Adult granulosa cell tumour of the ovary incidentally discovered during caesarean section in a pregnant patient after IVF: a rare case and a review of the literature
Kong Chi Pham1, Phuong Thi Minh Ta2 and Nhat Minh Huynh1
1Department of Gynecology, Da Nang Hospital for Women and Children, 402 Le Van Hien Street, Ngu Hanh Son District, Da Nang City 550000, Vietnam
2Department of Pathology, Da Nang Hospital for Women and Children, 402 Le Van Hien Street, Ngu Hanh Son District, Da Nang City 550000, Vietnam
Abstract
Adult granulosa cell tumours (AGCTs) of the ovary are very rare during pregnancy. To date, only five cases of ovarian AGCT in pregnancy have been reported in the literature and the patients all conceived spontaneously. We report a case of AGCT of the ovary that was incidentally discovered during a caesarean section in a patient undergoing In vitro fertilisation (IVF). To the authors’ knowledge, this is the first case of AGCT incidentally discovered during caesarean section in a pregnant patient after IVF. A 44-year-old primigravida with 39 weeks gestation was admitted to our hospital due to premature rupture of membranes in May 2019. She was treated by in vitro fertilisation due to being an elderly mother and she was pregnant after the first cycle. She was indicated for caesarean section due to conceiving following in vitro fertilisation and being an elderly mother. She gave birth to a 3,000 g baby boy and his Apgar scores were 8/1’–9/5’. When examining the adnexa, the left ovary had a tumour with a size of 7 × 4 × 4 cm. Left oophorectomy was performed and specimen sent to for histopathology. The histopathological diagnosis was an AGCT of the ovary. A month later, the patient received chemotherapy with Carboplatin and Paclitaxel for four cycles. After 32 months of follow-up, no recurrence was detected. In conclusion, AGCTs of the ovary are very rare during pregnancy. Pre-operative diagnosis is difficult. Conservative surgery should be considered in women who wish to have children. Patients should receive adequate counselling and long-term follow-up to ensure the highest survival rates and early detection of recurrence.
Keywords: Foetal outcome, granulosa cell tumour, in vitro fertilisation, maternal outcome, pregnancy
Correspondence to: Kong Chi Pham
Email: kongpc@danang.gov.vn
Published: 09/01/2023
Received: 20/06/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Granulosa cell tumour of the ovary is a rare malignancy, originating from ovarian sex cord stromal cells. The rate of ovarian granulosa cell tumour is 0.2/100,000 women, accounting for only 2%–5% of all malignant ovarian tumour cases. Based on clinical and histological features, ovarian granulosa cell tumours are divided into two types: adult (95%) and juvenile (5%) [1].
Ovarian granulosa cell tumour is very rare during pregnancy. The systematic review by Blake et al [2] showed that there were only 46 cases of ovarian sex cord stromal tumours in pregnancy reported in the literature from 1950 to 2012, of which 13 cases were granulosa cell tumour. According to Guidi’s et al [3] review published in 2021, only five cases of adult granulosa cell tumour (AGCT) of the ovary during pregnancy were reported in the literature and the patients all conceived spontaneously.
Clinical and subclinical features of AGCT are often nonspecific, with 1/5 of cases asymptomatic and incidentally detected [4]. Due to the rarity of the disease, consensus on the best treatment has not yet been reached. Surgery remains the gold standard in the treatment of AGCT of the ovary. The role of lymphadenectomy and chemotherapy has not been clearly defined [5]. The prognosis of granulosa cell tumour of the ovary is usually good, but the recurrence rate is high (36%); therefore, long-term follow-up is important. Disease stage is a significant prognostic factor for recurrence [6]. The relationship between ovarian stimulation in the treatment of infertility and ovarian cancer as well as granulosa cell tumour of the ovary has not been clearly defined [7, 8].
We report a case of an AGCT of the ovary that was incidentally discovered during a caesarean section in a patient undergoing In vitro fertilisation (IVF). To the authors’ knowledge, this is the first case of AGCT incidentally discovered during caesarean section in a pregnant patient after IVF One month later, the patient received chemotherapy with Carboplatin and Paclitaxel for four cycles and was re-examined according to the regimen. After 32 months of follow-up, no clinical or subclinical recurrence was detected.
Case report
A 44-year-old primigravida with 39 weeks gestation was admitted to our hospital due to premature rupture of membranes in May 2019. In her past obstetrical and gynaecological history, she had primary infertility for 3 years. Tests to investigate the cause of infertility for both the wife (basic endocrine including Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), oestrogen, testosterone, prolactin, anti-Mullerian hormone (AMH), gynaecological ultrasound and hysterosalpingography) and husband (endocrine and semen analysis) were normal. She was treated by in vitro fertilisation due to being an elderly mother and she was pregnant after the first cycle. Antenatal care in the first and second trimester at private hospitals did not detect any abnormality: fetal abnormality, and maternal status (internal and surgical medical history, uterus and ovaries problems). She was indicated for caesarean section due to conceiving following in vitro fertilisation and being an elderly mother. She gave birth to a 3,000 g baby boy and his Apgar scores were 8/1’–9/5’. When examining the adnexa, the left ovary had a tumour with a size of 7 × 4 × 4 cm, and the capsule was intact. Tumour was rough inside, hard shell removed (Figure 1). The right adnexa were normal. Peritoneum and omentum were also normal. Left oophorectomy was performed and specimen sent to for histopathology. We discussed with pathologist from Ho Chi Minh City Oncology Hospital – one of the largest Oncology hospital in Vietnam.
Macroscopically, the left ovarian tumour had a smooth surface, measured at 8 cm in its greatest diameter and was well demarcated. On the cut surface, it appeared yellowish solid, cystic, soft and focally haemorrhagic, with necrosis. On microscopic examination, it was small, bland cells with scant cytoplasm and pale, uniform angulated and grooved nuclei (coffee bean). The cells were arranged in various patterns, including diffuse, trabecular and corded, insular, microfollicular, Call-Exner Bodies (Figure 2a). Luteinised cells with moderate to abundant eosinophilic cytoplasm, conspicuous nucleoli, no nuclear grooves (Figure 2b). Mitotic activity was 4/10 high power fields. Immunohistochemistry was positive for inhibin (Figure 3a), negative for Epithelial membrane antigen (EMA) (Figure 3b). The final diagnosis was an AGCT of the ovary.
Magnetic resonance image (MRI) for the pelvic abdomen after caesarean section did not detect any abnormal signals. The patient was stage Ia. We consulted with oncologists, and the given regimen was adjuvant chemotherapy (Carboplatin + Paclitaxel).
A month later, she received adjuvant chemotherapy with four cycles of carboplatin (300 mg/m2) on day 1 and paclitaxel (175 mg/m2) on day 1 every 3 weeks. After 32 months follow-up, no recurrence has been detected in clinical or laboratory situation as well as Magnetic Resonance Imaging (MRI).
The study was approved by the Ethical Review Committee and performed in accordance with the principles of the Declaration of Helsinki. The patient provided written informed consent for the publication and the use of her images.
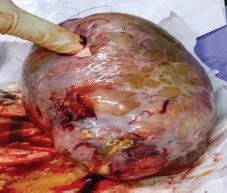
Figure 1. Mass of left ovary with smooth capsule
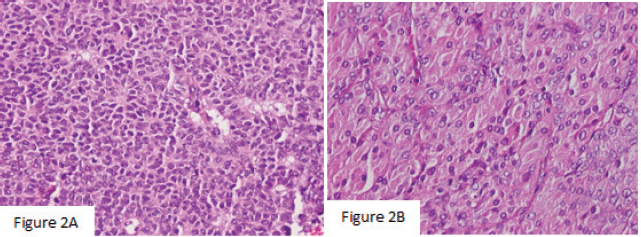
Figure 2. (a): The histology showed that the cells were arranged in various patterns, including diffuse, trabecular and corded, insular, microfollicular, Call-Exner Bodies (×200). (b): Luteinised cells with moderate to abundant eosinophilic cytoplasm, conspicuous nucleoli, no nuclear grooves (×400).
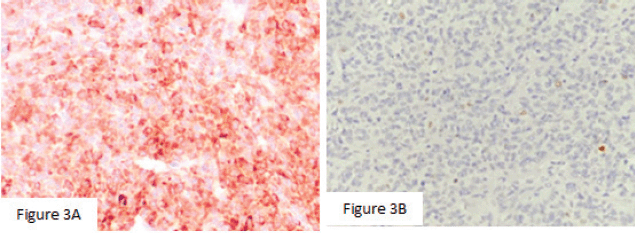
Figure 3. (a): Mitotic activity was 4/10 high power fields. (b): Immunohistochemistry was positive for inhibin (×400), negative for EMA (×200).
Discussion
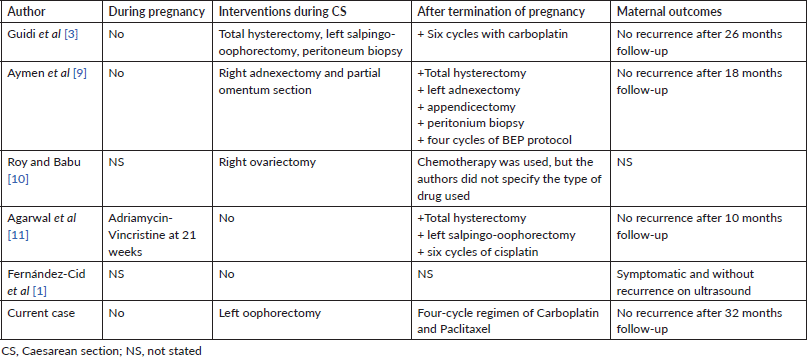
AGCT of the ovary accounts for 95% of granulosa cell tumour cases and is usually diagnosed in the peri- and postmenopausal stage, while the less common juvenile type is usually diagnosed at first three decades of life [1]. Five cases of ovarian AGCT in pregnancy were reviewed by Guidi et al [3] adding one our case. We summarised circumstances and time of detection, foetal outcomes in Table 1 and management, maternal outcomes in Table 2. There were three cases discovered by chance during caesarean section, two cases recurred and one case was discovered at 15 weeks gestation. Three cases had no symptoms, one case had abdominal pain because of threatened preterm birth, one case had nonspecific abdominal symptoms and one recurred case had enlarged and palpable tumour.
Table 1. Circumstances of discovery and foetal outcomes.
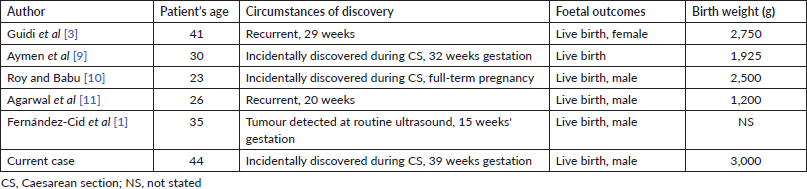
Table 2. Management and maternal outcomes.
Preoperative diagnosis of granulosa cell tumour is challenging for physicians. Clinical and laboratory features of granulosa cell tumour of the ovary are often nonspecific [4]. Granulosa cell tumour of the ovary has the ability to secrete oestrogen, causing symptoms such as abnormal vaginal bleeding in postmenopausal women and heavy menstrual bleeding in younger women. The most common clinical symptom was abnormal vaginal bleeding (44.6%), abdominal pain (25.8%) [12]. The International Ovarian Tumor Analysis gave two findings suggestive of granulosa cell tumour on ultrasound: (i) solid mass with heterogeneous echoes and (ii) polycystic solid mass with mixed or low echo, known as the ‘Swiss Cheese’ sign. However, because of the rarity of the disease, the value of these markers has not been determined. To date, only six reports have evaluated the sonographic features of granulosa cell tumour [13]. As for magnetic resonance, the reports are neither descriptive nor comprehensive in characterisation of granulosa cell tumour. Inhomogeneous signal intensity on both T1-weighted image (T1WI) and T2-weighted image (T2WI) and high signal intensity on Diffusion-weighted imaging (DWI) imaging are suggestive markers for the diagnosis of granulosa cell tumour of the ovary [14]. Today, the early detection of adult-type granulosa cell tumour of the ovary can be based on inhibin B and AMH tests. Because inhibin B levels fluctuate during the menstrual cycle and are also increased in some other ovarian cancers (e.g.: epithelial ovarian cancer), AMH is more specific for AGCT of the ovary [2, 3]. AMH has a high sensitivity (92%) and specificity (81%) in detecting macroscopic AGCT of the ovary. Combination of both hormones will have higher diagnostic value than inhibin alone [15]. Our patient had no specific symptoms and the tumour was discovered incidentally during caesarean section. According to Levin et al [4] 1/5 of AGCT of the ovary is asymptomatic and diagnosed incidentally, whereas most cases of juvenile granulosa cell tumour of the ovary are symptomatic at the time of diagnosis.
In the five cases of AGCT of the ovary reported above, the patients all conceived spontaneously. Meanwhile, our patient got pregnant after IVF treatment. Although AMH level and gynaecological ultrasound at the time of the infertility investigation as well as during ovarian stimulation and prenatal care screening for this patient did not detect any abnormalities, it is difficult to determine whether the granuloma was pre-existing or occurred during ovarian stimulation or during pregnancy. The relationship between the use of ovulation induction in infertility treatment and granulosa cell tumour has not been established. Several theories have been put forward to explain this association, such as (i) granulosa cell tumour is present in the ovary and when primed with hormones, they will grow, (ii) high levels of follicle-stimulating hormone oocytes have a carcinogenic effect on granulosa cells and (iii) the onset of granulosa cell tumour during ovarian stimulation is coincidental [8]. A recent Cochran library review (2019) found that infertility is an important risk factor for ovarian cancer. However, when evaluating the association between fertility drugs and ovarian cancer, other factors such as age, body mass index, genetic factors (e.g. family history of ovarian cancer) and causes of infertility should be considered [7].
Surgery remains the mainstay of treatment for primary or secondary AGCT including complete resection of the tumour, uterus, adnexa and staging procedures (peritoneal washings, biopsies and omentectomy). Routine dissection of pelvic and para-aortic lymph nodes is not recommended. Only large or suspicious lymph nodes are removed. For cases where the disease is localised (stage Ia) and where fertility is desired, the tumour adnexa can be removed, preserving the uterus and contralateral ovary. However, careful staging is required in these cases and endometrial biopsy is recommended to rule out metastases and/or concomitant endometrial disease. For early-stage cases, no additional medical treatments are needed. Chemotherapy may be indicated in advanced or inoperable cases, although efficacy and prognostic significance are uncertain. The role of adjuvant chemotherapy in AGCT remains controversial and does not appear to have a significant impact on patient outcomes [5].
Studies show that tumour size is related to disease prognosis, tumour size < 5 cm has a 10-year survival rate of 100%, this rate is 63% with size of 5–15 cm and >15 cm is 34%. Cells with atypical nuclei are considered the most reliable indicator in stage I. For later stages, in addition to atypical nuclei, the percentage of mitotic cells is a poor prognostic factor. However, the correlation between these two factors and the prognosis of the disease has not been determined yet [16]. Our patient had risk factors such as tumour size of 7 cm, histopathological findings with round or coffee bean-shaped nuclei, high number of atypical mitosis. In addition, the patient still wanted to preserve fertility, so the uterus and ovaries were kept. Therefore, this is an incomplete surgery. Based on the above factors, the patient received four cycles of adjuvant chemotherapy Carboplatin + Paclitaxel and was given a long-term follow-up. We stopped at the fourth chemotherapy cycle because of severe side effects – vomiting and neutropenia.
The prognosis of AGCT is generally good. The 5-, 10- and 20-year survival rates were 95.6%, 88.1% and 79.8%, respectively [12]. This tumour is slow-growing but has a high recurrence rate (36%), which can occur decades after initial diagnosis. Therefore, long-term follow-up is important [6]. In addition to gynaecological examination and vaginal ultrasound, AMH or inhibin can be measured during follow-up. However, the combination of AMH and inhibin B will increase the ability to detect disease recurrence [16]. In our patient, after a follow-up period of 36 months, no recurrence was detected in clinical, ultrasound and MRI as well as based on AMH levels.
Conclusion
In conclusion, our patient is the first case of ovarian AGCT incidentally discovered during caesarean section in a pregnant patient after IVF. The relationship between ovulation induction in the treatment of infertility and ovarian cancer as well as ovarian granulosa cell tumour has not been clearly defined. Pre-operative diagnosis is difficult. Conservative surgery should be considered in women who wish to have children. Depending on the stage of the disease and prognostic factors, the treatment method is surgery, chemotherapy, radiation therapy or multimodality. Patients should receive adequate counselling and long-term follow-up to ensure the highest survival rates and early detection of recurrence.
Acknowledgments
Dr Nguyen Van Thanh, who is Head of the Pathology Department, Ho Chi Minh City Oncology Hospital helped us perform immunohistochemical staining and make the histopathological diagnosis.
Abbreviation list
AGCT – adult granulosa cell tumor
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
The authors received no financial support for the research, authorship and publication of this article.
Authors’ contributions
Kong PC wrote the first draft of the paper. Nhat HM and Chi LHY collected the patient’s information. PC Kong reviewed the literature. All authors contributed to revising the manuscript and approving the final submission.
References
1. Fernández-Cid M, Pascual MA, and Graupera B, et al (2011) Adult granulosa cell tumour of the ovary associated with pregnancy J Obstet Gynaecol 31(3) 272–274 https://doi.org/10.3109/01443615.2010.550699 PMID: 21417664
2. Blake EA, Carter CM, and Kashani BN, et al (2014) Feto-maternal outcomes of pregnancy complicated by ovarian sex-cord stromal tumor: a systematic review of literature Eur J Obstet Gynecol Reprod Biol 175 1–177 https://doi.org/10.1016/j.ejogrb.2013.12.025 PMID: 24439718
3. Guidi S, Berghella V, and Scambia G, et al (2021) Adult granulosa cell tumor in pregnancy: a new case and a review of the literature Healthcare (Basel) 9(11) 1455 https://doi.org/10.3390/healthcare9111455 PMID: 34828500 PMCID: 8622987
4. Levin G, Zigron R, and Haj-Yahya R, et al (2018) Granulosa cell tumor of ovary: a systematic review of recent evidence Eur J Obstet Gynecol Reprod Biol 225 57–61 https://doi.org/10.1016/j.ejogrb.2018.04.002 PMID: 29665458
5. Färkkilä A, Haltia UM, and Tapper J, et al (2017) Pathogenesis and treatment of adult-type granulosa cell tumor of the ovary Ann Med 49(5) 435–447 https://doi.org/10.1080/07853890.2017.1294760 PMID: 28276867
6. Lenck C, Chopin N, and Gouy S, et al (2020) The French national network dedicated to rare gynecological cancers diagnosis and management could improve the quality of surgery in daily practice of granulosa cell tumors A TMRG and GINECO group study Gynecol Oncol 157(1) 78–84 https://doi.org/10.1016/j.ygyno.2020.02.012 PMID: 32131977
7. Rizzuto I, Behrens RF, and Smith LA (2019) Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility Cochrane Database Syst Rev 6(6) CD008215 PMID: 31207666 PMCID: 6579663
8. Willemsen W, Kruitwagen R, and Bastiaans B, et al (1993) Ovarian stimulation and granulosa-cell tumour Lancet 341(8851) 986–988 https://doi.org/10.1016/0140-6736(93)91071-S PMID: 8096944
9. Aymen FM, Majed G, and Hanene C, et al (2016) Advanced granulosa cell tumor and pregnancy: a case report, how to treat and how to preserve fertility? Endocrinol Metab Syndr 5250
10. Roy J and Babu AS (2014) Granulosa cell tumor of the ovary--an incidental finding during caesarean section--a rare case report Kathmandu Univ Med J (KUMJ) 12(45) 60–63 https://doi.org/10.3126/kumj.v12i1.13641 PMID: 25219997
11. Agarwal R, Radhakrishnan G, and Radhika AG, et al (2011) Pregnancy concomitant with metastatic adult granulosa cell tumor Arch Gynecol Obstet 284(3) 743–747 https://doi.org/10.1007/s00404-011-1958-y PMID: 21706344
12. Bryk S, Färkkilä A, and Bützow R, et al (2015) Clinical characteristics and survival of patients with an adult-type ovarian granulosa cell tumor: a 56-year single-center experience Int J Gynecol Cancer 25(1) 33–41 https://doi.org/10.1097/IGC.0000000000000304
13. Petrone M, Bergamini A, and Tateo S, et al (2020) Transvaginal ultrasound in evaluation and follow-up of ovarian granulosa cell tumors Int J Gynecol Cancer 30(9) 1384–1389 https://doi.org/10.1136/ijgc-2020-001276 PMID: 32474449
14. Zhang H, Zhang H, and Gu S, et al (2018) MR findings of primary ovarian granulosa cell tumor with focus on the differentiation with other ovarian sex cord-stromal tumors J Ovarian Res 11(1) 46 https://doi.org/10.1186/s13048-018-0416-x PMID: 29871662 PMCID: 5989475
15. Färkkilä A, Koskela S, and Bryk S, et al (2015) The clinical utility of serum anti-Müllerian hormone in the follow-up of ovarian adult-type granulosa cell tumors--a comparative study with inhibin B Int J Cancer 137(7) 1661–1671 https://doi.org/10.1002/ijc.29532 PMID: 25808251
16. Staats PN and Young RH (2019) Sex cord – stromal, steroid cell and other ovarian tumors with endocrine, paraendocrine, and paraneoplastic manifestations Blaustein’s Pathology of the Female Genital Tract 7th edn, eds RJ Kurman, LH Ellenson, and BM Ronnett (Switzerland: Springer Nature Switzerland) pp 967–1045 https://doi.org/10.1007/978-3-319-46334-6_15






