Second-line therapy in testicular germ cell tumours: results from a tertiary cancer care centre in India
Amit Joshi1, Devanshi Kalra1, Vijai Simha1, Nandini Menon1, Vanita Noronha1, Ganesh Bakshi2, Gagan Prakash2, Mahendra Pal2, Vedang Murthy3, Santosh Menon4, Nilesh Sable5, Archi Agrawal6, Pallavi Rane1 and Kumar Prabhash1
1Department of Medical Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 40012, India
2Department of Surgical Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 40012, India
3Department of Radiation Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 40012, India
4Department of Pathology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 40012, India
5Department of Radiology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 40012, India
6Department of Nuclear Medicine, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 40012, India
Abstract
Background: Malignant testicular neoplasms constitute about 1% of all cancers in males. This is one of the most common tumours in adolescents and young adult males. After the introduction of cisplatin-based chemotherapy, the survival of germ cell tumour patients, even those with poor prognostic risk factors, has significantly improved over the years. Second-line chemotherapy in patients who have progressed over the first-line cisplatin-based chemotherapy has shown convincing 5 years of overall survival (OS).
Methodology: This study is a retrospective analysis of testicular cancer patients from 2014 to 2020 who have received salvage chemotherapy treatment at Tata Memorial Centre. Patient demographics, tumour characteristics and treatment details were recorded in a specific format, and progression-free survival and OS were analysed along with response to therapy.
Results: A total of 46 testicular cancer patients from 2014 to 2020, who received second-line chemotherapy, were analysed from the database maintained at our hospital. The median age at diagnosis was 29.5 (18–60) years. Most of the patients (30, 65.2%) presented with lung metastasis and 11 (23.9%) patients with liver metastasis. Most of the patients (21, 45.6%) received vinblastine, ifosfamide and cisplatin, whereas 13 (28.2%) patients received paclitaxel, ifosfamide and cisplatin regimen and 7 (15.2%) patients received GemOx regimen as the second-line chemotherapy. Median OS was observed to be 33.97 months and median progression-free survival was 29.01 months.
Conclusion: Second-line chemotherapy in testicular germ cell tumours can result in long-term disease control and all patients who are fit to tolerate second-line therapy should be offered it. Patients with relapsed seminoma did better than relapsed non-seminomatous germ cell tumours.
Keywords: testicular cancer, second line therapy, TIP
Correspondence to: Amit Joshi
Email: dramitjoshi74@gmail.com
Published: 13/06/2022
Received: 12/10/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Testicular cancer is one of the most common tumours in adolescents and young adult males. Malignant testicular neoplasms constitute about 1% of all cancers in males [1]. They are classified according to the histological types of the cells, seminomatous and non-seminomatous [2]. Testicular cancers are highly chemotherapy-sensitive tumours. Especially after introducing cisplatin-based chemotherapies, the disease can be cured in 50%–60% of the cases, even if it presents as an advanced disease with poor prognostic risk factors [2].
Of all the testicular cancers, 95% are germ cell tumours [1]. Among these, about 50% are non-seminomatous germ cell tumours (NSGCT). It is one of the most common cancers in young men. The age-standardised incidence rate of testicular cancer is 6.7 and 5.6 per 100,000 population for Europe and the United States, respectively, whereas it is 0.5 per 100,000 population in India [2]. Globally, India has the lowest incidence, and hence there is a lack of literature regarding Indian data on testicular germ cell tumours.
When it comes to the management of relapsed testicular cancer patients, various options are available, such as conventional-dose chemotherapy, i.e., paclitaxel + ifosfamide + cisplatin (TIP) and etoposide + ifosfamide + cisplatin (VIP), and high-dose chemotherapy (HD-CT) regimens, such as carboplatin + etoposide and paclitaxel + ifosfamide + carboplatin + etoposide, followed by autologous bone marrow transplant [3].
Most patients receive second-line salvage chemotherapy after the progression of the disease over first-line therapy; an autologous bone marrow transplant is not feasible because of the unavailability of expertise, cost constraints and also due to lack of any randomised trial showing the superiority of one approach to another one [3].
In this article, we report a single-centre experience of managing testicular cancer patients who have received salvage second-line chemotherapy after the progression of the disease. We, at this moment, would like to describe the clinical presentation, diagnosis, treatment complexities and disease-free survival in the context of the patients who received second-line chemotherapy.
Materials and methods
Selection of cases
Case records of testicular cancer patients from 2014 to 2020 were retrospectively reviewed. Patients who had completed second-line chemotherapy were identified. Various parameters, such as patient demographics, tumour characteristics, treatment details recorded in a specific format, progression-free survival and overall survival (OS), were analysed.
Statistical analysis
Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) version 25 (IBM SPSS Statistics, Armonk, NY: IBM Corp). A descriptive analysis was carried out to analyse the demographic characteristics. Kaplan–Meier’s survival analysis computed the progression-free survival and OS.
Results
Forty-six testicular cancer patients from 2014 to 2020 who have received second-line chemotherapy were analysed from the database maintained at our hospital. All the patients were included for demographic analysis. The patients who had completed at least 1 year of follow-up after second-line treatment were analysed for progression-free and OS. The demographic characteristics were analysed descriptively using SPSS version 25.
Demographics
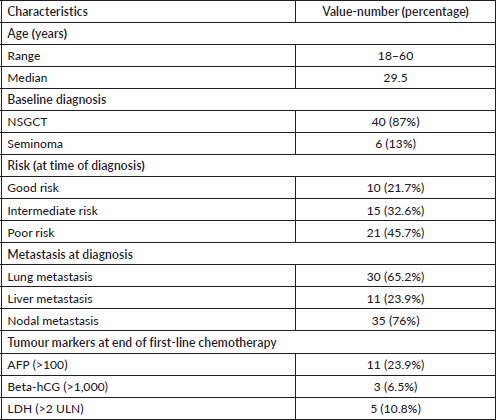
The median age was 29.5 years (18–60 years). 40 (87%) patients were diagnosed with NSGCT, whereas 6 (13%) patients were diagnosed with seminomatous germ cell tumours (Table 1).
21 (45.7%) patients had poor risk of the disease, 15 (32.6%) patients had intermediate risk and 10 (21.7%) patients had reasonable risk at the time of initial presentation. 12 (30%) non-seminomatous testicular cancer patients showed partial response (PR) to first-line chemotherapy and 2 (33.%) seminomatous testicular cancer patients had a partial answer to the first-line chemotherapy.
The median time to relapse after completion of first-line chemotherapy is 5.3 months (4.3–90.1 months).
Metastasis
Most of the patients (30, 65.2%) presented with lung metastasis, 11 (23.9%) patients with liver metastasis and 35 (76%) patients with nodal metastasis with the size of >5 cm in 8 patients and 13 (28.2%) patients had a nodal size between 2 and 5 cm.
Moreover, two patients had brain metastasis before starting second-line chemotherapy, and four developed it after or during second-line chemotherapy.
Table 1. Demographic characteristics.
Second-line chemotherapy details
Most of the patients (20, 43.4%) received vinblastine, ifosfamide and cisplatin (VeIP) regimen as per the physician’s decision, whereas 16 (34.7%) patients received TIP (paclitaxel, ifosfamide, cisplatin) regimen and 7 (15.2%) patients received GemOX (gemcitabine, oxalipaltin) regimen as the second-line chemotherapy. One of the patients underwent a transplant.
Most of the patients, 15 out of 20 (75%), completed 4 cycles of VIP, whereas 9 out of 16 (56%) patients completed 4 cycles of TIP as second-line chemotherapy at our centre. Progressive disease was the most common cause of not completing the planned therapy.
Toxicities
Second-line chemotherapy was relatively well tolerated. However, various toxicities are seen during treatment with second-line chemotherapy. Different toxicities were reported, i.e., 7 (15.2%) and 2 (0.043%) patients had developed grade III and grade IV anaemia, and 5 (10.8%) and 10 (21.7%) patients had developed grade III and grade IV neutropenia, respectively. Also, 9 (19.6%) and 3 (6.5%) patients had developed grade III and grade IV thrombocytopenia, respectively. Moreover, grade III diarrhoea and grade III hyponatraemia were reported in 2 (4.3%) patients.
10 (21.7%) patients underwent retroperitoneal lymph node dissection (RPLND) after completing second-line chemotherapy. Based on histopathology, three patients had mature teratoma and three had the viable disease. Also, four patients had no viable disease.
After completion of the second line of chemotherapy, currently, 18 patients are alive (39.1%), out of which 4 (22.2%) are active with the disease and 14 (77.7%) are alive without disease.
Patients with seminomatous testicular cancer showed better response to second-line chemotherapy than patients with non-seminomatous testicular cancer.
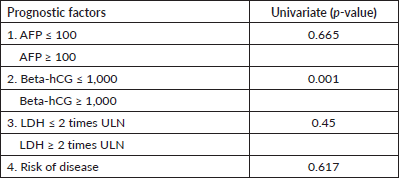
We tried to assess the correlation of survival with various prognostic factors. The univariate correlation was observed with elevated tumour markers, such as beta-hCG, alpha-fetoprotein (AFP) and lactate dehydrogenase (LDH), at the time of relapse on first-line therapy, i.e., only beta-hCG levels more than 1,000 at the time of relapse was found to be associated with worse survival (Table 2).
Response to the first line of chemotherapy
Out of 46 patients, 10 (21.7%) responded thoroughly to the first-line chemotherapy. 9 (22.5%) patients with NSGCT had a complete response (CR), whereas 1 (16.6%) patient with seminoma had a CR after first-line chemotherapy. PR was seen in 12 (30%) NSGCT patients and 2 (33.3%) seminoma patients. 22 (47.8%) patients had progressive disease after the first line of chemotherapy.
Table 2. Prognostic factors and OS.
Response to the second line of chemotherapy
Of a total of 46 patients, 7 (15.2%) patients had a stable disease, 5 (10.8%) patients showed CR and 18 (39.1%) patients had a partial answer to the second-line chemotherapy. Also, 10 (21.7%) patients progressed on second-line chemotherapy.
Survival and disease-free interval
10 (21.7%) out of 46 patients progressed after the second line of therapy. The patients who were lost to follow-up before completing 1-year post-second-line treatment, i.e., 9 out of 46 patients, were not included in the survival analysis. Median progression-free survival was 29.01 months, with a 2-year probability of survival of 53.4 months (35.7–71.1) (Figure 1).
OS was defined as the time from the start of second-line chemotherapy to the last follow-up date or date of death. The median OS was 33.97 months with 2 years probability of 59.6 months (42.2–76.9) (Figure 2). It was reported that the study’s median follow-up was 34.8 months.
Discussion
Despite the low incidence of testicular cancer in India and all the advances made in treatment options for testicular cancer, some patients had a recurrence of the disease. There is a lack of literature regarding the outcome of testicular cancer, especially with the role of second-line chemotherapy in treating this type of rare cancer. Hence, to understand the role of second-line chemotherapy and various factors affecting the treatment of patients undergoing second-line chemotherapy in our patient population, we conducted this retrospective analysis of second-line chemotherapy among testicular patients at a tertiary care centre in a developing country.
Our study specified 46 patients given second-line chemotherapy; out of this, 9 patients were lost to follow-up within 1 year of receiving therapy and were not included in the survival analysis. The study by Pico et al [4], included 263 patients randomised into 2 arms, and the survey by Loehrer et al [5] was conducted on 135 patients. Also, a study by Farhat et al [6] included 54 patients, signifying complete remission in 24 patients at completion of VIP chemotherapy (as mentioned in Table 3).
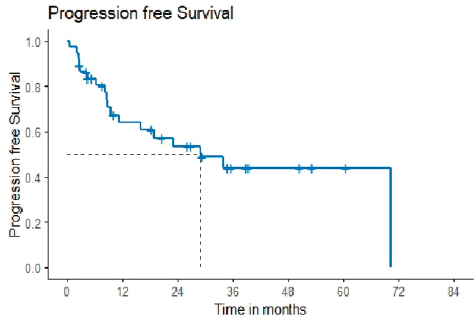
Figure 1. Progression-free survival.
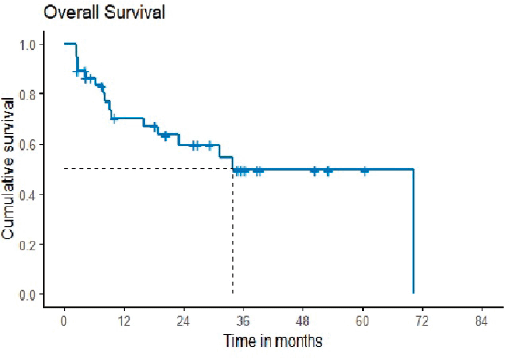
Figure 2. Overall survival.
Table 3. Studies conducted in refractory testicular cancer patients.

Using one of the predictive models to risk stratify our patients, i.e., Beyer et al’s [14] scoring method, 22 (47.8%) patients had good risk, 23 (50%) had intermediate risk and 1 (2.1%) had poor risk. Out of these, CR or PR to second-line chemotherapy was observed in 11 (50%) patients with good risk, 12 (52.1%) with intermediate risk, which is comparable to the study by Beyer et al [7] specifying 76.5% of the patients with good risk showing CR/PR and 58.6% of the patients with intermediate risk showing PR/CR to second-line chemotherapy.
Kondagunta et al [8] stated a median OS of 42 months. A study by McCaffrey et al [7] recorded a median OS of 18 months.
A few initial reports from the American and European groups that included VIP therapy in the second line demonstrated a CR in 25%–36% of the cohort. McCaffrey et al’s [7] study included 56 patients, and 36% of the patients had a CR. On the contrary, our study included only 10.8% of the patients who showed CR when given second-line chemotherapy. This difference in response to dual-line salvage therapy may be because, in our setting, we have analysed data of all the patients who have received salvage therapy irrespective of the timing of relapse (platin sensitive versus refractory disease), site of relapse (both visceral and non-visceral), different salvage chemotherapy protocol used (TIP/VI/GemOx) and performance status (PS) of patients [7].
Our study stated that 14 out of 46 patients had a late relapse. Late relapse refers to patients who relapsed after more than 2 years after completion of treatment [9]. Moreover, there is a lack of literature highlighting the late relapses in testicular cancer. Most relapses occur within the first 2 years of therapy; late relapse beyond 2 years is rare [9, 10].
Also, our study concludes that a total of 18 patients out of 46 are currently alive after completion of second-line chemotherapy, out of which 4 patients are active with the disease and the remaining 14 patients are alive without the disease; this is even after 9 patients were lost to follow-up within 1 year. A study by McCaffrey et al [7] specified that 23 patients out of 56 were alive; 19 of them were alive without any disease and 4 were active with the disease after completion of second-line chemotherapy.
Previous studies have shown moderately promising results in utilising HD-CT in the salvage treatment of refractory or relapsing germ cell tumours. Still, high-level evidence supporting HD-CT as a second or further-line treatment is lacking. One of the retrospective studies by Lorch et al [11] reported a favourable response rate of 55% after HD-CT as a second salvage treatment, with a remarkably long-term survival rate of 17%.
Although the standard second-line salvage therapy is still debatable, peripheral blood stem cell transplantation is also a salvage therapy option. It is commonly used in some parts of the world, especially in medical oncology centres in the USA.
The TIGER trial (A031102, E1407) is an ongoing trial with international collaboration of many centres in North America, Europe and Australia to determine the optimal initial salvage chemotherapy approach in patients with advanced germ cell tumour, which will help in giving a conclusion that either HD-CT, followed by autologous transplant, or salvage chemotherapy alone can be the standard of care in the initial salvage setting [12, 13].
The limitation of our study was that it is a small retrospective study; it does not include various factors that would be better recorded in a prospective study.
Most testicular cancer patients presented with advanced stages. Even with standard BEP (bleomycin, etoposide cisplatin) therapy and getting cured, only a few patients relapse, which can be successfully salvaged in a quarter of the patients and may get long-term control with acceptable toxicity at a community practice level.
Conclusion
Our study reports the various demographic factors of testicular patients who received second-line chemotherapy in the Indian context. Second-line chemotherapy in testicular germ cell tumours can result in long-term disease control. The high durable response proportion emphasises the importance of patient selection according to prognostic factors such as histopathology and tumour markers. It should be offered to all patients who have progressive disease on first-line therapy with good PS.
Acknowledgments
None.
Conflicts of interest
None.
Funding
None.
Disclosure of results at a meeting
None.
References
1. Singh D, Singh P, and Mandal A (2020) Epidemiology and treatment outcomes of testicular germ cell tumor at a tertiary care center in Patna, India: a retrospective analysis Asian Pacific J Cancer Care 5(1) 45–50 https://doi.org/10.31557/apjcc.2020.5.1.45-50
2. Yilmaz F, Soyer N, and Uslu R, et al (2017) Retrospective analysis of patients with relapsed or refractory testicular nonseminoma germ cell tumors treated with autologous stem cell transplantation Indian J Cancer 54(2) 415 https://doi.org/10.4103/ijc.IJC_284_17
3. Voss MH, Feldman DR, and Bosl GJ, et al (2011) A review of second-line chemotherapy and prognostic models for disseminated germ cell tumors Hematol Oncol Clin North Am 25(3) 557–576 https://doi.org/10.1016/j.hoc.2011.03.007 PMID: 21570609 PMCID: 3230321
4. Pico JL, Rosti G, and Kramar A, et al (2005) A randomized trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumors Ann Oncol 16(7) 1152–1159 https://doi.org/10.1093/annonc/mdi228 PMID: 15928070
5. Loehrer PJ, Gonin R, and Nichols CR, et al (1998) Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor J Clin Oncol 16(7) 2500–2504 https://doi.org/10.1200/JCO.1998.16.7.2500 PMID: 9667270
6. Farhat F, Cline S, and Théodore C, et al (1996) Cisplatin and ifosfamide with either vinblastine or etoposide as salvage therapy for refractory or relapsing germ cell tumor patients: the Institut Gustave Roussy experience Cancer 77 1193–1197 Date accessed: 03/08/21 https://doi.org/10.1002/(SICI)1097-0142(19960315)77:6<1193::AID-CNCR28>3.0.CO;2-W PMID: 8635143
7. McCaffrey JA, Mazumdar M, and Bajorin DF, et al (1997) Ifosfamide- and cisplatin-containing chemotherapy as first-line salvage therapy in germ cell tumors: response and survival J Clin Oncol 15(7) 2559–2563 https://doi.org/10.1200/JCO.1997.15.7.2559 PMID: 9215825
8. Kondagunta GV and Motzer RJ (2006) Chemotherapy for advanced germ cell tumors J Clin Oncol 24(35) 5493–5502 https://doi.org/10.1200/JCO.2006.08.7882 PMID: 17158534
9. George DW, Foster RS, and Hromas RA, et al (2003) Update on late relapse of germ cell tumor: a clinical and molecular analysis J Clin Oncol 21(1) 113–122 https://doi.org/10.1200/JCO.2003.03.019
10. Baniel J, Foster RS, and Gonin R, et al (1995) Late relapse of testicular cancer J Clin Oncol 13(5) 1170–1176 https://doi.org/10.1200/JCO.1995.13.5.1170 PMID: 7537800
11. Lorch A, Neubauer A, and Hackenthal M, et al (2010) High-dose chemotherapy (HDCT) as second-salvage treatment in patients with multiple relapsed or refractory germ-cell tumors Ann Oncol 21(4) 820–825 https://doi.org/10.1093/annonc/mdp366
12. McHugh DJ and Feldman DR (2018) Conventional-dose versus high-dose chemotherapy for relapsed germ cell tumors Adv Urol 2018 https://doi.org/10.1155/2018/7272541 PMID: 29736168 PMCID: 5874975
13. Oing C and Lorch A (2018) The role of salvage high-dose chemotherapy in relapsed male germ cell tumors Oncol Res Treat 41 365–369 https://doi.org/10.1159/000489135 PMID: 29843143
14. Beyer J, Kramar A, and Mandanas R, et al (1996) High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables J Clin Oncol 14(10) 2638–2645






