Management of the positive sentinel lymph node following neoadjuvant chemotherapy: results of a survey conducted with breast surgeons
Francisco Pimentel Cavalcante1,a, Felipe Zerwes2,b, Eduardo Camargo Millen3,c, Guilherme Novita4,d, Alessandra Borba Anton de Souza5,e, João Henrique Penna Reis6,f, Helio Rubens de Oliveira Filho7,g, Luciana Naíra de B L Limongi8,h, Barbara Pace Silva de Assis Carvalho9,i, Adriana Magalhães de Oliveira Freitas10,j, Monica Travassos Jourdan11,k, Vilmar Marques de Oliveira12,13,l, Ruffo Freitas-Junior14,m
1Breast Unit, Fortaleza General Hospital (HGF), Fortaleza, CE 60150160, Brazil
2School of Medicine, Pontificia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS 90450130, Brazil
3Instituto Oncoclinicas, Rio de Janeiro, RJ 22440040, Brazil
4Breast Unit, Hospital Israelita Albert Einstein, São Paulo, SP 01321001, Brazil
5Oncology Research Group-CNPq, PUCRS, Porto Alegre, RS 90610000, Brazil
6Breast Center, Instituto Orizonti and Hospital Mater Dei, Belo Horizonte, Minas Gerais, MG 30210080, Brazil
7School of Medicine, Federal University of Paraná, Curitiba, PR 80060900, Brazil
8Breast Unit, Real Hospital Português, Recife, PE 52010075, Brazil
9Breast Unit, Pace Hospital, Belo Horizonte, MG 30110062, Brazil
10Breast Unit, Larmony Mastologia, Florianópolis, SC 88015300, Brazil
11Breast Unit, Samaritano Botafogo (Américas Serviços Médicos), Rio de Janeiro, RJ 22270010, Brazil
12School of Medical Sciences, Santa Casa de São Paulo, São Paulo, SP 01224001, Brazil
13Breast Unit, Santa Casa de São Paulo, São Paulo, SP 01221010, Brazil
14CORA Advanced Centre for Diagnosis of Breast Cancer, Department of Obstetrics and Gynaecology, Federal University of Goiás, Goiânia, GO 74605050, Brazil
a https://orcid.org/0000-0002-7156-2890
b https://orcid.org/0000-0002-1643-727X
c https://orcid.org/0000-0002-2113-6324
d https://orcid.org/0000-0003-2983-3199
e https://orcid.org/0000-0002-6215-7076
f https://orcid.org/0000-0002-7754-9793
g https://orcid.org/0000-0003-4136-7047
h https://orcid.org/0000-0001-9014-4793
i https://orcid.org/0000-0003-1888-4433
j https://orcid.org/0000-0003-1068-4154
k https://orcid.org/0000-0003-0910-0270
l https://orcid.org/0000-0002-9478-5616
m https://orcid.org/0000-0003-4145-8598
Abstract
Introduction: Despite the lack of randomised evidence, there is a current trend towards omitting axillary surgery in cases of positive sentinel lymph node (SLN) following neoadjuvant chemotherapy (NACT). This study evaluated practice patterns of Brazilian breast surgeons when managing positive SLN following NACT.
Methods: This was a nationwide electronic survey of breast surgeons affiliated with the Brazilian Society of Mastology. Management approaches for positive SLN after NACT (axillary dissection (AD), regional nodal irradiation (RNI) or no additional treatment) were evaluated as a function of residual disease volume in the SLN (macro-metastasis, micro-metastasis or isolated tumour cells (ITC)).
Results: Survey response rate was 49%, with 799/1,627 questionnaires returned. Most respondents were <50 years old (61%), lived in south-eastern Brazil (50%), in a major city (67%), worked in an academic institute (80%) and were board-certified (80%). AD recommendation rate decreased according to residual nodal disease volume: 91% of respondents recommended AD for cases of macro-metastasis, 64% for micro-metastasis and 38% for ITC (p < 0.00001). Furthermore, 35% would recommend no additional surgery for micro-metastasis, while 27% would recommend no treatment at all for ITC (p < 0.00001). Not working in an academic institute was associated with RNI for micro-metastasis (p = 0.02), but not for macro-metastasis or ITC. Being board-certified did not affect axillary management.
Conclusion: Most respondents would recommend AD and/or RNI in residual nodal disease following NACT irrespective of disease volume. Nevertheless, a trend towards surgical de-escalation was found with low-volume disease (micro-metastasis and ITC). Ongoing randomised trials will clarify the impact of this trend.
Keywords: breast cancer, neoadjuvant chemotherapy, residual nodal disease, sentinel lymph node biopsy, axillary dissection, regional nodal irradiation
Correspondence to: Francisco Pimentel Cavalcante
Email: fpimentelcavalcante@gmail.com
Published: 18/02/2021
Received: 24/09/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sentinel lymph node (SLN) biopsy is the approach of choice in axillary surgery for patients with early breast cancer and clinically negative axilla [1, 2]. The technique provides excellent regional control compared to axillary dissection (AD) and a lower rate of lymphedema. Currently, AD has also been safely omitted during upfront surgery in cases in which there is only limited disease in the SLN [3–6]. The possibility of reducing the extent of axillary surgery has been evaluated in different situations in order to decrease rates of surgical morbidity while still guaranteeing accurate staging information, which is crucial for the planning of adjuvant treatment [7–9].
Systemic neoadjuvant chemotherapy (NACT), traditionally used in locally advanced breast cancer to facilitate breast-conserving surgery, has now been recommended to enable downstaging in clinically positive axillae. This strategy has shown acceptable false-negative rates comparable to upfront surgery with the resection of three or more negative SLN or the removal of the previously clipped node [7–11]. Nevertheless, omitting AD of positive SLN following NACT remains a much-debated issue.
An ongoing randomised clinical trial sponsored by the Alliance for Clinical Trials in Oncology [12] is evaluating the possibility of substituting AD for axillary radiation in patients with positive SLN following NACT. The concept that low-volume disease in the SLN can respond well to the local treatment either with radiotherapy or AD without involving any significant difference in overall survival (OS) was well documented in the AMAROS (After Mapping of the Axila: Radiotherapy or Surgery) trial [5]. However, randomised clinical trial data are still expected to clarify whether this concept is also valid in cases of residual low-volume disease in the SLN after NACT. The debate remains open on whether this state of disease could lead to a poorer prognosis and an increased likelihood of local recurrence, ultimately requiring AD. Until the results of the currently ongoing randomised clinical trial on this subject are available, the relevant breast societies still recommend AD if residual axillary disease after NACT is detected [12–14].
Regardless of these recommendations following NACT, in recent years, there has been a growing trend towards omitting additional axillary surgery despite the lack of evidence from randomised clinical trials focusing on this particular clinical endpoint. In routine clinical practice, breast surgeons are sometimes confronted with different situations such as when SLN biopsy is negative on frozen section but found to be positive/metastatic on paraffin section, with the surgeon then making the decision not to return the patient to the operating room to re-operate the axilla [15–18]. Indeed, although guidelines recommended AD if there is any residual disease after NACT, in practice, if residual disease is not detected at the time of frozen section biopsy but only in the definitive result, regional nodal irradiation (RNI) without AD can be considered by the multidisciplinary team [14]. Therefore, specific surveys on this subject are of the utmost importance in understanding what occurs in real world practice.
The principal objective of this study was to evaluate current trends among breast surgeons affiliated with the Brazilian Society of Mastology (SBM) with respect to their approach to axillary surgery following NACT with a positive SLN, particularly based on nodal disease volume.
Materials and methods
This is the second part of a nationwide electronic survey conducted between 25 June and 24 August 2020 with 1,627 breast surgeons affiliated with the SBM. While the first part of this survey referred to the management of positive SLN in upfront surgery (results in press), the current analysis refers to the management of the axilla in cases of positive SLN after NACT, in the presence of macro-metastasis, micro-metastasis or isolated tumour cells (ITC) in the SLN.
The SBM membership criteria require medical residency and/or board-certification in breast diseases. In Brazil, there is a specific medical residency program aimed at training specialists in breast surgery. Most of the SBM members work primarily on the treatment of breast cancer and most are board-certified by the Brazilian Medical Association. In this study, all the participants were specialists in breast cancer surgery and members of the SBM, although in some cases their specialist diploma had not been board-certified by the SBM itself.
The SBM provided data on the age and sex of its members, the region in which they worked, and whether or not they were board-certified. This allowed those who completed and returned the questionnaire to be compared with the group of non-respondents. The SBM adopts the international guideline recommendations for the technique of SLN biopsy. However, since most of the oncology institutes in the country do not have access to nuclear medicine services, blue dye alone tends to be used as a lymph node marker for the majority of patients [16, 17].
The survey contained questions aimed at obtaining participants’ demographic data: age, sex, the region in which they worked (southeast, northeast, south, north or mid-west of the country), whether they were board-certified in breast surgery and whether or not they worked in an academic institute. For each category of disease volume in the SLN (macro-metastasis, micro-metastasis or ITC), there were three possible answers: AD, RNI or no additional treatment. According to the Tumor size, Lymph Nodes, Metastasis (TNM) classification of malignant tumours, macro-metastasis was defined as >2 mm in the SLN, micro-metastasis as ≤2 and >0.2 mm, and ITC as ≤0.2 mm. The survey questionnaire is shown in Figure 1.
The internal review board of the SBM approved the study protocol and waived the requirement for informed consent since the questionnaires were to be answered anonymously. The data were analysed using Statistical Package for the Social Sciences, version 26.0. The demographic profile of the study sample and the management approach used by the respondents were evaluated using measures of absolute (n) and relative frequency (%). Relative frequencies were analysed according to the number of participants answering each individual question. The association between demographic characteristics and the management approach adopted was analysed using contingency tables and Pearson’s post-hoc chi-square test. Significance was set at 5% (p < 0.05).
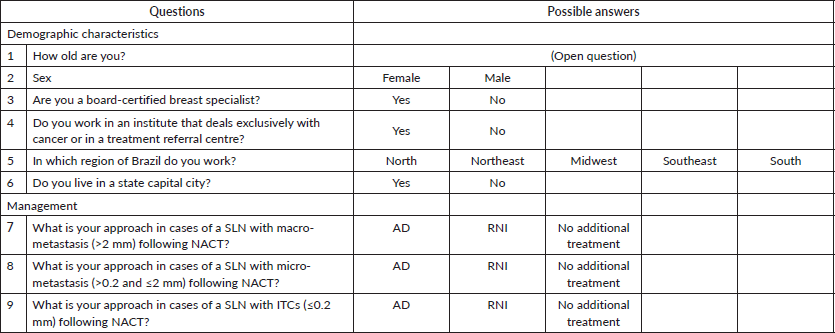
Figure 1. Box showing the survey questionnaire and the options given as possible answers.
Results
Of the 1,627 questionnaires sent out to SBM members, 799 were completed and returned, resulting in a response rate of 49%. Of the respondents, 61% were under 50 years of age, 49% were female, and 80% were board-certified breast surgeons. In relation to their place of work, 80% worked in an academic institute or in an institute working exclusively with breast cancer. Most (67%) lived in a state capital city and 50% lived in the southeast of the country compared to 21% in the northeast, 14% in the south, 9% in the mid-west and 3% in the north of Brazil (Table 1). The group of surgeons who answered the questionnaire was similar to the group of non-respondents in terms of sex (p = 0.13), the region of the country in which they lived/worked (p = 0.99) and board-certification (p = 13). The only significant difference between the two groups refers to a predominance of non-respondents in the subgroup of individuals aged ≥70 years (p < 0.01) (Table 2).
For the purposes of describing axillary management when there is residual disease following NACT, three different possibilities of residual disease are considered here: macro-metastasis, micro-metastasis and isolated tumor cells, as already mentioned in the methods section. There are also three different options of possible axillary management: AD, RNI and no treatment.
If macro-metastasis were present in the SLN following NACT, 91% (n = 732) of respondents would recommend AD compared to 6% who would recommend RNI alone and 2% who would recommend no additional axillary treatment. In the case of low-volume disease in the SLN, however, a significant change was seen: in cases of micro-metastasis, 35% of surgeons would not recommend any additional surgery (p < 0.00001), with 26.5% recommending RNI and 8.6% no further additional local treatment in the axilla. The same was also found with ITC, with 61% of respondents not recommending AD (only 38% would recommend AD, while 34% would recommend RNI and 27% would recommend no further treatment) (Tables 3 and 4).
Table 1. Demographic characteristics of the respondents.
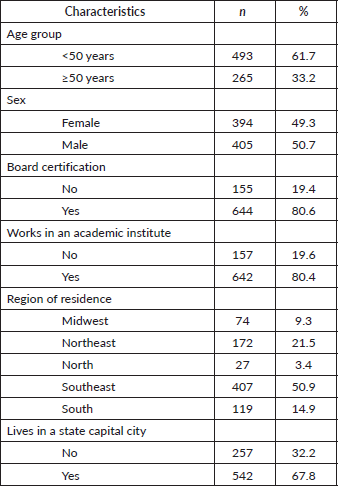
Table 2. Demographic characteristics of respondents versus non-respondents.
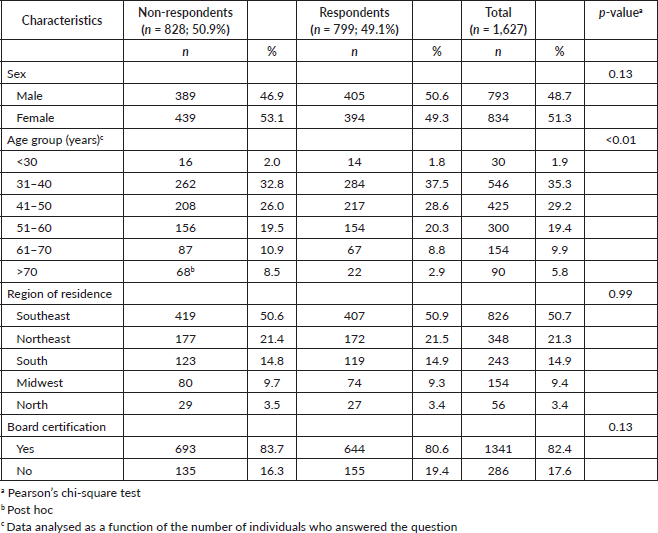
Table 3. Management of the positive SLN following NACT according to nodal disease volume.

Table 4. Surgical versus non-surgical management of positive SLN following NACT according to nodal disease volume.
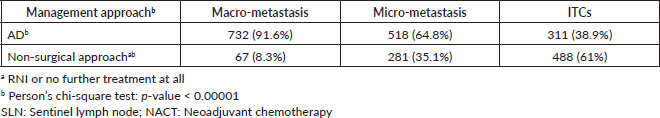
Table 5. Management of the positive SLN after NACT according to place of work and board-certification.
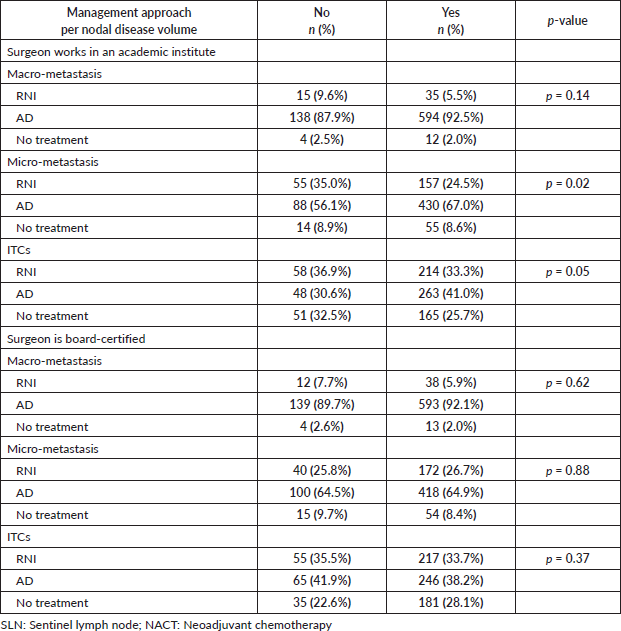
The extent of the treatment recommended decreased as a function of the volume of the disease. In cases in which ITC are present, 61% of respondents would recommend no surgical treatment compared to 35% if micro-metastasis is present and only 8% if there is macro-metastasis. For the purpose of analysis, RNI and no treatment have been grouped together as ‘no further surgical treatment’ (Table 4).
In the present study, not working in an academic institute was associated with recommending RNI in cases of micro-metastasis (p = 0.02); however, there was no such effect in cases of macro-metastasis (p = 0.14) or ITC (p = 0.05). On the other hand, whether the surgeon was board-certified had no effect on answers, irrespective of the volume of disease in the SLN (Table 5).
Discussion
Most Brazilian breast surgeons participating in this survey would recommend AD in cases of residual disease in the SLN following NACT, particularly when involving macro-metastasis. A recent survey conducted with North American breast cancer providers on the extrapolation of axillary management to situations not covered by the Z0011 criteria, including patients treated with systemic NACT, found that 85% of respondents believed that more data would have to be available for clinical practice to change [18]. On the other hand, increased interest in omitting AD in such cases has been noted. One study, conducted using data from the National Cancer Database (NCDB), evaluated the trend for axillary surgery following NACT in patients with clinically node positive disease prior to and following publication of the Z0011 study. The rate of SLN biopsy alone increased from 25.6% in 2006 to 33.2%
in 2012 in patients submitted to breast-conserving surgery (p < 0.01). That finding indicates that the results of the Z0011 in upfront surgery are being extrapolated to the situation of neoadjuvant treatment [15].
The Z0011 study confirmed the safety of omitting AD in patients with one or two positive SLNs in upfront surgery; however, that study excluded patients submitted to NACT [3]. There are no prospective, randomised studies on the oncologic safety of omitting AD in cases of residual disease in the SLN following NACT. A small retrospective study involving a short follow-up time in 161 patients with positive SLN after NACT detected no difference in regional control at 3 years, irrespective of the extent of axillary surgery; however, neither control of the disease nor survival was predicted [19]. The question remains whether AD would have an impact on the control of the disease or whether RNI could substitute it. A study conducted using data from the NCDB compared patients with positive SLN following NACT submitted to SLN dissection (removal of ≤4 lymph nodes) and RNI (n = 304) to a group submitted to AD and RNI (n = 1,313), following a design that was similar to that used in the ongoing ALLIANCE A011202 randomised, phase III, clinical trial [20]. Survival was poorer in the cases in which AD was omitted (HR = 1.7; 95% CI: 1.3–2.2; p < 0.001), with a 5-year survival rate of 71% compared to 77% in the AD group (p = 0.01). In that same study, an exploratory analysis showed SLN dissection to be comparable to AD in patients with hormone-positive tumours and with metastasis in a single lymph node, a finding more compatible with the results of the Z11 in upfront surgery. A retrospective study on patients receiving NACT between 2008 and 2013 in a single Korean institute involved only patients with one or two positive SLN following NACT who were followed up for a mean of 71 months. SLN biopsy alone (n = 98) was compared to AD (n = 98), with results showing no difference in OS (92.1% versus 91.1%; p = 0.809) [21]. Nevertheless, those studies could have involved biases with respect to lower-risk patients being selected for SLN biopsy alone, while recommending AD for those with greater residual nodal burden, thus affecting the results.
The presence of low-volume disease in the lymph node following NACT has also been a topic of debate. In the present analysis, 91% of the surgeons would recommend AD in cases of macro-metastasis; however, a trend was seen towards de-escalation with respect to axillary surgery in cases of micro-metastasis and ITC, with 35% and 61% of surgeons, respectively, not recommending any additional surgery. These findings could possibly reflect a belief that in cases of micro-metastasis and ITC following NACT the oncological outcome would be similar to that found in pathologically negative cases, as occurs in upfront surgery [19]. Historically, the presence of low-volume residual disease in the lymph node following NACT has generated controversy with respect to disease-free survival and OS. A retrospective review evaluated the burden of residual disease in positive SLN following NACT and showed a high additional tumour burden, irrespective of whether it consisted of micro-metastasis (59%) or macro-metastasis (63%), as a possible indicator for AD [22]. In another study conducted using the databases from the Dana-Farber/Brigham and Women’s Cancer Center and the NCDB, the presence of residual ITC and micro-metastasis was associated with poorer disease-free survival when compared to the absence of disease in the lymph node. That study concluded that following NACT, a low volume of residual disease implied a poorer prognosis compared to negative nodes, particularly in patients with triple-negative and HER2 tumours [23]. In fact, the burden of residual disease in the axilla following NACT and a positive SLN appears to be high, irrespective of tumour subtype [24]. On the other hand, a study of cancer records in the Netherlands involving 1,347 patients with an initially positive lymph node treated with NACT and submitted to AD showed comparable disease-free and OS rates in ypN0 and ypNitc/mic patients, but significantly different rates in ypN0 and ypN1-3 patients, with this latter group being associated with a less favourable prognosis [25]. Another study conducted with 98 patients submitted to AD following NACT and with residual disease in the SLN reported similar results in the group with micro-metastasis and in the group with negative SLN, with these groups having a more favourable outcome when compared to the cases involving macro-metastasis [26]. Again, the question is whether AD can be omitted in such cases. During the 2021 St. Gallen International Breast Cancer Consensus Conference, the topic of axillary surgery following NACT and residual disease in the SLN generated much debate. According to 73% of the panellists, AD should be recommended whenever macro-metastasis is identified; however, 60% opposed AD when micro-metastasis was present and 89% opposed any additional axillary surgery when the only finding in the SLN was ITC [27]. These results show a tendency towards de-escalation in axillary surgery, particularly in cases of low-volume disease in the SLN following NACT; however, the question of micro-metastasis was fiercely debated, showing that, indeed, the lack of randomised clinical trials on this issue still leaves many surgeons uncomfortable at omitting AD.
There are some limitations associated with the present study. Since it consists of a survey, it is impossible to guarantee that these results would be applicable in real life. The response rate of 49% is another limitation; however, there was no statistically significant difference between the group of surgeons who completed and returned the questionnaire and those who did not. The fact that no question was included in the survey on how to manage a positive SLN after neoadjuvant endocrine therapy (NET) may represent a further limitation. In our opinion, however, these are different circumstances compared to NACT, particularly in initially clinically node-negative (cN0) cases. In general, pathologic complete response following NET is not expected and the prognosis is therefore different [28]. A study conducted with data from the NCDB and from a single institute evaluated the burden of residual disease following NET and the type of axillary surgery performed: SLN biopsy or AD. More than 90% of the patients in both cohorts had cN0 axilla at the initial presentation and had fewer than three positive lymph nodes at final pathology, with no difference in OS irrespective of the type of axillary surgery [29].
This finding suggests that patients who are initially cN0 and SLN-positive following NET could be similar in profile to those of the Z0011 study; hence, AD could be omitted.
Conclusions
The present results suggest that the majority of respondents would recommend AD or RNI when residual disease is identified in the SLN following NACT irrespective of the volume of residual disease in the lymph node. There was, however, a trend towards de-escalation of surgery in cases of low-volume disease in the SLN. Further studies are required to increase understanding of this type of case, and the results of randomised clinical trials on clinical outcomes will be crucial in optimising axillary surgery. While randomised evidence is awaited, surveys, real world data and survival analyses in cohort studies may contribute to improving understanding of this trend towards extrapolating conservative management in axillary surgery from upfront surgery studies to this scenario after NACT.
Acknowledgments
The authors are grateful to all the affiliated members of the SBM who participated in this study. We also thank Fernanda Pereira Alves and Jéssica Telles Bonavita for their administrative work in providing the relevant demographic data on the SBM members.
Abbreviations
AD Axillary dissection
ITC Isolated tumour cells
NACT Neoadjuvant chemotherapy
NET Neoadjuvant endocrine therapy
RNI Regional nodal irradiation
SBM Brazilian Society of Mastology
SLN Sentinel lymph node
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
None.
Authors’ contributions
Francisco Pimentel Cavalcante, Felipe Zerwes, Eduardo Camargo Millen and Ruffo Freitas-Junior: conceptualisation, methodology. Francisco Pimentel Cavalcante, Felipe Zerwes, Eduardo Camargo Millen, Guilherme Novita, João Henrique Penna Reis, Alessandra
Borba Anton de Souza and Ruffo Freitas-Junior: formal analysis, investigation. All authors: data curation, writing - Original draft preparation, writing - reviewing and editing.
References
1. Giuliano AE, Haigh PI, and Brennan MB, et al (2000) Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node- negative breast cancer J Clin Oncol 18 2553–2559 https://doi.org/10.1200/JCO.2000.18.13.2553 PMID: 10893286
2. Krag DN, Anderson SJ, and Julian TB, et al (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial Lancet Oncol 8 881–888 https://doi.org/10.1016/S1470-2045(07)70278-4 PMID: 17851130
3. Giuliano AE, Ballman KV, and McCall L, et al (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial JAMA 318 918–926 https://doi.org/10.1001/jama.2017.11470 PMID: 28898379 PMCID: 5672806
4. Galimberti V, Cole BF, and Zurrida S, et al (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial Lancet Oncol 14 297–305 https://doi.org/10.1016/S1470-2045(13)70035-4 PMID: 23491275 PMCID: 3935346
5. Donker M, van Tienhoven G, and Straver ME, et al (2014) Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial Lancet Oncol 15 1303–1310 https://doi.org/10.1016/S1470-2045(14)70460-7 PMID: 25439688 PMCID: 4291166
6. Sávolt Á, Péley G, and Polgár C, et al (2017) Eight-year follow up result of the OTOASOR trial: the optimal treatment of the axilla – surgery or radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial Eur J Surg Oncol 43 672–679 https://doi.org/10.1016/j.ejso.2016.12.011
7. Boughey JC, Suman VJ, and Mittendorf EA, et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial JAMA 310 1455–1461 https://doi.org/10.1001/jama.2013.278932 PMID: 24101169 PMCID: 4075763
8. Kuehn T, Bauerfeind I, and Fehm T, et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study Lancet Oncol 14 609–6018 https://doi.org/10.1016/S1470-2045(13)70166-9 PMID: 23683750
9. Boileau JF, Poirier B, and Basik M, et al (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study J Clin Oncol 33 258–264 https://doi.org/10.1200/JCO.2014.55.7827
10. Kahler-Ribeiro-Fontana S, Pagan E, and Magnoni F, et al (2021) Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up Eur J Surg Oncol 47 804–812 https://doi.org/10.1016/j.ejso.2020.10.014
11. Enokido K, Watanabe C, and Nakamura S, et al (2016) Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer Clin Breast Cancer 16 299–304 https://doi.org/10.1016/j.clbc.2016.02.009 PMID: 26993216
12. Boughey J and Alliance for Clinical Trials on Oncology (2021) Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy [https://clinicaltrials.gov/ct2/show/NCT01901094]
13. Mamounas EP, Bandos H, and White JR, et al (2019) NRG oncology/NSABP B-51/RTOG 1304: phase III trial to determine if chest wall and regional nodal radiotherapy (CWRNRT) post mastectomy (Mx) or the addition of RNRT to whole breast RT post breast-conserving surgery (BCS) reduces invasive breast cancer recurrence-free interval (IBCR-FI) in patients (pts) with pathologically positive axillary (PPAx) nodes who are ypN0 after neoadjuvant chemotherapy (NC) J Clin Oncol 37(Suppl. 15) TPS600 https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS600
14. Brackstone M, Baldassarre FG, and Perera FE, et al (2021) Management of the axilla in early-stage breast cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline J Clin Oncol 39 3056–3082 https://doi.org/10.1200/JCO.21.00934 PMID: 34279999
15. Kantor O, Pesce C, and Liederbach E, et al (2017) Are the ACOSOG Z0011 trial findings being applied to breast cancer patients undergoing neoadjuvant chemotherapy? Breast J 23 554–562 https://doi.org/10.1111/tbj.12793 PMID: 28295828
16. Buzaid AC, Achatz MI, and Amorim GLS, et al (2020) Challenges in the journey of breast cancer patients in Brazil Braz J Oncol 16 1–10
17. Resende HM, Lichtenfels M, and Soares IC, et al (2021) Sentinel lymph node biopsy using single-agent mapping tracer (blue dye) after neoadjuvant chemotherapy in a Brazilian cohort of breast cancer patients. Real world evidence Acta Cir Bras 36 e360608 https://doi.org/10.1590/acb360608 PMID: 34231654 PMCID: 8253600
18. Weiss A, Cooley V, and Al-Hilli Z, et al (2021) Extrapolation of ACOSOG Z0011 trial results – a survey of breast cancer providers Breast J 27 537–542 https://doi.org/10.1111/tbj.14226 PMID: 33720478
19. Ling DC, Iarrobino NA, and Champ CE, et al (2019) Regional recurrence rates with or without complete axillary dissection for breast cancer patients with node-positive disease on sentinel lymph node biopsy after neoadjuvant chemotherapy Adv Radiat Oncol 5 163–170 https://doi.org/10.1016/j.adro.2019.09.006
20. Almahariq MF, Levitin R, and Quinn TJ, et al (2021) Omission of axillary lymph node dissection is associated with inferior survival in breast cancer patients with residual N1 nodal disease following neoadjuvant chemotherapy Ann Surg Oncol 28 930–940 https://doi.org/10.1245/s10434-020-08928-2
21. Chun JW, Kim J, and Chung IY, et al (2021) Sentinel node biopsy alone for breast cancer patients with residual nodal disease after neoadjuvant chemotherapy Sci Rep 11 9056 https://doi.org/10.1038/s41598-021-88442-x PMID: 33907236 PMCID: 8079673
22. Moo TA, Edelweiss M, and Hajiyeva S, et al (2018) Is low-volume disease in the sentinel node after neoadjuvant chemotherapy an indication for axillary dissection? Ann Surg Oncol 25 1488–1494 https://doi.org/10.1245/s10434-018-6429-2 PMID: 29572705 PMCID: 5930130
23. Wong SM, Almana N, and Choi J, et al (2019) Prognostic significance of residual axillary nodal micrometastases and isolated tumor cells after neoadjuvant chemotherapy for breast cancer Ann Surg Oncol 26 3502–3509 https://doi.org/10.1245/s10434-019-07517-2 PMID: 31228134
24. Moo TA, Pawloski KR, and Flynn J, et al (2021) Is residual nodal disease at axillary dissection associated with tumor subtype in patients with low volume sentinel node metastasis after neoadjuvant chemotherapy? Ann Surg Oncol https://doi.org/10.1245/s10434-021-09910-2
25. van Nijnatten TJ, Simons JM, and Moossdorff M, et al (2017) Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: isolated tumor cells and micrometastases carry a better prognosis than macrometastases Breast Cancer Res Treat 163 159–166 https://doi.org/10.1007/s10549-017-4157-0 PMID: 28213782 PMCID: 5387009
26. Canavese G, Tinterri C, and Carli F, et al (2021) Correlation between outcome and extent of residual disease in the sentinel node after neoadjuvant chemotherapy in clinically fine-needle proven node-positive breast cancer patients Eur J Surg Oncol 47 1920–1917 https://doi.org/10.1016/j.ejso.2021.04.039 PMID: 33972144
27. Thomssen C, Balic M, and Harbeck N, et al (2021) St. Gallen/Vienna 2021: a brief summary of the consensus discussion on customizing therapies for women with early breast cancer Breast Care 16 135–143 https://doi.org/10.1159/000516114 PMID: 34002112 PMCID: 8089428
28. Hammond JB, Parnall TH, and Scott DW, et al (2020) Gauging the efficacy of neoadjuvant endocrine therapy in breast cancer patients with known axillary disease J Surg Oncol 122 619–622 https://doi.org/10.1002/jso.26047
29. Kantor O, Wakeman M, and Weiss A, et al (2021) Axillary management after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer Ann Surg Oncol 28 1358–1367 https://doi.org/10.1245/s10434-020-09073-6






