49, 52] Africa (n = 2, 3.8%), [23, 26] and South America (n = 2, 3.8%) [20, 40]. This geographical distribution is visualised as hotspots on a world map in Figure 3. Canada (n = 11, 20.8%), [8, 11, 12, 14, 16, 17, 28, 50, 53–55] United States of America (n = 9, 17.0%) [4, 6, 10, 25, 33, 34, 37, 51, 56], and China (n = 6, 11.3%) [5, 7, 13, 18, 29, 44] in consecutive order, are the top three countries for VR research in oncology.
Of the 24 studies related to surgical oncology, 19 (79.2%) focused on the use of VR in supporting surgical trainees with competencies, [7–12, 14–17, 19, 20, 54] and the other five (20.8%) focused on pre-operative surgical planning [5, 13, 25, 30, 33]. VR was applied across different modalities of surgery, including open procedures (n = 14, 58.3%), [5, 8, 11–14, 16, 17, 26, 28–30, 33, 54], minimally invasive (n = 5, 20.8%) [7, 9, 10, 19, 22], and robotic (n = 5, 20.8%) procedures [15, 20, 21, 25, 32]. Neurosurgical oncology procedures were the most studied in terms of VR application (n = 13, 54.2%), followed by urology (n = 8, 33.3%), and gynaecology (n = 2, 8.3%), as illustrated in Figure 4.
In terms of characteristics of VR-assisted surgical training tools, 11 (57.9%) out of the 19 articles described VR-assisted tools which have high fidelity, meaning clinicians were able to practice through simulation of full length operations instead of abstract psychomotor tasks [7–10, 12, 15, 17, 19, 22, 26, 32]. 12 (63.2%) out of 19 articles further identified VR-assisted surgical training tools paired with haptic feedback to enhance the realism of the simulation to operation [8, 10, 11, 14, 16, 17, 19, 20, 22, 28, 54]. As such, clinicians received sensory feedback using transmitted resistance when they encountered tissues or objects during the simulation.
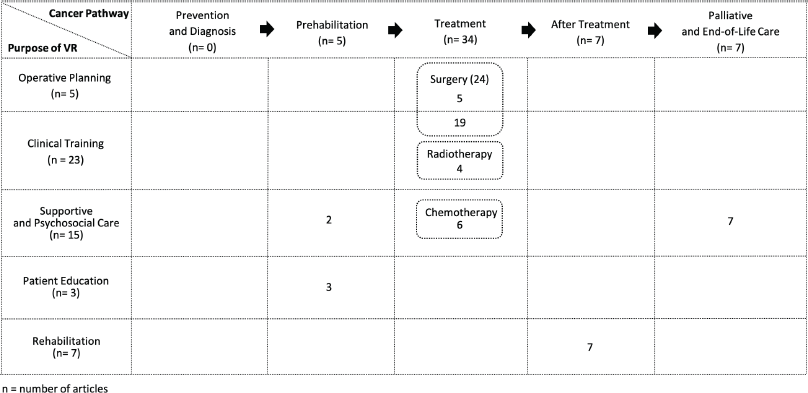
Figure 2. Swimlane diagram of the use of VR in the cancer care pathway.
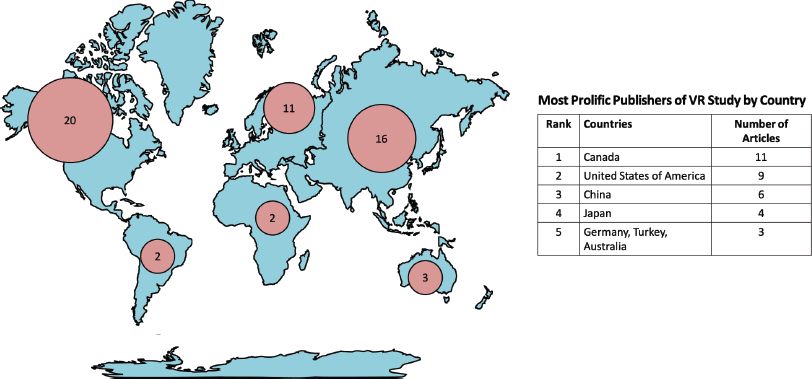
Figure 3. Number of VR studies related to oncology by continent and country.
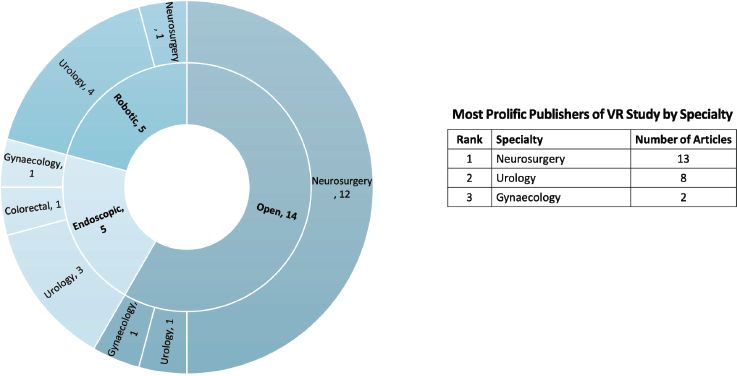
Figure 4. Number of VR studies by surgical modality and specialty.
While all the VR-assisted surgical tool illustrated face and content validity, only 12 (63.2%) exhibited construct validity, thus discriminating clinicians of various levels of expertise [11, 12, 14–17, 21, 22, 26, 32, 54]. Only one (5.3%) study demonstrated predictive validity, where the VR-assisted training was shown to improve clinicians’ future performance [21]. However, the study only has a sample size of three, undermining the internal and external validity of its result.
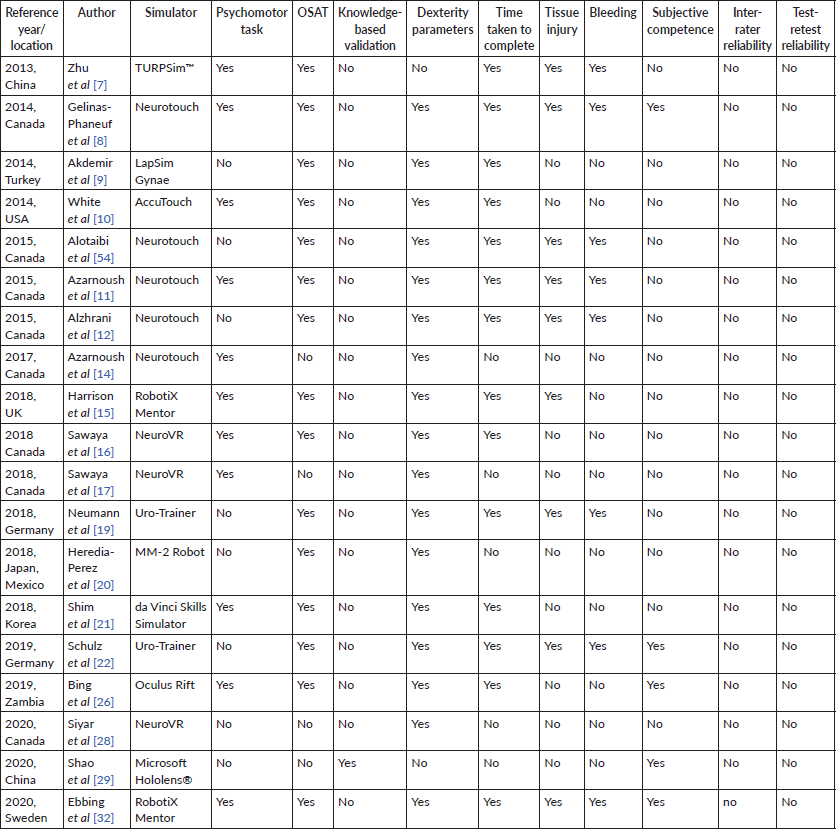
In addition to the characteristics of VR-assisted surgical training tool shown in Table 1, a range of methods used to validate VR-assisted surgical training were consolidated in Table 2. Surgical competency was mostly validated using dexterity parameters (n =17, 89.5%) [8–12, 14–17, 20–22, 26, 28, 32, 54] and objective structured assessment of technical skills (OSATS) (n = 14, 73.7%) [7–12, 15, 16, 19–22, 26, 32, 54]. Psychomotor tasks were used in 11 out of 19 of the studies (57.9%) [7, 8, 10, 11, 14–17, 21, 26, 32]. One study (5.3%) used knowledge-based validation instead [29].
14 out of 19 studies (73.7%) recorded the time taken to complete the task, [7–12, 15, 17, 19, 21, 22, 26, 32] while 9 (47.4%) and 8 (42.1%) included post-operative complications, specifically tissue injury [7, 8, 11, 12, 15, 19, 22, 32, 54] and bleeding [7, 8, 11, 12, 19, 22, 32, 54] respectively, as part of the training validation. Only five (26.3%) studies assessed the subjective competence of clinicians using the Likert Scale [8, 19, 22, 26, 29]. No studies demonstrated test-retest reliability in its validation process or assessed for inter-rater reliability.
The application of VR in surgical oncology was not limited to surgical training, but also operative optimisation in clinical setting. Five out of 24 studies related to surgical oncology highlighted the use of VR in operative planning. Of the five studies, three (60%) were used in pre-operative setting [5, 30, 33] and two (40%) in intra-operative setting [13, 25]. It is worth noting that only one study (20%) reported an improvement in surgical outcome in terms of resection rate and the preservation of neural function [13]. The other four (80%) highlighted its use in providing anatomic information [5, 25, 30, 33] and of these, two (40%) used the information to advise surgical approach [5, 30]. A summary table for these five studies is attached as Appendix C.
Table 1. Characteristics of VR-assisted surgical training tool.
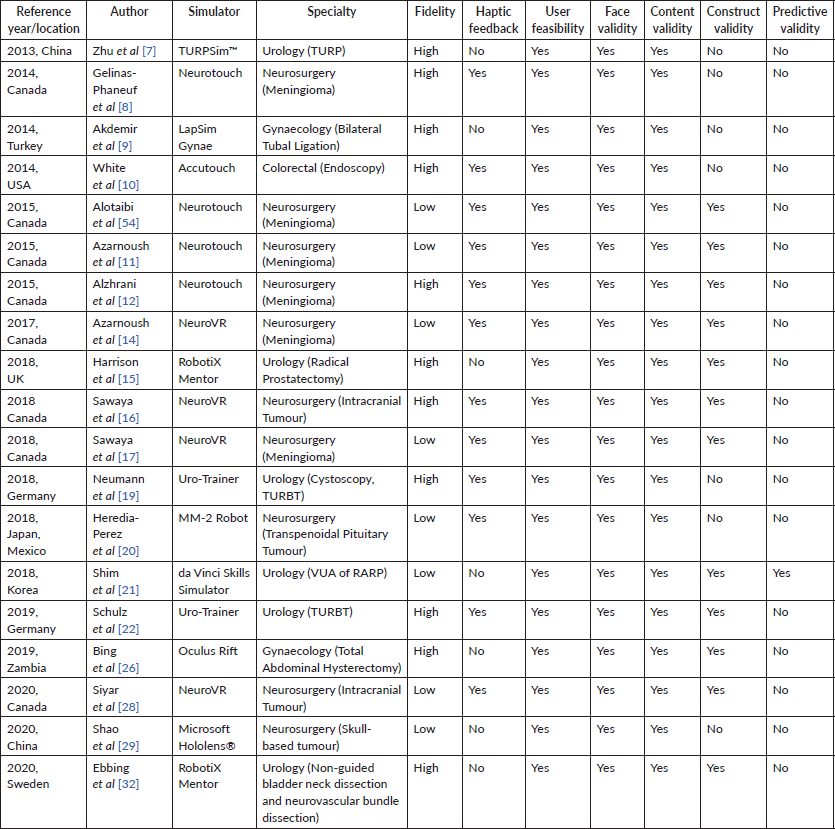
Table 2. Method validation of VR-assisted surgical training.
Discussion
From a nascent technology reserved for experts from niche computer field, VR tool has matured and evolved into a popular technological gadget in the general consumer market as its creation fundamentally changes how one engages with virtuality for entertainment, education and communication.
In particular, VR in medicine has emerged as an epiphenomenon of the rapid pace of its technological advancement as its potential was widely regarded as undertapped since an early stage. Nonetheless, our comprehensive search discovered surprisingly little literature related to its application in surgical oncology and, more widely, oncology. The literature reflects a widely disparate range of applications at different stages of cancer pathway and for different purposes, such as providing supportive and psychosocial care and facilitating rehabilitation. However, surgical oncology remains the main focus both for surgical training and operative planning.
Application gap between high-risk, complex and low-risk, open procedures
As a surgical oncology speciality, neuro-oncology led in the research and adoption of VR, primarily in surgical training. Most studies highlighted the benefit of VR simulation for neurosurgery trainees in gaining skill-based experience in common yet high-risk neurosurgeries, which are often heavily led by consultants in the operating theatre. Trainees were able to practice and receive feedback from senior colleagues on a wide range of surgical skills using VR simulation, without detriment to patients and with hope to translate acquired simulation-based skills to surgical proficiency. Their competencies were often subsequently tested through psychomotor tasks or OSATS to consolidate learning.
Besides the high risk attribute of procedure, technically complex surgeries, such as those requiring endoscopic and robotic equipment, are also bona fide early adopters of VR simulation in surgical training. This is due to the fact that surgeons generally require more time and practice to acquire new skillsets required to use endoscopy or robot, which they often initially lack human intuition in without prior clinical exposure and experience. This review identified uro-oncology as the leading surgical specialty on this front, driven primarily by novel endoscopic and robotic approach to prostatectomy.
This pattern of adoption of VR overshadows the larger potential of VR simulation as a platform to develop surgical training towards high demand, low-risk, open-approach cancer surgeries such as mastectomy and colectomy, that are sorely lacking in capacity in LMICs.
Accessibility gap between high-income and LMICs
There were very few studies of VR conducted by and for LMICs (except for China). This reflects a yawning socio-techncological gap between the direction of travel of VR in surgical oncology and the actual development need of VR to build surgical capacity and capability globally.
Nonetheless, one study from Zambia [26] stood out as an anomaly, as it trialled the use of a cost-efficient VR (Oculus Rift, each cost less than USD1,500) as a surgical training tool for trainees to learn open radical abdominal hysterectomy for cervical cancer in local setting. It demonstrated how the VR simulation, when co-designed and co-developed with local experts, can teach surgical novices and may lead to shorter surgical training time and better surgical outcome. Appendix D attached includes two images, depicting the VR of an operating theatre mimicking the real setting of a Zambian teaching hospital and that of an intricate step of an open abdominal hysterectomy using equipment available locally. This example sheds optimism on the potential of VR as the scalable and sustainable solution to addressing global surgical training needs in oncology when its design fits the demand. To replicate such practice, more publically funded development and implementation of VR are necessary to drive its adoption. Otherwise, the tendency for VR to service solely high-income countries and/or technically complex procedures (MIS/robotics) is likely to prevail.
Evaluation and method validation gap in VR-assisted surgical training
Given the relative novelty of VR as a surgical training tool, there remains a dearth of evidence that actually showed VR simulation improves cancer surgical training, surgical quality and better patient outcomes. Evaluation gap is defined by the lack of standardisation of ideal qualities in VR-assisted surgical training tool, which make them user-friendly, realistic and effective in developing surgical skills. Based on definition set by Lewis et al [57], most VR in studies have attained face and content validity with the adoption of high-fidelity simulation, advanced VR lens for orientation and haptic feedback. However, many still struggle to achieve contsruct and predictive validity, which enable surgical trainees to track their progress at different level of proficiency and to measure translation of their acquired surgical proficiency into better surgical care and patient outcomes based on real-life performance, respectively.
Such evaluation gap is thought to be tightly correlated to a lack of gold standard to validate VR-assisted surgical training tools, also known as method validation gap. This review identified heterogenous methods adopted to validate surgical training, most commonly through psychomotor tasks and OSATS that measure dexterity parameters, time taken to complete task and/or complication rate. Such practice is often enabled by the inbuilt features of the simulators but often lacks in inter-rater and test-retest reliability, which are crucial in distinguishing the level of proficiency of trainee through a peer-review process and in testing their future operative performance.
Thus, to bridge these gaps, we recommend future studies related to VR-assisted surgical training to include a validation process, which requires more than one senior reviewer and to follow up on trainees’ surgical proficiency as well as patient outcome to establish test-retest reliability and predictive validity, respectively.
VR in non-surgical oncology
Outside the scope of surgical oncology, the application of VR emerges in a myriad of other oncology fields- most prominently radiotherapy, neuropsychiatry and rehabilitation. These three modalities of treatment share the common feature of utilising the fully immersive experience of VR to engage with patients meaningfully for an extended period of time. To illustrate, half (three out of six) of the radiotherapy-related studies demonstrated how VR was adopted to help patients comprehend the intricate process of radiotherapy and vivaciously experience the treatment setting prior to starting, thus easing anxiety. On the other hand, 13 out of 15 studies which used VR as a neuropsychiatric intervention showed how VR could be effective in easing pain through distraction and alleviate patient mood. Creatively, mainstream VR consoles, such as Xbox 360 and Wii, have also over time adapted their games to help patients rehabilitate limb function and improve energy level after cancer treatment.
Conclusion
The emergence of VR in medicine opens a window of opportunity to build capacity and capability in surgical oncology in a wide range of resource settings using low cost novel tools. However, current VR development is still overly focused on high-income settings, with complex procedures such as MIS and robotics, instead of open-approach procedures needed in many LMIC settings. The asynchrony between the development of VR in surgical oncology and the actual global cancer surgery demand means that the technology is not effectively, efficiently and equitably applied to realise its capacity-building potential. Future research should prioritise the use of affordable VR technology in high-demand surgical procedures and further explore its use in other common cancer domains such as breast, colorectal and lung cancer. This process can be accelerated with the adoption of a more standardised and complete method validation of VR-assisted surgical training tool, in a bid to make VR a real and effective digital adjunct to traditional, didactic surgical training.
Acknowledgment and financial declaration
This work was funded by the Wellcome Trust Grant [215723/Z/19/Z] held by RS, EB and GP.
Conflicts of interest
N/A.
Authors’ contributions
PN, RS and AA conceived, designed, and supervised the study. PN and RS searched, screened, and assessed the publications. PN extracted, analysed and visualised the data. PN and RS drafted the manuscript and interpreted the findings. EB, RS, AA, AC, BC, MM, MM and GP reviewed and edited drafts of the manuscript. RS accessed and verified the data. All authors read the manuscript, provided feedback, and approved the final version.
References
1. GBD 2015 Mortality and Causes of Death Collaborators (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015 Lancet 388(10053) 1459–1544 https://doi.org/10.1016/S0140-6736(16)31012-1
2. Sullivan R, Alatise OI, and Anderson BO, et al (2015) Global cancer surgery: delivering safe, affordable, and timely cancer surgery Lancet Oncol 16(11) 1193–1224 https://doi.org/10.1016/S1470-2045(15)00223-5 PMID: 26427363
3. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136(5) E359–E386 https://doi.org/10.1002/ijc.29210
4. Schneider SM, Kisby CK, and Flint EP (2011) Effect of virtual reality on time perception in patients receiving chemotherapy Support Care Cancer 19(4) 555–564 https://doi.org/10.1007/s00520-010-0852-7
5. Tang HL, Sun HP, and Gong Y, et al (2012) Preoperative surgical planning for intracranial meningioma resection by virtual reality Chin Med J (Engl) 125(11) 2057–2061 PMID: 22884077
6. Glaser S, Warfel B, and Price J, et al (2012) Effectiveness of virtual reality simulation software in radiotherapy treatment planning involving non-coplanar beams with partial breast irradiation as a model Technol Cancer Res Treat 11(5) 409–414 https://doi.org/10.7785/tcrt.2012.500256 PMID: 22417058
7. Zhu H, Zhang Y, and Liu JS, et al (2013) Virtual reality simulator for training urologists on transurethral prostatectomy Chin Med J (Engl) 126(7) 1220–1223 PMID: 23557547
8. Gelinas-Phaneuf N, Choudhury N, and Al-Habib AR, et al (2014) Assessing performance in brain tumor resection using a novel virtual reality simulator Int J Comput Assist Radiol Surg 9(1) 1–9 https://doi.org/10.1007/s11548-013-0905-8
9. Akdemir A, Sendag F, and Oztekin MK (2014) Laparoscopic virtual reality simulator and box trainer in gynecology Int J Gynaecol Obstet 125(2) 181–185 https://doi.org/10.1016/j.ijgo.2013.10.018 PMID: 24576509
10. White I, Buchberg B, and Tsikitis VL, et al (2014) A virtual reality endoscopic simulator augments general surgery resident cancer education as measured by performance improvement J Cancer Educ 29(2) 333–336 https://doi.org/10.1007/s13187-014-0610-5 PMID: 24493635
11. Azarnoush H, Alzhrani G, and Winkler-Schwartz A, et al (2015) Neurosurgical virtual reality simulation metrics to assess psychomotor skills during brain tumor resection Int J Comput Assist Radiol Surg 10(5) 603–618 https://doi.org/10.1007/s11548-014-1091-z
12. AlZhrani G, Alotaibi F, and Azarnoush H, et al (2015) Proficiency performance benchmarks for removal of simulated brain tumors using a virtual reality simulator NeuroTouch J Surg Educ 72(4) 685–696 https://doi.org/10.1016/j.jsurg.2014.12.014 PMID: 25687956
13. Sun GC, Wang F, and Chen XL, et al (2016) Impact of virtual and augmented reality based on intraoperative magnetic resonance imaging and functional neuronavigation in glioma surgery involving eloquent areas World Neurosurg 96 375–382 https://doi.org/10.1016/j.wneu.2016.07.107 PMID: 27521727
14. Azarnoush H, Siar S, and Sawaya R, et al (2017) The force pyramid: a spatial analysis of force application during virtual reality brain tumor resection J Neurosurg 127(1) 171–181 https://doi.org/10.3171/2016.7.JNS16322
15. Harrison P, Raison N, and Abe T, et al (2018) The validation of a novel robot-assisted radical prostatectomy virtual reality module J Surg Educ 75(3) 758–766 https://doi.org/10.1016/j.jsurg.2017.09.005
16. Sawaya R, Alsideiri G, and Bugdadi A, et al (2018) Development of a performance model for virtual reality tumor resections J Neurosurg 131(1) 192–200 https://doi.org/10.3171/2018.2.JNS172327 PMID: 30074456
17. Sawaya R, Bugdadi A, and Azarnoush H, et al (2018) Virtual reality tumor resection: the force pyramid approach Oper Neurosurg (Hagerstown) 14(6) 686–696 https://doi.org/10.1093/ons/opx189
18. Zhou Z, Jiang S, and Yang Z, et al (2019) Personalized planning and training system for brachytherapy based on virtual reality Virtual Reality 23(4) 347–361 https://doi.org/10.1007/s10055-018-0350-7
19. Neumann E, Mayer J, and Russo GI, et al (2019) Transurethral resection of bladder tumors: next-generation virtual reality training for surgeons Eur Urol Focus 5(5) 906–911 https://doi.org/10.1016/j.euf.2018.04.011
20. Heredia-Perez SA, Harada K, and Padilla-Castaneda MA, et al (2019) Virtual reality simulation of robotic transsphenoidal brain tumor resection: evaluating dynamic motion scaling in a master-slave system Int J Med Robot 15(1) e1953 https://doi.org/10.1002/rcs.1953
21. Shim JS, Noh TI, and Kim JY, et al (2018) Predictive validation of a robotic virtual reality simulator: the tube 3 module for practicing vesicourethral anastomosis in robot-assisted radical prostatectomy Urology 122 32–36 https://doi.org/10.1016/j.urology.2018.08.013 PMID: 30144481
22. Schulz GB, Grimm T, and Buchner A, et al (2019) Validation of a high-end virtual reality simulator for training transurethral resection of bladder tumors J Surg Educ 76(2) 568–577 https://doi.org/10.1016/j.jsurg.2018.08.001
23. Bani Mohammad E and Ahmad M (2019) Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: a randomized control trial Palliat Support Care 17(1) 29–34 https://doi.org/10.1017/S1478951518000639
24. Taubert M, Webber L, and Hamilton T, et al (2019) Virtual reality videos used in undergraduate palliative and oncology medical teaching: results of a pilot study BMJ Support Palliat Care 9(3) 281–285 https://doi.org/10.1136/bmjspcare-2018-001720 PMID: 30808627 PMCID: 6817702
25. Mehralivand S, Kolagunda A, and Hammerich K, et al (2019) A multiparametric magnetic resonance imaging-based virtual reality surgical navigation tool for robotic-assisted radical prostatectomy Turk J Urol 45(5) 357–365 https://doi.org/10.5152/tud.2019.19133 PMID: 31509508 PMCID: 6739087
26. Bing EG, Parham GP, and Cuevas A, et al (2019) Using low-cost virtual reality simulation to build surgical capacity for cervical cancer treatment J Glob Oncol 5 1–7 PMID: 31070982 PMCID: 6550092
27. Gunn T, Rowntree P, and Starkey D, et al (2021) The use of virtual reality computed tomography simulation within a medical imaging and a radiation therapy undergraduate programme J Med Radiat Sci 68(1) 28–36 https://doi.org/10.1002/jmrs.436 PMCID: 7890924
28. Siyar S, Azarnoush H, and Rashidi S, et al (2020) Tremor assessment during virtual reality brain tumor resection J Surg Educ 77(3) 643–651 https://doi.org/10.1016/j.jsurg.2019.11.011
29. Shao X, Yuan Q, and Qian D, et al (2020) Virtual reality technology for teaching neurosurgery of skull base tumor BMC Med Educ 20(1) 3 https://doi.org/10.1186/s12909-019-1911-5 PMID: 31900135 PMCID: 6942358
30. Zawy Alsofy S, Sakellaropoulou I, and Stroop R (2020) Evaluation of surgical approaches for tumor resection in the deep infratentorial region and impact of virtual reality technique for the surgical planning and strategy J Craniofac Surg 31(7) 1865–1869 https://doi.org/10.1097/SCS.0000000000006525 PMID: 32433127
31. Chirico A, Maiorano P, and Indovina P, et al (2020) Virtual reality and music therapy as distraction interventions to alleviate anxiety and improve mood states in breast cancer patients during chemotherapy J Cell Physiol 235(6) 5353–5362 https://doi.org/10.1002/jcp.29422 PMID: 31957873
32. Ebbing J, Wiklund PN, and Akre O, et al (2021) Development and validation of non-guided bladder-neck and neurovascular-bundle dissection modules of the RobotiX-Mentor(R) full-procedure robotic-assisted radical prostatectomy virtual reality simulation Int J Med Robot 17(2) e2195 https://doi.org/10.1002/rcs.2195
33. Tapiero S, Karani R, and Limfueco L, et al (2020) Evaluation of interactive virtual reality as a preoperative aid in localizing renal tumors J Endourol 34(11) 1180–1187 https://doi.org/10.1089/end.2020.0234 PMID: 32597217
34. Scates D, Dickinson JI, and Sullivan K, et al (2020) Using nature-inspired virtual reality as a distraction to reduce stress and pain among cancer patients Environ Behav 52(8) 895–918 https://doi.org/10.1177/0013916520916259
35. Sharifpour S, Manshaee G, and Sajjadian I (2020) Effects of virtual reality therapy on perceived pain intensity, anxiety, catastrophising and self-efficacy among adolescents with cancer Couns Psychother Res 21(1) 218–226 https://doi.org/10.1002/capr.12311
36. Semerci R, Akgun Kostak M, and Eren T, et al (2021) Effects of virtual reality on pain during venous port access in pediatric oncology patients: a randomized controlled study J Pediatr Oncol Nurs 38(2) 142–151 https://doi.org/10.1177/1043454220975702
37. Hoffman AJ, Brintnall RA, and Brown JK, et al (2014) Virtual reality bringing a new reality to postthoracotomy lung cancer patients via a home-based exercise intervention targeting fatigue while undergoing adjuvant treatment Cancer Nurs 37(1) 23–33 https://doi.org/10.1097/NCC.0b013e318278d52f
38. Tsuda K, Sudo K, and Goto G, et al (2016) A feasibility study of virtual reality exercise in elderly patients with hematologic malignancies receiving chemotherapy Intern Med 55(4) 347–352 https://doi.org/10.2169/internalmedicine.55.5275 PMID: 26875958
39. Yoon J, Chun MH, and Lee SJ, et al (2015) Effect of virtual reality-based rehabilitation on upper-extremity function in patients with brain tumor: controlled trial Am J Phys Med Rehabil 94(6) 449–459 https://doi.org/10.1097/PHM.0000000000000192
40. Aguirre-Carvajal M (2018) Psychological and functional effects in mastectomized patients treated with virtual reality Cancer Ther Oncol Int J 9(3) 84–88
41. Atef D, Elkeblawy MM, and El-Sebaie A, et al (2020) A quasi-randomized clinical trial: virtual reality versus proprioceptive neuromuscular facilitation for postmastectomy lymphedema J Egypt Natl Canc Inst 32(1) 29 https://doi.org/10.1186/s43046-020-00041-5 PMID: 32537717
42. Niewolak K, Fielek D, and Pecyna P, et al (2020) Medical resort treatment extended with modern feedback exercises using virtual reality improve postural control in breast cancer survivors Acta Balneol 62 92–98 https://doi.org/10.36740/ABal202002104
43. Feyzioglu O, Dincer S, and Akan A, et al (2020) Is Xbox 360 Kinect-based virtual reality training as effective as standard physiotherapy in patients undergoing breast cancer surgery? Support Care Cancer 28(9) 4295–4303 https://doi.org/10.1007/s00520-019-05287-x PMID: 31907649
44. Li WH, Chung JO, and Ho EK (2011) The effectiveness of therapeutic play, using virtual reality computer games, in promoting the psychological well-being of children hospitalised with cancer J Clin Nurs 20(15–16) 2135–2143 https://doi.org/10.1111/j.1365-2702.2011.03733.x PMID: 21651633
45. Banos RM, Espinoza M, and Garcia-Palacios A, et al (2013) A positive psychological intervention using virtual reality for patients with advanced cancer in a hospital setting: a pilot study to assess feasibility Support Care Cancer 21(1) 263–270 https://doi.org/10.1007/s00520-012-1520-x
46. Duivon M, Perrier J, and Joly F, et al (2018) Impact of breast cancer on prospective memory functioning assessed by virtual reality and influence of sleep quality and hormonal therapy: PROSOM-K study BMC Cancer 18(1) 866 https://doi.org/10.1186/s12885-018-4762-2 PMID: 30176833 PMCID: 6122719
47. Niki K, Okamoto Y, and Maeda I, et al (2019) A novel palliative care approach using virtual reality for improving various symptoms of terminal cancer patients: a preliminary prospective, multicenter study J Palliat Med 22(6) 702–707 https://doi.org/10.1089/jpm.2018.0527 PMID: 30676847
48. Cîmpean AI (2019) A pilot study to compare cognitive behavioral therapy with virtual reality vs. standard cognitive behavioral therapy for patients who suffer from cervical cancer J Evid-Based Psychother 19(1) 115–127 https://doi.org/10.24193/jebp.2019.1.7
49. Tennant M, Youssef GJ, and McGillivray J, et al (2020) Exploring the use of immersive virtual reality to enhance psychological well-being in pediatric oncology: a pilot randomized controlled trial Eur J Oncol Nurs 48 101804 https://doi.org/10.1016/j.ejon.2020.101804 PMID: 32949941
50. Garrett BM, Tao G, and Taverner T, et al (2020) Patients perceptions of virtual reality therapy in the management of chronic cancer pain Heliyon 6(5) e03916 https://doi.org/10.1016/j.heliyon.2020.e03916 PMID: 32426540 PMCID: 7226660
51. Marquess M, Johnston SP, and Williams NL, et al (2017) A pilot study to determine if the use of a virtual reality education module reduces anxiety and increases comprehension in patients receiving radiation therapy J Radiat Oncol 6(3) 317–322 https://doi.org/10.1007/s13566-017-0298-3
52. Jimenez YA, Cumming S, and Wang W, et al (2018) Patient education using virtual reality increases knowledge and positive experience for breast cancer patients undergoing radiation therapy Support Care Cancer 26(8) 2879–2888 https://doi.org/10.1007/s00520-018-4114-4 PMID: 29536200
53. Johnson K, Liszewski B, and Dawdy K, et al (2020) Learning in 360 degrees: a pilot study on the use of virtual reality for radiation therapy patient education J Med Imaging Radiat Sci 51(2) 221–226 https://doi.org/10.1016/j.jmir.2019.12.008 PMID: 32046946
54. Alotaibi FE, AlZhrani GA, and Mullah MA, et al (2015) Assessing bimanual performance in brain tumor resection with NeuroTouch, a virtual reality simulator Neurosurgery 11(Suppl 2) 89–98; discussion PMID: 25599201
55. Birnie KA, Kulandaivelu Y, and Jibb L, et al (2018) Usability testing of an interactive virtual reality distraction intervention to reduce procedural pain in children and adolescents with cancer [formula: see text] J Pediatr Oncol Nurs 35(6) 406–416 https://doi.org/10.1177/1043454218782138 PMID: 29950139
56. Glennon C, McElroy SF, and Connelly LM, et al (2018) Use of virtual reality to distract from pain and anxiety Oncol Nurs Forum 45(4) 545–552 https://doi.org/10.1188/18.ONF.545-552 PMID: 29947355
57. Lewis TM, Aggarwal R, and Rajaretnam N, et al (2011) Training in surgical oncology – the role of VR simulation Surg Oncol 20(3) 134–139 https://doi.org/10.1016/j.suronc.2011.04.005 PMID: 21605972
Supplementary information
Figshare Link: https://doi.org/10.6084/m9.figshare.22207594.v1






