Acute dermatitis in adult female patients receiving hypofractionated radiotherapy for breast cancer: experience from a low- and middle-income country
Yumna Ahmed, Agha Muhammad Hammad Khan, Fatima Shaukat, Rabia Tahseen, Maria Tariq, Bilal Mazhar, Sehrish Abrar and Nasir Ali
Department of Radiation Oncology, Aga Khan University Hospital, Karachi 74800, Pakistan
Abstract
Radiotherapy (RT) is an important component of treatment in the management of breast cancer patients. The radiation treatment paradigm has been shifted towards hypofractionated RT. This study aims to determine the severity of acute dermatitis in patients receiving hypofractionated RT for breast cancer at a tertiary care university hospital in Pakistan. Patients with biopsy-proven invasive breast carcinoma or DCIS who were referred for radical radiotherapy after discussion in the breast tumour board were retrospectively reviewed. Physical assessment of the patients for evaluation of the severity of radiation dermatitis will be carried out in the first week, last week and on the first follow-up after 1 month of completion of RT, according to the Radiation Therapy Oncology Group/European Organisation For Research And Treatment Of Cancer (RTOG/EORTC) criteria. We identified 92 female patients in 6 months at Aga Khan University Hospital, with a mean age of 53.1 years. Most of the treated patients had clinical stage 3 (64%) cancer, while others were stage 2 (42%), stage 1 (2%) and stage 0 (2%). The surgeries performed were mastectomy in 59 patients and breast-conserving surgery in 33 patients. Histology was Intra Ductal Carcinoma (IDC) (95%), DCIS (3%) and Invasive Lobular Carcinoma (ILC) (2%). Most of the patients received chemotherapy (96%). Radiotherapy dose was 4256 cGy in 16 fractions, followed by a boost of 10 Gy. The radiation techniques used were intensity-modulated radiotherapy (47.8%) and three-dimensional conformal radiotherapy (52.2%). Most of the patients experienced no toxicity (59%), while grade I toxicity was observed in 29% of the patients and grade II toxicity was observed in 11%. Only 1% of the patients experienced grade III skin toxicity. Hypofractionated radiation therapy is beneficial because of the shorter overall treatment time which reduces the socio-economic burden, not only for patients but also for radiotherapeutic institutions. However, extended follow-up is to be reported for long-term toxicity and other consequences.
Keywords: hypofractionated, breast cancer, radiation, dermatitis, toxicity
Correspondence to: Yumna Ahmed
Email: yumna.ahmed@aku.edu
Published: 15/06/2022
Received: 14/02/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer is the leading cause of morbidity and mortality in women globally [1, 2]. Pakistan has the highest incidence of breast cancer in Asia, with 1 in every 9 women at risk of developing breast cancer [3, 4]. According to a report, breast cancer accounts for about 34.6% of all cancer cases in Karachi [5], and Pakistani women present at advanced stages with more aggressive diseases. The late presentation may partly be due to a lack of awareness and knowledge about early symptoms and screening for risk factors [5]. Adjuvant radiotherapy (RT) after surgical resection of tumour improves loco-regional control and survival [6, 7]; hence, it is currently the standard of care for all women with breast cancer after breast conservation surgery (BCS) [8]. In early breast cancer (EBC), the 5-year local relapse rate dropped from 4.1% to 1.3% if radiation was offered in their management plan [9]. RT is generally a part of their treatment plan for locally advanced breast cancer (LABC) patients after mastectomy, especially in all T3 and axillary node-positive diseases [10].
Historically, the conventional regimen for adjuvant breast RT was 50 Gray (Gy), at 2 Gy per fraction. Recently, there has been an increasing global interest in the ‘hypofractionated’ regimen which delivers more than 2 Gy/fraction of radiation while reducing the total cumulative dose and reducing the number of treatment days favouring the low- and middle-income country (LMIC) [11]. The hypofractionated regimen offers similar clinical outcomes but fewer hospital visits, providing a practical and cost-effective treatment approach [12]. The skin is susceptible to damage by ionising radiation since it is a highly proliferative and self-renewing organ [13], hence nearly all patients who receive RT for breast cancer experience some degree of radiation dermatitis varying from mild to brisk erythema with or without moist desquamation and, occasionally, ulceration of the skin [14, 15]. They may suffer from dryness, redness, itching, burning, pain and discomfort, which may affect daily life activities and in severe cases, cause interruptions in treatment [16]. In an analysis of the toxicity of hypofractionated RT in breast carcinoma, all patients experienced some degree of acute dermatitis but the most commonly observed severity was grade I experienced by 80% of the patients, grade II by 18% and less than 2% had grade III and IV dermatitis, making it an ideal fractionated schedule in terms of the toxicity profile [12].
This study aims to determine the severity of acute dermatitis in adult female patients receiving hypofractionated radiation therapy for breast cancer to fill the gap in local data especially since the hypofractionated regimen potentially offers comparable outcomes with fewer hospital visits and lower costs in resource-constrained regions.
Methodology
This is a single institution, retrospective chart review to determine the safety of hypofractionated radiation therapy as part of breast cancer treatment. Institutional Ethical Review Committee’s approval was obtained. All patients with biopsy-proven invasive carcinoma or in situ lesion arising from breast cancer, who were referred by a breast surgeon or medical oncologist for radical radiotherapy with a hypofractionated schedule, were enrolled in the study. The inclusion criteria were a) patients with non-metastatic breast cancer after breast-conserving surgery or mastectomy; b) age between 20 and 80 years; and (c) carcinoma in situ or invasive breast cancer proven by histology of the surgical specimen. Patients who had received previous radiotherapy in the same region or had a history of systemic lupus erythematosus or scleroderma and those with bilateral breast cancer were excluded. A report of 6 months from 1 December 2018 to 30 May 2019 was documented.
Considering 80% grade 1 toxicity from the previous studies, we enrolled 92 patients after (obtaining informed consent) for a 95% confidence level and 8% bound on error of estimation using non-probability consecutive sampling.
Data collection
The acute phase of radiation dermatitis was observed from medical records for first and last week of treatment and on first follow-up after 1-month completion of radiation therapy.
During each visit, examination of the severity of reactions was graded from 0 to IV, according to the RTOG/EORTC acute radiation morbidity scoring criteria, version 2.0 [17].
Data were collected by the researcher from hospital medical records and documented as required in the study proforma.
Radiation planning and delivery
A hypofractionated course of radiation in our study was defined as a definitive dose to the whole breast of 2.6 Gy/fraction. Patients were simulated with a computed tomography scan in the supine position. Our clinical pathway permitted optional boost radiotherapy of 10 Gy in 4–5 fractions using electron beam for post-mastectomy chest wall scar boost or photon beam lumpectomy boost radiotherapy in patients who underwent BCS. Treatment plans were created using 2D, 3D or intensity-modulated radiotherapy (IMRT) techniques as per the physician’s choice and considering patients’ financial constraints to meet the institutional dosimetric criteria, limiting WB-CTV V105 to 95% (95% of the PTV receiving 95% of the prescribed dose), although D90 >90% was also deemed acceptable to meet homogeneity goals.
Data analysis
SPSS (version 21.0) was used for statistical analysis. A descriptive analysis was carried out. Mean and standard deviation were calculated for quantitative variables, i.e., age and frequency of occurrence of dermatitis. Percentages were calculated for qualitative variables, i.e., stage, type of surgery, chemotherapy, radiation technique and severity of acute dermatitis. Stratification of outcome variables was performed for effect modifiers like age, disease pathology, stage of disease, type of surgery, chemotherapy and radiation technique. Post-stratification chi-square test was applied. A p-value less than or equal to 0.05 was considered statistically significant.
Results
92 patients received hypofractionated radiotherapy at our institution during this duration. The median age of female patients was 53.1 years (range = 22–80 years). An equal number of patients were treated for right and left breast cancers. Most patients had IDC 88 (95.7%) as the primary pathology. Most of the patients had stage III cancer (55.4, 51%), followed by stage II (37, 40.2%). One-third of the patients underwent BCS and received hypofractionated radiotherapy while two-thirds of the patients received radiation to the chest wall after mastectomy. Total radiation dose of 42.56 Gy at 2.66 Gy per day was delivered in all cases. A radiation boost of 10 Gy in 2 Gy daily fractions was delivered to 83 (90.2%) patients who were younger than 50 years of age, IDC grade 3 or positive margin disease. IMRT was the most used technique comprising 46 (50%) patients, followed by the three-dimensional conformal radiotherapy (3D CRT) technique in 44 (47.8%) patients. Two-dimensional (2D) was used in two (2.2%) patients. Most of our patients (89, 96%) already received chemotherapy before starting radiotherapy during the course of their treatment either in the neoadjuvant or adjuvant setting after definitive surgery. The patient and treatment characteristics are listed in Table 1.
Early (1st week) treatment
Most (80, 87%) patients had no dermatitis. Grade I dermatitis was present in 12 (13%) patients. None of the patients had grade II, III or IV dermatitis within the first week of radiotherapy (Figure 1).
Late (last week) treatment
Grade 1 dermatitis was mostly reported during the last week of radiation therapy constituting 44% (n = 41) of the patients, while 24% (n = 22) had grade II dermatitis and approximately one-third of the patients experienced no toxicity (32%, n = 27).
Post-treatment (1-month follow-up)
60% (n = 56) had no dermatitis at the post-treatment 1-month follow-up, while 26% (n = 24) had grade 1 dermatitis, 11% (n = 10) had grade 2 and 2.2% (n = 2) had grade 3 dermatitis.
Table 1. Patient and treatment characteristics
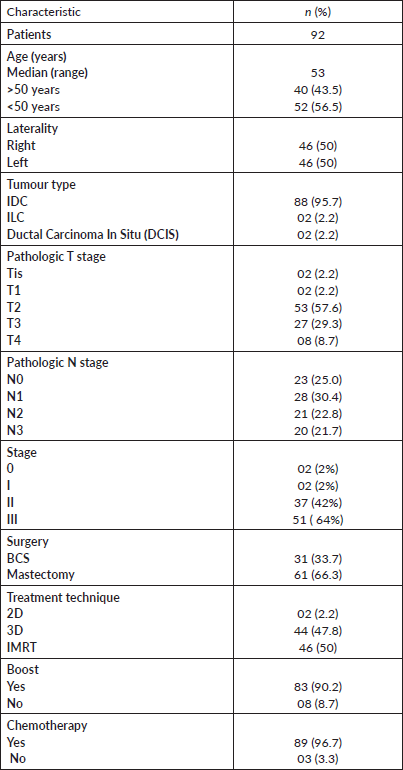
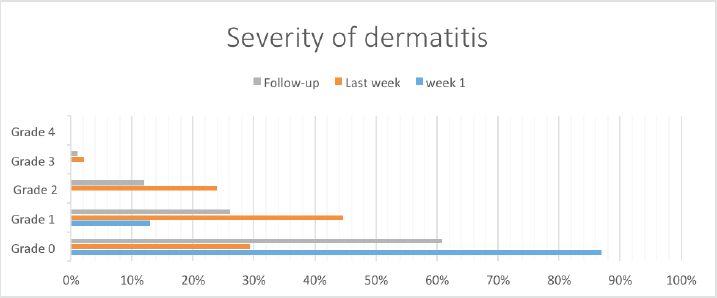
Figure 1. Bar chart illustrating the severity of dermatitis over time.
Stratification of severity of dermatitis with different variables
There was no significant difference in the severity of dermatitis during the first and last week of radiation and on follow-up after radiation relative to age and radiation boost. Grade 2 toxicity was significantly associated with the 3D CRT technique plan during the last week of radiotherapy (p = 0.05). However, no other association between severity of dermatitis and technique was found during the first week and on follow-up.
Grade 1 toxicity during follow-up after RT was significantly associated with mastectomy (p = 0.01). In a chest wall patient (undergoing mastectomy), the most common site of local recurrence is skin; so in these patients, a bolus is placed to increase the skin dose resulting in grade 2 skin dermatitis. Only patients with cT4 disease were found to be associated with grade 3 toxicity during follow-up (p = 0.03).
Discussion
Breast cancer has the highest incidence among women worldwide. Approximately 90,000 new cases of breast cancer are diagnosed in Pakistan each year [18]. RT plays a core role in management, not only by reducing local recurrence but also by improving overall survival in both EBC and LABC patients [19]. In LMICs like Pakistan with limited public health resources and a significant lack of awareness about screening methods, which results in advanced stage presentation, RT treatment becomes a necessity for many with a long waiting list. A shift in adaptation to a hypofractionated schedule from the conventional was confirmed in a meta-analysis quoting 17 studies and concluded that there was no significant difference in local recurrence and improved toxicity and appearance outcomes noted in the hypofractionated with long-term follow-up [19, 20].
The first published phase II clinical trial of hypofractionated radiotherapy was published in 1987 with comparable loco-regional control and an acceptable cosmetic outcome [21].
A similar outcome was reported for 150 patients who underwent hypofractionated radiotherapy by Ortholan et al [22, 23]. Whelan et al also demonstrated the same finding in a better cohort of 1200 patients who received 50 Gy in 25 fractions or 42.56 Gy in 16 fractions with a 10-year follow-up. Grade 3 or higher acute dermatitis was only present in 3% of each group [24, 25]. A 12-year follow-up of a randomised
Canadian trial of 1234 women with early breast cancer reported the same outcome comparable between two groups of patients [26]. The Chinese randomised trial used hypofractionated RT in EBC patients who underwent breast-conserving surgery and showed no different toxicity than conventional arm [27]. All the studies mentioned above showed good tolerability of hypofractionated radiotherapy in breast cancer but cosmetic results have been somewhat mixed and likely depend on the specific schedule used [28]. Our study evaluated toxicity with a single regimen of hypofractionated radiotherapy and most of the patients had locally advanced diseases requiring chemotherapy. We offered a hypofractionated schedule to all our patients who had undergone BCS or modified radical mastectomy with the intention of reducing treatment time and comparing acute toxicity without compromising the oncologic result. The results from our study are consistent with international studies showing reduced skin toxicity with the hypofractionated RT schedule [29]. A total of 92 patients enrolled in our study were administered 2.66 Gy per fraction in an adjuvant setting after surgery and most of them did not develop toxicity in the first week. There is also insufficient data to evaluate the tolerability of shorter fractionation when used with different radiotherapy techniques, chemotherapy and type of surgical procedures [29]. There is a significant association between grade II and grade III dermatitis with 3D CRT and mastectomy, respectively. In addition to reduced toxicity, the hypofractionated schedule proved to be economically feasible because of the shorter overall treatment time, not only for patients but also beneficial for the limited number of hospitals providing radiation therapy to cancer patients in the region. Thus, implementation of shortened RT regimens would help decrease the burden on such institutions and help maintain mechanical and human resources required to meet the increasing demand for radiotherapy. Recently, further reduction of treatment time with a 1-week schedule of radiotherapy after primary surgery for EBC reported grade II as the most commonly observed acute toxicity (47%) as in our study and is proven safe in terms of normal tissue effects up to 5 years [30, 31]. The prevalence of acute dermatitis in our study suggested that severity is more intense during the last week of radiation (70%) and settled in one-third of the patients on follow-up after 4 weeks of radiation (28%). The grade III toxicity (2%) was only observed in patients who underwent mastectomy and had T4 disease so these patients should be kept under invigilation for clinical evaluation. However, since long-term follow-up was not done, only acute toxicity was assessed during and after completion of treatment. The impact of hypofractionated regimen on loco-regional control rates, distant metastasis-free survival rates, overall survival rates and long-term morbidities were not documented in our study.
Our study suggests excellent tolerability of hypofractionated RT for acute dermatitis in our populations. Considering this question has not been explored from our part of the world in both subsets of patients. Our study also evaluated the severity of acute dermatitis and its relation with time throughout the course of radiation therapy and follow-up.
Conclusion
Hypofractionated radiation therapy is a very promising regimen because of the shorter overall treatment time which reduces the socio-economic burden, not only for patients but also for radiotherapeutic institutions in LMICs. Further building upon this database will help find outcomes related to disease control and late effects concerning the dose of radiation therapy.
Funding
No funding was received for this article.
Conflicts of interest
The authors declare no conflicts of interest in this work.
Acknowledgments
None.
Authors’ contributions
Dr Yumna developed the concept and design of the study, acquired data for analysis and interpreted the data.
Dr Hammad and Dr Fatima drafted the article.
Dr Rabia, Dr Maria and Dr Sehrish revised the article critically for important intellectual content.
Dr Bilal and Dr Nasir finally approved the version to be published.
References
1. Kumar S, Imam AM, and Manzoor NF, et al (2009) Knowledge, attitude and preventive practices for breast cancer among health care professionals at Aga Khan Hospital Karachi J Pak Med Assoc 59(7) 474 PMID: 19579739
2. Parkin DM and Fernández LM (2006) Use of statistics to assess the global burden of breast cancer Breast J 12 S70–S80 https://doi.org/10.1111/j.1075-122X.2006.00205.x PMID: 16430400
3. Bhurgri Y, Kayani N, and Faridi N, et al (2007) Patho-epidemiology of breast cancer in Karachi ‘1995-1997’ Asian Pac J Cancer Prev 8(2) 215–220 PMID: 17696734
4. Hadi NI and Jamal Q (2016) Comparison of clinicopathological characteristics of lymph node positive and lymph node negative breast cancer Pak J Med Sci 32(4) 863 PMID: 27648029 PMCID: 5017092
5. Bhurgri Y, Bhurgri A, and Hassan SH, et al (2000) Cancer incidence in Karachi, Pakistan: first results from Karachi cancer registry Int J Cancer Manag 85(3) 325–329 https://doi.org/10.1002/(SICI)1097-0215(20000201)85:3<325::AID-IJC5>3.0.CO;2-J
6. Group EBCTC (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials Lancet Oncol 378(9804) 1707–1716 https://doi.org/10.1016/S0140-6736(11)61629-2
7. Overgaard M, Hansen PS, and Overgaard J, et al (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy N Engl J Med 337(14) 949–955 https://doi.org/10.1056/NEJM199710023371401
8. Kirwan C, Coles C, and Bliss J, et al (2016) It’s PRIMETIME. Postoperative avoidance of radiotherapy: biomarker selection of women at very low risk of local recurrence Clin Oncol 28(9) 594–596 https://doi.org/10.1016/j.clon.2016.06.007
9. Kunkler IH, Williams LJ, and Jack WJ, et al (2015) Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial Lancet Oncol 16(3) 266–273. https://doi.org/10.1016/S1470-2045(14)71221-5 PMID: 25637340
10. Cooke AL, Diaz-Abele J, and Hayakawa T, et al (2017) Radiation therapy versus no radiation therapy to the neo-breast following skin-sparing mastectomy and immediate autologous free flap reconstruction for breast cancer: patient-reported and surgical outcomes at 1 year—a Mastectomy Reconstruction Outcomes Consortium (MROC) substudy Int J Radiat Oncol Biol Phys 99(1) 165–172 https://doi.org/10.1016/j.ijrobp.2017.05.001 PMID: 28816143
11. Mortimer JW, McLachlan CS, and Hansen CJ, et al (2016) Use of hypofractionated post‐mastectomy radiotherapy reduces health costs by over $2000 per patient: an Australian perspective J Med Imaging Radiat Oncol 60(1) 146–153. https://doi.org/10.1111/1754-9485.12405
12. MiShRa R, KhuRana R, and Mishra H, et al (2016) Retrospective analysis of efficacy and toxicity of hypo-fractionated radiotherapy in breast carcinoma J Clin Diagn Res 10(8) XC01 PMID: 27656543 PMCID: 5028454
13. Zhao H, Zhu W, and Jia L, et al (2015) Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy Br J Radiol 89(1058) 20150665 https://doi.org/10.1259/bjr.20150665 PMID: 26607642 PMCID: 4985212
14. Kole AJ, Kole L, and Moran MS (2017) Acute radiation dermatitis in breast cancer patients: challenges and solutions Breast Cancer (Dove Med Press) 9 313
15. De Langhe S, Mulliez T, and Veldeman L, et al (2014) Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy BMC Cancer 14(1) 711 https://doi.org/10.1186/1471-2407-14-711 PMID: 25252713 PMCID: 4192342
16. Fernández‐Castro M, Martín‐Gil B, and Peña‐García I, et al (2017) Effectiveness of semi‐permeable dressings to treat radiation–induced skin reactions. A systematic review Eur J Cancer 26(6) e12685 https://doi.org/10.1111/ecc.12685
17. Trotti A, Byhardt R, and Stetz J, et al (2000) Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy IJROBP 1 47(1) 13–47
18. Khan MO (2009) The necessity of awareness of breast cancer amongst women in Pakistan. JPMA J Pak Med Assoc 59(11) 804
19. Clarke M, Collins R, and Darby S, et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials Lancet 366 2087 https://doi.org/10.1016/S0140-6736(05)67887-7 PMID: 16360786
20. Early Breast Cancer Trialists’ Collaborative Group, Darby S, and McGale P, et al (2011 Nov) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials Lancet 378(9804) 1707–1716 https://doi.org/10.1016/S0140-6736(11)61629-2 PMID: 22019144 PMCID: 3254252
21. Haviland JS, Owen JR, and Dewar JA, et al (2013) The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials Lancet Oncol 14 1086 https://doi.org/10.1016/S1470-2045(13)70386-3 PMID: 24055415
22. Rostom AY, Pradhan DG, and White WF (1987) Once weekly irradiation in breast cancer Int J Radiat Oncol Biol Phys 13(4) 551–555 https://doi.org/10.1016/0360-3016(87)90070-8 PMID: 3558045
23. Ortholan C, Hannoun-Lévi JM, and Ferrero JM, et al (2005) Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients Int J Radiat Oncol Biol Phys 61(1) 154–162 https://doi.org/10.1016/j.ijrobp.2004.04.059 PMID: 15629606
24. Whelan TJ, Levine M, and Julian J, et al (2000) The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial Cancer 88(10) 2260-6.000000000000000 https://doi.org/10.1002/(SICI)1097-0142(20000515)88:10<2260::AID-CNCR9>3.0.CO;2-M PMID: 10820347
25. Whelan T, MacKenzie R, and Julian J, et al (2002) Randomized trial of regional irradiation schedules after lumpectomy for women with lymph node-negative breast cancer J Nat Cancer Inst 94 1143–1150 https://doi.org/10.1093/jnci/94.15.1143
26. Whelan T, MacKenzie R, and Julian J, et al (2002) Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer J Natl Cancer Inst 94(15) 1143–1150 https://doi.org/10.1093/jnci/94.15.1143 PMID: 12165639
27. Wang SL, Fang H, and Hu C, et al (2020) Hypofractionated versus conventional fractionated radiotherapy after breast-conserving surgery in the modern treatment era: a multicenter, randomized controlled trial from China J Clin Oncol 38(31) 3604–3614 https://doi.org/10.1200/JCO.20.01024 PMID: 32780661
28. James ML, Lehman M, and Hider PN, et al (2010) Fraction size in radiation treatment for breast conservation in early breast cancer Cochrane Database Syst Rev (11) Art. No.: CD003860 https://doi.org/10.1002/14651858.CD003860.pub3 PMID: 21069678
29. Jagsi R, Griffith KA, and Vicini F, et al (2020) Toward improving patients’ experiences of acute toxicity from breast radiotherapy: insights from the analysis of patient-reported outcomes in a large multicenter cohort J Clin Oncol 38(34) 4019 https://doi.org/10.1200/JCO.20.01703 PMID: 32986529
30. Brunt AM, Wheatley D, and Yarnold J, et al (2016) Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial Radiother Oncol 120(1) 114–118 https://doi.org/10.1016/j.radonc.2016.02.027 PMID: 27046390 PMCID: 4998960
31. Brunt AM, Haviland JS, and Wheatley DA, et al (2020) Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial Lancet 395(10237) 1613–1626 https://doi.org/10.1016/S0140-6736(20)30932-6






