Biomarkers for precision immunotherapy in the metastatic setting: hope or reality?
Elham Sajjadi1,2, Konstantinos Venetis1,2, Cristian Scatena3 and Nicola Fusco1,2
1Divison of Pathology, European Institute of Oncology (IEO) IRCCS, University of Milan, Via Giuseppe Ripamonti 435, 20141 Milan, Italy
2Department of Oncology and Hemato-Oncology, University of Milan, Via Festa del Perdono 7, 20122 Milan, Italy
3Division of Pathology, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Via Roma 57, 56126 Pisa, Italy
Abstract
Precision immunotherapy is a crucial approach to improve the efficacy of anti-cancer treatments, particularly in the metastatic setting. In this respect, accurate patient selection takes advantage of the multidimensional integration of patients’ clinical information and tumour-specific biomarkers status. Among these biomarkers, programmed death-ligand 1, tumour-infiltrating lymphocytes, microsatellite instability, mismatch repair and tumour mutational burden have been widely investigated. However, novel tumour-specific biomarkers and testing methods will further improve patients’ outcomes. Here, we discuss the currently available strategies for the implementation of a precision immunotherapy approach in the clinical management of metastatic solid tumours and highlight future perspectives.
Keywords: biomarkers, immunotherapy, PD-L1, TILs, mismatch repair, TMB
Correspondence to: Nicola Fusco
Email: nicola.fusco@unimi.it
Published: 03/12/2020
Received: 30/06/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Immune checkpoint inhibition has been increasingly applied in several solid tumours, with significant survival benefits, providing a precise patient selection [1–3]. Hence, not all the patients, even in the presence of similar clinical characteristics, would respond in the same way to the same immunotherapy protocol [4]. Furthermore, the toxicity and adverse events of such agents are not uncommon and should be taken into account while assessing the patient’s eligibility [5, 6]. In this scenario, the application of tailored immunotherapy schemes is of great importance.
In this era of histology-agnostic approvals, the identification of tumour-specific biomarkers and interpretation guidelines is a growing opportunity [7, 8]. Currently, the most studied immune-related biomarkers include programmed death-ligand 1 (PD-L1), tumour-infiltrating lymphocytes (TILs), microsatellite instability (MSI), mismatch repair (MMR) and tumour mutational burden (TMB) [9]. The level of approval of these tests is shown in Figure 1. There are currently multiple lines of evidence on the overall better response rate of TMB-high, MSI-high and PD-L1POS tumours treated with immunotherapy [10, 11]. Additionally, there are several indications that candidate complementary and/ or surrogate biomarkers (e.g. phosphatase and tensin homologue ) may contribute to an optimal patient selection [12–16]. Novel means of mutation measurement as comprehensive genomic profiling (CGP) are currently being explored in this setting [17].
Tumour-specific biomarkers, coupled with companion diagnostics (CDx), may enhance the process of precise patients’ selection, leading to a higher probability of reaching satisfying clinical outcomes [18]. In this review article, we illustrate the impacts and gaps of biomarkers suggested by previous clinical trials and translational research studies in immuno-oncology treatments. A particular focus will be given on the hopes and facts behind the concept of ‘precision immunotherapy’.
Immunotherapy in clinical practice
Cancer cells can evade the immune system through downregulation or loss of tumour antigens and alterations in the expression of costimulatory and coinhibitory molecules [19, 20]. Under normal conditions, antigens conjugated with major histocompatibility complex (MHC) molecules are presented on the surface of cancer cells. These antigens can be recognised by T cells possessing the same MHC alleles through T-cell receptors–antigen/MHC interactions [21]. For an optimal T cell response, a second signal mediated by co-stimulatory molecules is required. CD28 binds to CD80 and/or CD86, which are present on the surface of activated antigen-presenting cells [22]. Cytotoxic T-lymphocyte antigen 4 (CTLA4) is homologous to CD28 and similarly binds to CD80 or CD86, preventing the attachment of CD28 to these surface proteins. In other words, CTLA4 is a negative regulatory molecule of T cell activation [22, 23]. The pharmacologic inhibition of CTLA4 is one of the possible approaches employed in cancer immunotherapy [22]. The checkpoint axis programmed cell death protein 1 (PD-1)/PD-L1 is another widely explored target [24]. When PD-1 binds to its ligands named as PD-L1 and PD-L2, T cells undergo a negative regulatory process referred to as immune checkpoint [25]. Antibodies that block PD-1 or PD-L1 lead to activation of T cells which can subsequently recognise and attack cancer cells [26]. The therapeutic antibody ipilimumab, targeting CTLA-4, is the first approved checkpoint inhibitor for clinical use in melanoma [27]. Additionally, anti-PD-1 molecules for the management of malignancies such as non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), Hodgkin lymphoma, melanoma, urothelial carcinoma, metastatic colorectal cancer and hepatocellular carcinoma are Food and Drugs Administration (FDA)-consented to be prescribed [28], as summarised in Table 1. Clinical use of immune checkpoint inhibitors (ICPis) may bring along undesired side effects termed as immune-related adverse events (irAEs) [29]. Reportedly, anti-CTLA-4 therapy often results in more severe side effects comparing to other immunotherapy agents [30]. Organs such as intestine, liver, lung, skin and endocrine glands are frequently affected by immunotherapy toxicity [31]. Around 13%–17% of NSCLC patients treated with anti-PD-1 experienced grade 3 or higher toxicities [32]. Yet, less than 20% of patients show high-grade toxicity when treated with anti-PD-1 and/or anti-PD-L1 [33]. Most of the side effects are tackled by corticosteroids and other adjunctive medications effectively [34].

Figure 1. Schematic representation of the main fields of applications of MMR/MSI, PD-L1, TMB testing in patients’ selection for immunotherapy. Tumours are depicted in the columns, while the application of the test in the rows. The colour-coded circles refer to the selected testing method provided on the bottom left legend. The circles are distributed among different anatomical sites based on their clinical utility, as reported on the column placed on the left. MMR, mismatch repair; MSI, microsatellite instability; PD-L1, programmed cell death ligand 1; TMB, tumour mutational burden; IHC, immunohistochemistry; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma.
Table 1. Summary of immune checkpoint blockade therapies which have been approved by the FDA for being applied in clinical practices. https://www.fda.gov/

The quantum leap of immune-related biomarkers
Programmed death ligand 1 (PD-L1)
In 2015, FDA approved pembrolizumab as the first PD-1 inhibitor in NSCLC [35]. Since then, different clones of the antibody against PD-1 ligand, such as SP142 (Ventana Medical Systems), SP263 (Ventana Medical Systems) and 22C3 (Dako North America, Inc.) were validated as specific biomarkers for patient selection [36]. Immunohistochemistry (IHC) assessment of PD-L1 is employed for patient selection in several cancers [37]. PD-L1 evaluation differs in each tumour type, thus a conclusive protocol may not fit all malignancies. For instance, tumour proportion score (TPS) which is functional in lung cancer cannot be tailored for head and neck cancer, and vice versa for the combined positive score (CPS) [38]. TPS considers PD-L1-positivity merely in neoplastic cells, whereas CPS considers the positivity of tumour cells, lymphocytes and macrophages. CPS equals the number of PD-L1 positive tumour cells and lymphocytes, divided by the total number of viable tumour cells, multiplied by 100. Another example is represented by triple-negative breast cancers (TNBC), where the CDx test for this indication was PD-L1 (SP142) IHC Assay by using the immune cell (IC) scoring system [39]. IC scoring was considered as positive, for those with the presence of PD-L1POS ICs that covered more than 1% of the tumour area (tumour cells, associated intratumoural and contiguous peritumoural stroma) [2]. The PD-L1 scoring systems are shown in Figure 2. Pre-analytical and informative phases of PD-L1 testing have been coordinated in NSCLC where the propagative application of PD-L1 testing in clinical practices indicated coinciding results, mostly by using the 22C3 antibody clone [40]. PD-L1 plays a significant role in the NSCLC treatment profile. In this malignancy, PD-L1 expression is assessed by TPS of membrane expression [41]. Based on KEYNOTE‑042 (NCT02220894), pembrolizumab is approved as the first-line treatment of stage III NSCLC patients with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic aberrations, while also the tumour must express PD-L1 (TPS ≥1%) [42].
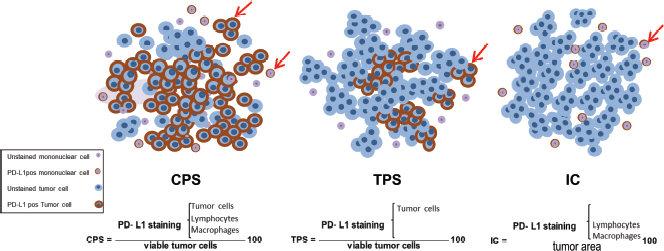
Figure 2. Schematic representation of the available scoring criteria for PD-L1 assessment. CPS counts for both tumour and mononuclear cells which are PD-L1pos among total viable tumour cells, multiplied by 100. While TPS and IC are contributed to PD-L1pos, tumour cells and mononuclear cells, respectively, divided by total number of viable tumour cells. PD-L1 stained and unstained tumour and mononuclear cells are depicted on the left bottom legend. PD-L1, programmed cell death ligand 1, CPS, combined positive score; TPS, tumour proportion score; IC, immune cell.
PD-L1 assessment is debated among scientists. In head and neck squamous cell carcinoma (HNSCC), irrespective of PD-L1 expression status, immunotherapy with nivolumab and pembrolizumab is consented by FDA for the second-line treatment of recurrent and/or metastatic HNSCC [43]. These agents show a greater overall survival (OS) in comparison with the standard, single-agent treatment in those with platinum-refractory, recurrent or metastatic HNSCC [44]. According to Ferris et al individuals treated with nivolumab, regardless of tumour PD-L1 expression, appeared to have greater OS than those treated with standard therapy. However, they noted that patients with a tumour PD-L1 expression level of more than 1% may benefit more from nivolumab therapy than those whose PD-L1 level was less than 1% [45].
In HNSCC, CPS is recommended for PD-L1 evaluation, where CPS > 20 represents a significantly longer OS [46]. In melanoma, tumours with PD-L1-overexpression are related to a fairly high response rate (>50%) and longer progression-free survival (PFS) and OS [47]. In PD-L1 positive, advanced and refractory gastric cancer (GC), those treated with pembrolizumab presented a greater objective response rate (ORR). Added to this, PD-L1 negative cases had also shown responses [48]. Regarding RCC, a meta-analysis comprising 4,063 patients suggested a greater OS and PFS in PD-L1 positive tumours [49]. In urothelial carcinoma, patients with high PD-L1 expression had a greater ORR and OS rate [50]. In TNBC, studies indicate that PD-L1 is highly expressed which suggests a potential role for immunotherapy [51]. Pembrolizumab implies durable anti-cancer effects in a small subset of PD-L1 positive metastatic TNBC [52].
Tumour-infiltrating lymphocytes (TILs)
Leukocytes are thought to be involved in both protumour and antitumour activities [53–55]. Molecular factors formed by ICs may lead to cancer cells’ fate of death or survival [4]. Lymphocytes migrated within tumour stroma or the tumour itself are termed as TILs [56]. In 2014, the International TILs Working Group (ITWG) suggested a standardised methodology for evaluating TILs with detailed information and instruction with step to step tutorial in breast cancer setting [57]. Later on, in 2017, other solid tumours were also included in the ITWG study framework [58] along with other studies confirmations or updates [59, 60]. Accordingly, TILs assessment is performed on haematoxylin and eosin slides by considering both the stromal and the intra-tumour cell compartments [61]. Stromal TILs (sTILs) refer to the area occupied by mononuclear inflammatory cells over the total stromal area, while intra-tumoural TILs (iTILs) are related to the tumour cell area [62]. sTILs and iTILs ought to be reported separately to avoid the effect of tumour cell density and growth pattern on the TIL count. Another reason for reporting individually is that in many tumours the density of TIL is different in both compartments [57]. After defining stromal and intra-tumoural areas with low magnification, the type of inflammatory infiltrates is supposed to be determined [61]. Based on the tumour type, either TILs subtypes or one of them needs to be evaluated. For example, in breast cancer, only sTILs provide valuable information [57]. Apart from TILs, other complementary biomarkers such as the CD4, CD8 and forkhead box P3 are of great relevance in the assessment of TILs function [63, 64].
The lymphocytic infiltration in primary cutaneous melanoma was originally noted by Clark et al in almost half a decade ago [65]. Later on, Day et al [65] provided data that highlighted the prognostic significance of infiltrated lymphocytes within tumours . The College of American Pathologist has divided TILs in melanoma into three groups, namely Brisk (i.e. diffuse permeation of the invasive tumour), non-Brisk (i.e. focally infiltrating lymphocytes) and not identified subsets [66]. A recent meta-analysis demonstrated non-brisk TILs as a favourable prognostic biomarker in melanoma [64].
In breast cancer, the presence of TILs has been thoroughly investigated, leading to interesting insights. Specifically, increased levels of TILs in TNBC have been associated with better OS and disease-free survival [67]. Another interesting study suggested that sTILs can identify a subset of stage I TNBC patients with exceptional prognosis without adjuvant chemotherapy [68]. Moreover, early-stage HER2POS breast cancer patients with the presence of TILs have been found to benefit when treated with trastuzumab and chemotherapy [69, 70]. However, according to De Angelis et al [71] HER2POS breast cancers with the presence of TILs above the threshold of 60%, established by the authors, were marginally associated with higher pathologic complete response rate when treated with lapatinib plus trastuzumab.
In GC, sTILs positivity has been associated with favourable prognosis [72, 73]. According to a systematic review and quantitative meta-analysis, including 43 studies, it has been suggested that high-density TILs also present a favourable prognosis in colorectal cancer [74]. In patients with high-grade serous carcinoma of the ovary, TILs levels may be associated with chemotherapeutic sensitivity [75]. Interestingly, TILs have also been reported as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic NSCLC or metastatic melanoma [76]. However, in RCC, high TILs expression has been suggested to be correlated with poor prognosis [77]. All these studies make evident the extremely important role of TILs across different cancer types while they highlight the need for the discovery of essential information hidden behind TILs evaluation.
Finally, in malignant pleural mesotheliomas (MPMs), low CD4POS and high CD8POS sTILs are associated with poor patients’ survival [78]. In MPMs PD-L1 CPS > 1, stromal CD8HIGH seems to be a poor prognostic factor, while stromal CD4POS peritumoural TILs correlate with a worse prognosis [78]. In these tumours, a high CD4POS/CD8POS ratio in the immune microenvironment is an independent prognostic factor for survival. All these recent observations provide novel insights into the clinical scenario of immune-related biomarkers in MPM.
MMR deficiency and MSI
During the DNA recombination process, strands may detach and reanneal incorrectly, leading to mismatches [79]. However, during evolution, cells have developed strategies to identify and repair these errors. Within this DNA repair network, the mismatch repair (MMR) system is capable of solving insertion/deletion or base-base disparities on DNA [79, 80]. Six MMR proteins—mutL homologue 1 (MLH1), mutL homologue 3 (MLH3), mutS homologue 2 (MSH2), mutS homologue 3 (MSH3), mutS homologue 6 (MSH6) and postmeiotic segregation increased 2 (PMS2)—work coordinately within five complexes to repair mismatches [81]. Deficiency in the compartments of this system may result in modifications in repeated-sequence motifs, termed as microsatellites [79, 80].
Replication errors are more probable in microsatellites due to their repeated structure [82]. Hence, they are considered a potential biomarker for identifying MMR malfunction. The presence of multiple alterations in the length of microsatellites is defined as MSI [83]. MMR/MSI testing is utilised mainly to identify potential Lynch syndrome families. MLH1, MSH2, MSH6 and PMS2 proteins are assessed by IHC antibodies. This evaluation is preferred as one of the most cost-effective and available means of measurement [84]. MSI detection is generally performed through polymerase chain reaction (PCR) approaches by amplifying microsatellite markers with PCR-based methods and detecting MSI by measuring the length of the fragments [85]. Next-generation sequencing (NGS) with higher sensitivity is also being used to detect MSI in various malignancies [86]. In colorectal [87], ovarian [88], endometrial [89] and GC [90], MMR malfunction/MSI is reported as a prognostic biomarker. Contrary, it has been shown that in breast cancer, IHC and MSI testing are not interchangeable tests meaning that each type of cancer requires different and optimised management [8, 91].
The role of gene signature evaluation has become more blatant when FDA related novel immunotherapies to MMR and MSI status regardless of primary tumour site [92]. For the first time in 2017, the FDA approved the use of immunotherapy based on patients’ MMR/MSI status. Accordingly, MMR-deficient and MSI-high metastatic colorectal cancer with progression following treatment with fluoropyrimidine, oxaliplatin and irinotecan were permitted for anti-PD-1 treatment. This accelerated approval was related to nivolumab (OPDIVO, Bristol-Myers Squibb Company) [93]. Later on, in 2018, another accelerated approval was granted, adding ipilimumab (YERVOY, Bristol-Myers Squibb Company Inc.) as a combination therapy to nivolumab of those patients previously noted in 2017 [94] (Table 1).
Tumour mutational burden (TMB)
The concept of TMB refers to the number of somatic coding DNA mutations in the tumour exome [95]. TMB is noted as a beneficial biomarker in tumour immunotherapy [96]. Genetically unstable characteristics of cancer cells raise the possibility of somatic mutations resulting in neoantigens [97]. Diverse types of tumours display a different load of somatic mutations [97]. To date, melanoma and NSCLC show the highest frequencies of mutations [98]. As PD-L1 expression is reported to be highly heterogeneous, predicting the efficacy of immune checkpoint inhibitors (ICPis) in NSCLC is not yet feasible by this biomarker. Hence TMB has shown a new perspective in identifying the most fitting candidates for immunotherapy [99]. According to Hellmann et al [100], combination therapy of nivolumab and ipilimumab results in a greater PFS in high TMB cases. Remarkably, this study considered patients regardless of PD-L1 expression . Another study indicated a positive relation between atezolizumab efficacy and high level of TMB, resulting in improved ORR and duration of response in other tumours [101]. These findings suggested the importance of TMB assessment regardless of PD-L1 expression.
Generally, TMB is performed on the DNA extracted from tumour tissue, however, the analysis of circulating tumour DNA (ctDNA) is being investigated in the clinical practice, particularly in follow-up settings [102, 103]. The gold standard method for assessing TMB is whole-exome sequencing (WES) by using NGS technology [104]. This technology estimates the neoantigen load based on somatic nonsynonymous coding mutations [95]. WES highlights the presence of mutations in around 22,000 genes which makes it an expensive and time-consuming application to run [95, 97]. Targeted NGS panels are being used routinely in the clinic for oncogenic mutation detection [97]. A standardised guideline that clearly states methods and analytical validation are of importance as there are several platforms with similar targeted panels and technologies [105].
CGP assays
CGP is a targeted assay with great value in personalised cancer care transformation [106]. This assay identifies genomic alterations including mutations, copy number variants (amplification) and fusions (rearrangements), associated with targeted therapy opportunities in clinically relevant cancer genes [107]. TMB reports the number of mutations per megabase. However, there is no agreed threshold in existing assays with similar intended use [108]. Friends of Cancer Research and Quality Assurance Initiative Pathology joined to come up with harmonise and standardise TMB testing results [109]. FoundationOne® CDx is an approved CDx test by FDA [110]. This CDx identifies genetic alterations in 324 genes, MSI and TMB by extracting DNA from formalin-fixed paraffin-embedded tumour tissue specimens. The sequenced DNA is then evaluated for the presence or absence of mutations [108]. Another FDA-approved testing panel is IMPACT which utilises NGS to identify the presence of mutations in 468 unique genes, as well as other molecular changes [111]. This assay has more than 99% accuracy with the ability to detect mutations at a frequency of 2 to 5 percent [111]. Rizvi et al [112] showed that TMB quantified by targeted NGS closely correlates with TMB as quantified by WES. However, not all NGS panels may be well suited to estimate TMB.
Biomarkers and precision immunotherapy future prospectives (hope)
ICPis therapies have significantly improved precise treatment in several types of solid tumours [113]. Immunotherapy based on immune checkpoints is being widely expanded in clinical practice by gaining FDA approval in different antibody settings [114]. As listed in Table 1, PD-L1 was approved by the FDA as a biomarker in the line of predicting response to ICPis in several solid tumours [115]. FDA has also approved the application of other biomarkers such as MMR and MSI for colorectal cancer in both monotherapy and combination therapy [116]. Added to these, several other biomarkers and therapies are under the process of accelerated approval which is expected to add more value to ICPis therapy in the near future (Table 1).
Mechanisms associated with ICPIs resistance and predictive biomarkers for ICPis therapy are being actively studied [117]. Immunotherapy efficacy is strictly related to the tumour microenvironment (TME) [118]. Hence, studying components within TME is of interest in forthcoming studies. For instance, myeloid-derived suppressor cells (MDSCs), as a component of TME, are associated with ICPIs inhibition [119]. Reportedly, immunotherapy response can be improved by blocking MDSC activity [120]. Also, a correlation between MDSCs expression and poor OS and PFS is noted [121]. Another perspective issue focuses on stimulating T cell responses in which elevated co-stimulatory molecules result in favourable anti-tumour alterations [121]. For example, inducible T-cell co-stimulator, an indicator of T cell-mediated immune response, that enables early prediction of therapeutic response over multiple treatment regimens [122]. The combination of epigenetic modulator inhibitors with ICPis represents another promising approach in cancer management; as epigenetic alterations may downregulate tumour antigens by disturbing immune recognition [123]. Hong et al [124] used nivolumab in order to target epigenetic modulators which significantly increased apoptosis. The application of neoantigen vaccines as modulators of the immune microenvironment is another upcoming topic. Neoantigens resulted in mutations, may give rise to immune responses [125]. As a result, synthesised peptides may induce CD4POS and CD8POS T cell responses [126]. Reportedly, low mutation load and low T cell infiltrating TME are suitable candidates for vaccination [127]. Genetically engineered oncolytic viruses are also of interest. OVs destroy tumour cells by selectively replicating in these cells and inducing systematic anti-tumour immune responses [128]. Several clinical trials are under investigation in combining OV with cancer immunotherapies [129]. Last but not least, gut microbial alterations may lead to the additional possibility of cancer treatment. The gut microbiome is considered as a potential biomarker for ICPis response [121]. Modulation of the gut microbiome to enhance therapeutic response is being tested in multiple ongoing clinical studies [130]. Accordingly, antibiotic consumption before ICPIs had worse OS than unexposed patients [131].
Several studies suggest potential improvement of ICPis efficacy in combination with treatments such as chemotherapy, radiation and targeted therapy. These treatments can modulate the TME resulting in increased immunogenicity [132, 133]. Thus, upcoming findings in novel combinations of therapeutic agents may hopefully unravel the current gap of partial effectiveness of single-agent ICPis therapy [134]. Chemotherapy and radiotherapy are not only able to kill cancer cells directly but also present immunomodulatory properties [135]. Destruction of cancer cells with chemotherapy agents can be followed by the release of tumour-associated antigens that activate immune response as well as reduction of immunosuppressive cells such as MDSCs and Tregs [136, 137]. Radiation not only causes the release of tumour antigens but also improves antigen presentation and TIL infiltration stimulating an immune response [138]. Interestingly, studies have tested the efficacy of either chemotherapy plus ICPis or administration of ICPis after radiotherapy reporting encouraging results [139–141], while high-expectation clinical trials are ongoing (e.g. NCT04262687, NCT03453892). Targeted therapy presents similar immunomodulatory effects [132]. A phase 2 ongoing trial (NCT02954536) evaluated the safety profile and activity of pembrolizumab in combination with trastuzumab and chemotherapy in first-line HER2-positive metastatic gastric, oesophageal and gastroesophageal junction cancer. The response rate of 91% and median OS (27·3 months) were improved compared to the response rate (47%) and median OS (16 months) previously reported for chemotherapy plus trastuzumab. According to this trial, pembrolizumab can be safely combined with trastuzumab and chemotherapy and has promising activity in HER2-positive metastatic esophagogastric cancer [142]. Trastuzumab in combination with pembrolizumab may enhance HER2-specific T-cell responses and improve T cell and dendritic cell trafficking [142]. Other benefits of targeted therapy along with immunotherapy cross-talk could be seen in anti-PD-1 antibody treatment in combination with lenvatinib. This combinatory treatment mainly targets vascular endothelial growth factor and fibroblast growth factor receptors in patients with advanced endometrial cancers. In this study, lenvatinib reduced tumour-associated macrophages and increased the percentage of activated CD8POS T cells secreting interferon [143].
A promising application of ICPis can also be found in neoadjuvant therapy as recent publications note neoadjuvant immunotherapy may result in better clinical efficacy over an adjuvant application ICPis may also be used in the neoadjuvant setting since recent studies support that neoadjuvant immunotherapy can result in better clinical efficacy compared to the corresponding adjuvant therapy [144]. Added to all dated advancements, common means of time-consuming and painful tissue biopsies may be replaced by ctDNA in the peripheral blood [145, 146]. Most tumours are highly heterogeneous and may change during the progression of the disease. To define optimal therapeutic strategies, temporal sampling is mandatory. However, tissue biopsies are not always easy to perform since the tumour site may not be accessible and may not be representative of the whole tumour. Thus, the innovative approach of ‘liquid biopsy’ is gaining more and more attention. The fast turnover of tumour cells leads to a constant release in the peripheral blood of circulating tumour cells (CTCs) and cell-free ctDNA [147]. CTCs are believed to be passively spread from the primary and/or metastatic tumour sites into the bloodstream and may be responsible for the establishment of distant metastases. The liquid biopsy approach allows a repetitive and less invasive interrogation of tumours’ evolution, making sample collection much easier and efficient both for patients and clinicians [148]. All these improvements which are usually based on well-validated principles of certain biomarkers give hope for better results in precision immunotherapy.
Pitfalls in biomarker-based patients’ selection (reality)
ICPis have drastically transformed cancer treatment profiles by giving hope to physicians in cancer management [149]. However, a significant proportion of patients do not benefit from immunotherapy (with an ORR of only 20% to 23%) [150]. Biomarkers are therefore applied for the finest patient selection. Yet, assortment based on a single biomarker does not appear to be highly efficient [3]. Thus far, numerous gaps should be considered carefully to achieve optimal therapeutic benefit [151]. As stated by Pagni et al [4] ‘we do need biomarkers’ to target immune-related pathways in precise therapy. PD-L1 plays a great role as a biomarker [151]. Despite the availability and low cost of PD-L1 assessment by IHC, several technical issues are related to this method. Firstly, the IHC assessment of PD-L1 has limited accuracy due to tumour heterogeneity [151]. Moreover, several antibody clones produced by different companies are used in clinical trials; this variety of antibody clones is mystifying [153]. Added to this, different scoring methodologies—iTILs, sTILs, pTILs—which vary in different tumour types, potentially lead to confusion [154]. Besides, the PD-L1 assessment by itself does not grant to come up with an optimal therapeutic strategy [155].
Resistance to pharmacotherapy is a major issue that prevents a significant subset of patients from responding to PD-1/PD-L1 blockade. Thus, tumour immune microenvironment classification may lighten up the reasons behind [156]. When PD-L1 expression is accompanied by the presence of TILs, it characterises an adaptive resistance of tumours related to the PD-1 pathway (type-I). When both PD-L1 and TILs are not sufficient, termed as immunologically ignorant, ICs do not migrate toward cancer cells (type II). Positive PD-L1 and negative TILs lead to the induction of PD-L1 expression in tumour cells (type III). Contrary, low PD-L1 expression with optimal TILs is referred to as tolerance since the present TILs do not induce PD-L1 expression (type IV) [157]. Ultimately, the goal is to harmonise the patient’s TME with sufficient PD-L1 and TILs [158]. Added to these, not only ICPis response may remain temporarily, with the median duration of response of 1 to 2 years in NSCLC, but it can also result in resistance after the initial response [159]. The mechanisms behind therapeutic resistance are essential to address details of current misfunctions. Yet, introducing proper immunotherapeutic agents and related biomarkers to highlight malfunction is of necessity [160].
Several studies have reported TILs as a potential prognostic and predictive marker in various types of cancer [66]. Even though the TILs working group recommended standardised methodologies for the assessment of immuno-oncology biomarkers/TILs in different malignancies, the efficacy of this evaluation is suggested to be assessed by a large cohort of studies on all solid tumours [161]. MMR-wise, different methods of evaluation such as IHC, MSI and TMB are introduced to evaluate MMR status, hence a single method of assessment could provide more uniform and reliable results [81]. Several institutions perform TMB measurements mostly based on targeted NGS [97]. Despite WES is the gold standard method, usually, it is time-consuming and not affordable to run routinely [104]. Moreover, dedicated platforms are not available in all pathology laboratories [162]. As an alternative, panel-based NGS assays are of use to measure TMB. However, TMB levels are variable among each tumour type and cut-off values need to be established to reliably assess this emerging biomarker. [163]. Regarding adverse events, likewise other medications, ICPis administration brings along unwanted effects [164]. Auto-immune reactions are among the most common side effects and they can be presented as simple skin rashes but also as severe neurologic, hematologic, cardiac and respiratory implications [165]. These can be initiated by nonspecific activations of the immune system through different mechanisms. It is of note that about 2% of irAEs lead to treatment-related deaths, varying by ICPis [33]. Above all, further irAE may have not been documented yet as ICPis have only recently been introduced in therapeutic schemes. Consequently, a more detailed investigation is needed to fully approve ICPis safety profile [149].
The excessive cost of immunotherapy can be considered another important limiting factor [162]. Despite great importance, the economical aspect of this therapy has not been shielded to date [166]. ICPis therapies ought to be bearable so that not only patients can benefit from the latest therapies but also scientists could implement expanded databases for additional validations of their investigations.
Conclusion
Cancer is a complicated malignancy that involves several mechanisms and immune-related pathways. Therefore, a combination of innovative therapeutic strategies that rely on precise biomarkers has to be developed to profoundly address this issue [167]. Precision immunotherapy has already started to light up a new era in cancer management. It is fair to conclude that several struggles are yet to be addressed in patients’ selection for immunotherapy. We highlight the importance of implementing tumour-specific tests and precise guidelines in routine clinical practice for optimal therapeutic outcomes.
List of abbreviations
PD-L1, Programmed death-ligand 1; TILs, Tumour-infiltrating lymphocytes; MSI, Microsatellite instability; MMR, Mismatch repair; TMB, Tumour mutational burden; CGP, Comprehensive genomic profiling; MHC, Major histocompatibility complex; CTLA4, Cytotoxic T-lymphocyte antigen 4; PD-1, Checkpoint axis programmed cell death protein 1; NSCLC, Non-small cell lung cancer; RCC, Renal cell carcinoma; FDA, Food and Drugs Administration; irAEs, Immune-related adverse events; ICPis, Immune checkpoint inhibitors; IHC, Immunohistochemistry; TPS, Tumour proportion score; CPS, Combined positive score; TNBC, Triple-negative breast cancers; ICs, Immune cells; HNSCC, Head and neck squamous cell carcinoma; OS, Overall survival; ORR, Objective response rate; ITWG, International TILs Working Group; sTILs, Stromal TILs; iTILs, intra-tumoural TILs; GC, Gastric cancer; MLH1, mutL homologue 1; MLH3, mutL homologue 3; MSH2, mutS homologue 2; MSH3, mutS homologue 3; MSH6, mutS homologue 6; PMS2, postmeiotic segregation increased 2; NGS, Next-generation sequencing; ctDNA, circulating tumour DNA; WES, Whole exome sequencing; CDx, companion diagnostic; TME, Tumour microenvironment; MDSCs, Myeloid-derived suppressor cells; PFS, Progression-free survival; CTCs, Circulating tumour cells
Conflicts of interest
Nicola Fusco has received honoraria for consulting/advisory role from Merck Sharp & Dohme (MSD), Boehringer Ingelheim and Novartis. These companies had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript and/or in the decision to publish the results. All the other authors declare no conflicts of interest.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. Napolitano M, Schipilliti FM, and Trudu L, et al (2019) Immunotherapy in head and neck cancer: The great challenge of patient selection Crit Rev Oncol Hematol 144 102829 https://doi.org/10.1016/j.critrevonc.2019.102829 PMID: 31739116
2. Reisenbichler ES, Han G, and Bellizzi A, et al (2020) Prospective multi-institutional evaluation of pathologist assessment of PD-L1 assays for patient selection in triple negative breast cancer Mod Pathol 33(9) 1746–1752 https://doi.org/10.1038/s41379-020-0544-x PMID: 32300181
3. Iivanainen S and Koivunen JP (2020) Possibilities of improving the clinical value of immune checkpoint inhibitor therapies in cancer care by optimizing patient selection Int J Mol Sci 21(2) https://doi.org/10.3390/ijms21020556 PMID: 31952311 PMCID: 7014370
4. Pagni F, Guerini-Rocco E, and Schultheis AM, et al (2019) Targeting immune-related biological processes in solid tumors: we do need biomarkers Int J Mol Sci 20(21) https://doi.org/10.3390/ijms20215452 PMID: 31683784 PMCID: 6862285
5. Brahmer JR, Lacchetti C, and Schneider BJ, et al (2018) Management of Immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline J Clin Oncol 36(17) 1714–1768 https://doi.org/10.1200/JCO.2017.77.6385 PMID: 29442540 PMCID: 6481621
6. Choi J and Lee SY (2020) Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors Immune Netw 20(1) e9 https://doi.org/10.4110/in.2020.20.e9 PMID: 32158597 PMCID: 7049586
7. Gambardella V, Tarazona N, and Cejalvo JM, et al (2020) Personalized medicine: recent progress in cancer therapy Cancers (Basel) 12(4) https://doi.org/10.3390/cancers12041009
8. Venetis K, Sajjadi E, and Haricharan S, et al (2020) Mismatch repair testing in breast cancer: the path to tumor-specific immuno-oncology biomarkers Transl Cancer Res 9(7) https://doi.org/10.21037/tcr-20-1852
9. Signorelli D, Giannatempo P, and Grazia G, et al (2019) Patients selection for immunotherapy in solid tumors: overcome the naïve vision of a single biomarker Biomed Res Int 2019 https://doi.org/10.1155/2019/9056417
10. Samstein RM, Lee CH, and Shoushtari AN, et al (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types Nat Genet 51(2) 202–206 https://doi.org/10.1038/s41588-018-0312-8 PMID: 30643254 PMCID: 6365097
11. Salmaninejad A, Valilou SF, and Shabgah AG, et al (2019) PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy J Cell Physiol 234(10) 16824–16837 https://doi.org/10.1002/jcp.28358 PMID: 30784085
12. Lopez G, Noale M, and Corti C, et al (2020) PTEN expression as a complementary biomarker for mismatch repair testing in breast cancer Int J Mol Sci 21(4) https://doi.org/10.3390/ijms21041461
13. Fusco N, Sajjadi E, and Venetis K, et al (2020) PTEN alterations and their role in cancer management: are we making headway on precision medicine? Genes 11(7) 719 https://doi.org/10.3390/genes11070719 PMCID: 7397204
14. Costa C, Wang Y, and Ly A, et al (2020) PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer Cancer Discov 10(1) 72–85 https://doi.org/10.1158/2159-8290.CD-18-0830
15. Razavi P, Dickler MN, and Shah PD, et al (2020) Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors Nat Cancer 1(4) 382–393 https://doi.org/10.1038/s43018-020-0047-1 PMID: 32864625 PMCID: 7450824
16. Vidotto T, Melo CM, and Castelli E, et al (2020) Emerging role of PTEN loss in evasion of the immune response to tumours Br J Cancer 122(12) 1732–1743 https://doi.org/10.1038/s41416-020-0834-6 PMID: 32327707 PMCID: 7283470
17. Malone ER, Oliva M, and Sabatini PJB, et al (2020) Molecular profiling for precision cancer therapies Genome Med 12(1) 8 https://doi.org/10.1186/s13073-019-0703-1 PMID: 31937368 PMCID: 6961404
18. Lee EY and Kulkarni RP (2019) Circulating biomarkers predictive of tumor response to cancer immunotherapy Expert Rev Mol Diagn 19(10) 895–904 https://doi.org/10.1080/14737159.2019.1659728 PMID: 31469965 PMCID: 6773262
19. Labani-Motlagh A, Ashja-Mahdavi M, and Loskog A (2020) The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses Front Immunol 11 940 https://doi.org/10.3389/fimmu.2020.00940 PMID: 32499786 PMCID: 7243284
20. Gil Del Alcazar CR, Alečković M, and Polyak K (2020) Immune escape during breast tumor progression Cancer Immunol Res 8(4) 422–427 https://doi.org/10.1158/2326-6066.CIR-19-0786 PMID: 32238387 PMCID: 7138346
21. He Q, Jiang X, and Zhou X, et al (2019) Targeting cancers through TCR-peptide/MHC interactions J Hematol Oncol 12(1) 139 https://doi.org/10.1186/s13045-019-0812-8 PMID: 31852498 PMCID: 6921533
22. Van Coillie S, Wiernicki B, and Xu J (2020) Molecular and cellular functions of CTLA-4 Adv Exp Med Biol 1248 7–32 https://doi.org/10.1007/978-981-15-3266-5_2 PMID: 32185705
23. Hosseini A, Gharibi T, and Marofi F, et al (2020) CTLA-4: From mechanism to autoimmune therapy Int Immunopharmacol 80 106221 https://doi.org/10.1016/j.intimp.2020.106221 PMID: 32007707
24. Han Y, Liu D, and Li L (2020) PD-1/PD-L1 pathway: current researches in cancer Am J Cancer Res 10(3) 727–742 PMID: 32266087 PMCID: 7136921
25. He X and Xu C (2020) Immune checkpoint signaling and cancer immunotherapy Cell Res 30(8) 660–669 https://doi.org/10.1038/s41422-020-0343-4 PMID: 32467592 PMCID: 7395714
26. Zhang JY, Yan YY, and Li JJ, et al (2020) PD-1/PD-L1 based combinational cancer therapy: icing on the cake Front Pharmacol 11 722 https://doi.org/10.3389/fphar.2020.00722 PMID: 32528284 PMCID: 7247431
27. Weiss SA, Wolchok JD, and Sznol M (2019) Immunotherapy of melanoma: facts and hopes Clin Cancer Res 25(17) 5191–5201 https://doi.org/10.1158/1078-0432.CCR-18-1550 PMID: 30923036 PMCID: 6726509
28. Jiang Y, Chen M, and Nie H, et al (2019) PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations Hum Vaccin Immunother 15(5) 1111–1122 https://doi.org/10.1080/21645515.2019.1571892 PMID: 30888929 PMCID: 6605868
29. Das S and Johnson DB (2019) Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors J Immunother Cancer 7(1) 306 https://doi.org/10.1186/s40425-019-0805-8 PMID: 31730012 PMCID: 6858629
30. Palmieri DJ and Carlino MS (2018) Immune checkpoint inhibitor toxicity Curr Oncol Rep 20(9) 72 https://doi.org/10.1007/s11912-018-0718-6 PMID: 30066230
31. Postow MA, Sidlow R, and Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade N Engl J Med 378(2) 158–168 https://doi.org/10.1056/NEJMra1703481 PMID: 29320654
32. O’Kane GM, Labbé C, and Doherty MK, et al (2017) Monitoring and management of immune‐related adverse events associated with programmed cell death protein‐1 axis inhibitors in lung cancer Oncologist 22(1) 70–80 https://doi.org/10.1634/theoncologist.2016-0164 PMCID: 5313273
33. Puzanov I, Diab A, and Abdallah K, et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group J Immunother Cancer 5(1) 95 https://doi.org/10.1186/s40425-017-0300-z PMID: 29162153 PMCID: 5697162
34. Doroshow DB, Sanmamed MF, and Hastings K, et al (2019) Immunotherapy in non-small cell lung cancer: facts and hopes Clin Cancer Res 25(15) 4592–4602 https://doi.org/10.1158/1078-0432.CCR-18-1538 PMID: 30824587 PMCID: 6679805
35. Peters S, Kerr KM, and Stahel R (2018) PD-1 blockade in advanced NSCLC: A focus on pembrolizumab Cancer Treat Rev 62 39–49 https://doi.org/10.1016/j.ctrv.2017.10.002
36. Davis AA and Patel VG (2019) The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors J Immunother Cancer 7(1) 278 https://doi.org/10.1186/s40425-019-0768-9 PMID: 31655605 PMCID: 6815032
37. Cooks T, Theodorou SD, and Paparouna E, et al (2019) Immunohisto(cyto)chemistry: an old time classic tool driving modern oncological therapies Histol Histopathol 34(4) 335–352
38. Burtness B, Harrington KJ, and Greil R, et al (2018) KEYNOTE-048: Phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) Ann Oncol 29 https://doi.org/10.1093/annonc/mdy424.045
39. Atezolizumab Combo Approved for PD-L1-positive TNBC Cancer Discov. 9. United States: ©2019 (Philadelphia: American Association for Cancer Research) 2019. p Of2
40. Vigliar E, Malapelle U, and Bono F, et al (2019) The reproducibility of the immunohistochemical PD-L1 testing in non-small-cell lung cancer: a multicentric italian experience Biomed Res Int 2019 6832909 https://doi.org/10.1155/2019/6832909 PMID: 31111063 PMCID: 6487144
41. Udall M, Rizzo M, and Kenny J, et al (2018) PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics Diagn Pathol 13(1) 12 https://doi.org/10.1186/s13000-018-0689-9 PMID: 29426340 PMCID: 5807740
42. Mok TSK, Wu YL, and Kudaba I, et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial Lancet 393(10183) 1819–1830 https://doi.org/10.1016/S0140-6736(18)32409-7 PMID: 30955977
43. Cohen EEW, Bell RB, and Bifulco CB, et al (2019) The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J Immunother Cancer 7 https://doi.org/10.1186/s40425-019-0662-5
44. Mehra R, Seiwert TY, and Gupta S, et al (2018) Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012 Br J Cancer 119(2) 153–159 https://doi.org/10.1038/s41416-018-0131-9 PMID: 29955135 PMCID: 6048158
45. Ferris RL, Licitra L, and Fayette J, et al (2019) Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by prior cetuximab use Clin Cancer Res 25(17) 5221–5230 https://doi.org/10.1158/1078-0432.CCR-18-3944 PMID: 31239321
46. Kulangara K, Hanks DA, and Waldroup S, et al (2017) Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx J Clin Oncol 35(15_suppl) e14589 https://doi.org/10.1200/JCO.2017.35.15_suppl.e14589
47. Kythreotou A, Siddique A, and Mauri FA, et al (2018) PD-L1 J Clin Pathol 71(3) 189–194 https://doi.org/10.1136/jclinpath-2017-204853
48. Fuchs CS, Doi T, and Jang RW, et al (2018) Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial JAMA Oncol 4(5) e180013 https://doi.org/10.1001/jamaoncol.2018.0013 PMID: 29543932 PMCID: 5885175
49. Roviello G, Corona SP, and Nesi G, et al (2019) Results from a meta-analysis of immune checkpoint inhibitors in first-line renal cancer patients: does PD-L1 matter? Ther Adv Med Oncol 11 1758835919861905 https://doi.org/10.1177/1758835919861905 PMID: 31428205 PMCID: 6683319
50. Wu X, Gu Z, and Chen Y, et al (2019) Application of PD-1 blockade in cancer immunotherapy Comput Struct Biotechnol J 17 661–674 https://doi.org/10.1016/j.csbj.2019.03.006 PMID: 31205619 PMCID: 6558092
51. Tomioka N, Azuma M, and Ikarashi M, et al (2018) The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC) Breast Cancer 25(1) 34–42 https://doi.org/10.1007/s12282-017-0781-0
52. Planes-Laine G, Rochigneux P, and Bertucci F, et al (2019) PD-1/PD-L1 targeting in breast cancer: the first clinical evidences are emerging—a literature review Cancers (Basel) 11 https://doi.org/10.3390/cancers11071033
53. Simon SCS, Utikal J, and Umansky V (2019) Opposing roles of eosinophils in cancer Cancer Immunol Immunother 68(5) 823–833 https://doi.org/10.1007/s00262-018-2255-4
54. Olingy CE, Dinh HQ, and Hedrick CC (2019) Monocyte heterogeneity and functions in cancer J Leukoc Biol 106(2) 309–322 https://doi.org/10.1002/JLB.4RI0818-311R PMID: 30776148 PMCID: 6658332
55. Giese MA, Hind LE, and Huttenlocher A (2019) Neutrophil plasticity in the tumor microenvironment Blood 133(20) 2159–2167 https://doi.org/10.1182/blood-2018-11-844548 PMID: 30898857 PMCID: 6524564
56. Roncati L, Gasparri P, and Gallo G, et al (2020) Appendix tumor microenvironment Adv Exp Med Biol 1226 87–95 https://doi.org/10.1007/978-3-030-36214-0_7 PMID: 32030678
57. Hendry S, Salgado R, and Gevaert T, et al (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, tils in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research Adv Anat Pathol 24(5) 235–251 https://doi.org/10.1097/PAP.0000000000000162 PMID: 28777142 PMCID: 5564448
58. Hendry S, Salgado R, and Gevaert T, et al (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors Adv Anat Pathol 24(6) 311–335 https://doi.org/10.1097/PAP.0000000000000161 PMID: 28777143 PMCID: 5638696
59. Kojima YA, Wang X, and Sun H, et al (2018) Reproducible evaluation of tumor-infiltrating lymphocytes (TILs) using the recommendations of International TILs Working Group 2014 Ann Diagn Pathol 35 77–79 https://doi.org/10.1016/j.anndiagpath.2018.05.007 PMID: 29886396
60. Dieci MV, Radosevic-Robin N, and Fineberg S, et al (2018) Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer Semin Cancer Biol 52(Pt 2) 16–25 https://doi.org/10.1016/j.semcancer.2017.10.003
61. Iseki Y, Shibutani M, and Maeda K, et al (2018) A new method for evaluating tumor-infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H-E)-stained tumor sections PLoS One 13(4) e0192744 https://doi.org/10.1371/journal.pone.0192744 PMID: 29698402 PMCID: 5919485
62. Liu J, Xu Y, and Yu M, et al (2019) Increased stromal infiltrating lymphocytes are associated with circulating tumor cells and metastatic relapse in breast cancer patients after neoadjuvant chemotherapy Cancer Manag Res 11 10791–1800 https://doi.org/10.2147/CMAR.S220327
63. Orhan A, Vogelsang RP, and Andersen MB, et al (2020) The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: a systematic review and meta-analysis Eur J Cancer 132 71–84 https://doi.org/10.1016/j.ejca.2020.03.013 PMID: 32334338
64. Fu Q, Chen N, and Ge C, et al (2019) Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis Oncoimmunology 8(7) 1593806 https://doi.org/10.1080/2162402X.2019.1593806 PMID: 31143514 PMCID: 6527267
65. Antohe M, Nedelcu RI, and Nichita L, et al (2019) Tumor infiltrating lymphocytes: the regulator of melanoma evolution Oncol Lett 17(5) 4155–4161 PMID: 30944610 PMCID: 6444298
66. Badalamenti G, Fanale D, and Incorvaia L, et al (2019) Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol 343 103753 https://doi.org/10.1016/j.cellimm.2018.01.013
67. Gao G, Wang Z, and Qu X, et al (2020) Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis BMC Cancer 20(1) 179 https://doi.org/10.1186/s12885-020-6668-z PMID: 32131780 PMCID: 7057662
68. Park JH, Jonas SF, and Bataillon G, et al (2019) Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy Ann Oncol 30(12) 1941–1949 https://doi.org/10.1093/annonc/mdz395 PMID: 31566659
69. Dieci MV, Conte P, and Bisagni G, et al (2019) Association of tumor-infiltrating lymphocytes with distant disease-free survival in the ShortHER randomized adjuvant trial for patients with early HER2 breast cancer Ann Oncol 30(3) 418–423 https://doi.org/10.1093/annonc/mdz007 PMID: 30657852 PMCID: 6442655
70. Ignatiadis M, Van den Eynden G, and Roberto S, et al (2019) Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: a TRYPHAENA substudy J Natl Cancer Inst 111(1) 69–77 https://doi.org/10.1093/jnci/djy076 PMCID: 6335115
71. De Angelis C, Nagi C, and Hoyt CC, et al (2020) Evaluation of the predictive role of tumor immune infiltrate in patients with HER2-positive breast cancer treated with neoadjuvant anti-HER2 therapy without chemotherapy Clin Cancer Res 26(3) 738–745 https://doi.org/10.1158/1078-0432.CCR-19-1402 PMCID: 7002194
72. Liu JY, Yang GF, and Chen FF, et al (2019) Evaluating the prognostic significance of tumor-infiltrating lymphocytes in solid tumor: practice of a standardized method from the International Immuno-Oncology Biomarkers Working Group Cancer Manag Res 11 6815–6827 https://doi.org/10.2147/CMAR.S201538 PMID: 31440080 PMCID: 6664256
73. Zheng X, Song X, and Shao Y, et al (2017) Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis Oncotarget 8(34) 57386–57398 https://doi.org/10.18632/oncotarget.18065 PMID: 28915679 PMCID: 5593650
74. Idos GE, Kwok J, and Bonthala N, et al (2020) The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis Sci Rep 10(1) 3360 https://doi.org/10.1038/s41598-020-60255-4 PMID: 32099066 PMCID: 7042281
75. Choi KU, Kim A, and Kim JY, et al (2020) Differences in immune-related gene expressions and tumor-infiltrating lymphocytes according to chemotherapeutic response in ovarian high-grade serous carcinoma J Ovarian Res 13(1) 65 https://doi.org/10.1186/s13048-020-00667-y PMID: 32513298 PMCID: 7278194
76. Uryvaev A, Passhak M, and Hershkovits D, et al (2018) The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma Med Oncol 35(3) 25 https://doi.org/10.1007/s12032-018-1080-0 PMID: 29388007
77. Kawashima A, Kanazawa T, and Kidani Y, et al (2020) Tumour grade significantly correlates with total dysfunction of tumour tissue-infiltrating lymphocytes in renal cell carcinoma Sci Rep 10(1) 6220 https://doi.org/10.1038/s41598-020-63060-1 PMID: 32277125 PMCID: 7148296
78. Fusco N, Vaira V, and Righi I, et al (2020) Characterization of the immune microenvironment in malignant pleural mesothelioma reveals prognostic subgroups of patients Lung Cancer 150 53–61 https://doi.org/10.1016/j.lungcan.2020.09.026 PMID: 33065463
79. Motegi A, Masutani M, and Yoshioka KI, et al (2019) Aberrations in DNA repair pathways in cancer and therapeutic significances Semin Cancer Biol 58 29–46 https://doi.org/10.1016/j.semcancer.2019.02.005 PMID: 30922960
80. Trenner A and Sartori AA (2019) Harnessing DNA double-strand break repair for cancer treatment Front Oncol 9 1388 https://doi.org/10.3389/fonc.2019.01388
81. Corti C, Sajjadi E, and Fusco N (2019) Determination of mismatch repair status in human cancer and its clinical significance: does one size fit all? Adv Anat Pathol 26(4) 270–279 https://doi.org/10.1097/PAP.0000000000000234 PMID: 30932972
82. Yamamoto H and Imai K (2019) An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine Semin Oncol 46(3) 261–270 https://doi.org/10.1053/j.seminoncol.2019.08.003 PMID: 31537299
83. Viale G, Trapani D, and Curigliano G (2017) Mismatch repair deficiency as a predictive biomarker for immunotherapy efficacy Biomed Res Int 2017 https://doi.org/10.1155/2017/4719194 PMID: 28770222 PMCID: 5523547
84. Yurgelun MB and Hampel H (2018) Recent advances in lynch syndrome: diagnosis, treatment, and cancer prevention Am Soc Clin Oncol Educ Book 38 101–109 https://doi.org/10.1200/EDBK_208341 PMID: 30231390
85. Baudrin LG, Deleuze JF, and How-Kit A (2018) Molecular and computational methods for the detection of microsatellite instability in cance Front Oncol 8 621 https://doi.org/10.3389/fonc.2018.00621
86. Kok M, Chalabi M, and Haanen J (2019) How I treat MSI cancers with advanced disease ESMO Open 4(Suppl 2) e000511 https://doi.org/10.1136/esmoopen-2019-000511 PMID: 31231574 PMCID: 6555602
87. Gong Q, Zhang HH, and Sun SB, et al (2018) Mismatch repair-deficient status associates with favorable prognosis of Eastern Chinese population with sporadic colorectal cancer Oncol Lett 15(5) 7007–7013 PMID: 29725427 PMCID: 5920361
88. Zhao C, Li S, and Zhao M, et al (2018) Prognostic values of DNA mismatch repair genes in ovarian cancer patients treated with platinum-based chemotherapy Arch Gynecol Obstet 297(1) 153–159 https://doi.org/10.1007/s00404-017-4563-x PMCID: 5762798
89. Tangjitgamol S, Kittisiam T, and Tanvanich S (2017) Prevalence and prognostic role of mismatch repair gene defect in endometrial cancer patients Tumour Biol 39(9) 1010428317725834 https://doi.org/10.1177/1010428317725834 PMID: 28946809
90. Wang L, Zhang Q, and Ni S, et al (2018) Programmed death-ligand 1 expression in gastric cancer: correlation with mismatch repair deficiency and HER2-negative status Cancer Med 7(6) 2612–2620 https://doi.org/10.1002/cam4.1502 PMID: 29673110 PMCID: 6010739
91. Fusco N, Lopez G, and Corti C, et al (2018) Mismatch Repair protein loss as a prognostic and predictive biomarker in breast cancers regardless of microsatellite instability JNCI Cancer Spectr 2(4) pky056 https://doi.org/10.1093/jncics/pky056
92. Zhang L, Peng Y, and Peng G (2018) Mismatch repair-based stratification for immune checkpoint blockade therapy Am J Cancer Res 8(10) 1977–1988 PMID: 30416850 PMCID: 6220134
93. Ganesh K, Stadler ZK, and Cercek A, et al (2019) Immunotherapy in colorectal cancer: rationale, challenges and potential Nat Rev Gastroenterol Hepatol 16(6) 361–375 https://doi.org/10.1038/s41575-019-0126-x PMID: 30886395 PMCID: 7295073
94. Lichtenstern CR, Ngu RK, and Shalapour S, et al (2020) Immunotherapy, inflammation and colorectal cancer Cells 9(3) https://doi.org/10.3390/cells9030618 PMID: 32143413 PMCID: 7140520
95. Fancello L, Gandini S, and Pelicci PG, et al (2019) Tumor mutational burden quantification from targeted gene panels: major advancements and challenges J Immunother Cancer 7(1) 183 https://doi.org/10.1186/s40425-019-0647-4 PMID: 31307554 PMCID: 6631597
96. Nagahashi M, Shimada Y, and Ichikawa H, et al (2019) Next generation sequencing-based gene panel tests for the management of solid tumors Cancer Sci 110(1) 6–15 https://doi.org/10.1111/cas.13837 PMCID: 6317963
97. Chan TA, Yarchoan M, and Jaffee E, et al (2019) Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic Ann Oncol 30(1) 44–56 https://doi.org/10.1093/annonc/mdy495 PMCID: 6336005
98. Stenzinger A, Allen JD, and Maas J, et al (2019) Tumor mutational burden standardization initiatives: Recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions Genes Chromosomes Cancer 58(8) 578–588 https://doi.org/10.1002/gcc.22733 PMID: 30664300 PMCID: 6618007
99. Greillier L, Tomasini P, and Barlesi F (2018) The clinical utility of tumor mutational burden in non-small cell lung cancer Transl Lung Cancer Res 7(6) 639–646 https://doi.org/10.21037/tlcr.2018.10.08 PMID: 30505708 PMCID: 6249623
100. Hellmann MD, Ciuleanu TE, and Pluzanski A, et al (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden N Engl J Med 378(22) 2093–2104 https://doi.org/10.1056/NEJMoa1801946 PMID: 29658845 PMCID: 7193684
101. Legrand F, Gandara DR, and Mariathasan S, et al (2018) Association of high tissue TMB and atezolizumab efficacy across multiple tumor types J Clin Oncol 36 12000 https://doi.org/10.1200/JCO.2018.36.15_suppl.12000
102. Hofman P (2017) Liquid biopsy and therapeutic targets: present and future issues in thoracic oncology Cancers (Basel) 9(11) https://doi.org/10.3390/cancers9110154
103. Fumagalli C, Bianchi F, and Raviele PR, et al (2017) Circulating and tissue biomarkers in early-stage non-small cell lung cancer Ecancermedicalscience 11 717 https://doi.org/10.3332/ecancer.2017.717 PMID: 28194229 PMCID: 5295844
104. Bartha Á and Győrffy B (2019) Comprehensive outline of whole exome sequencing data analysis tools available in clinical oncology Cancers (Basel) 11(11) https://doi.org/10.3390/cancers11111725
105. Truesdell J, Miller VA, and Fabrizio D (2018) Approach to evaluating tumor mutational burden in routine clinical practice Transl Lung Cancer Res 7(6) 678–681 https://doi.org/10.21037/tlcr.2018.10.10 PMID: 30505712 PMCID: 6249621
106. Signorovitch J, Zhou Z, and Ryan J, et al (2019) Budget impact analysis of comprehensive genomic profiling in patients with advanced non-small cell lung cancer J Med Econ 22(2) 140–150 https://doi.org/10.1080/13696998.2018.1549056
107. Nesline MK, DePietro P, and Dy GK, et al (2019) Oncologist uptake of comprehensive genomic profile guided targeted therapy Oncotarget 10(45) 4616–4629 https://doi.org/10.18632/oncotarget.27047 PMID: 31384390 PMCID: 6659802
108. Büttner R, Longshore JW, and López-Ríos F, et al (2019) Implementing TMB measurement in clinical practice: considerations on assay requirements ESMO Open 4(1) e000442 https://doi.org/10.1136/esmoopen-2018-000442 PMID: 30792906 PMCID: 6350758
109. Stenzinger A, Endris V, and Budczies J, et al (Harmonization and standardization of panel-based tumor mutational burden measurement: real-world results and recommendations of the quality in pathology study J Thorac Oncol 15(7) 1177–1189 PMID: 32119917
110. Wu HX, Wang ZX, and Zhao Q, et al (2019) Designing gene panels for tumor mutational burden estimation: the need to shift from ‘correlation’ to ‘accuracy’ J Immunother Cancer 7(1) 206 https://doi.org/10.1186/s40425-019-0681-2 PMID: 31387639 PMCID: 6685228
111. @US_FDA (2017) FDA Unveils a Streamlined Path for the Authorization of Tumor Profiling Tests Alongside Its Latest Product Action (Maryland: FDA)
112. Rizvi H, Sanchez-Vega F, and La K, et al (2018) Molecular determinants of response to anti–programmed cell death (PD)-1 and anti–programmed death-ligand 1 (PD-L1) blockade in patients with non–small-cell lung cancer profiled with targeted next-generation sequencing J Clin Oncol 36 633–641 https://doi.org/10.1200/JCO.2017.75.3384 PMID: 29337640 PMCID: 6075848
113. Havel JJ, Chowell D, and Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy Nat Rev Cancer 19(3) 133–150 https://doi.org/10.1038/s41568-019-0116-x PMID: 30755690 PMCID: 6705396
114. Vaddepally RK, Kharel P, and Pandey R, et al (2020) Review of indications of fda-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence Cancers (Basel) 12(3) https://doi.org/10.3390/cancers12030738
115. Christofi T, Baritaki S, and Falzone L, et al (2019) Current perspectives in cancer immunotherapy Cancers (Basel) 11(10) https://doi.org/10.3390/cancers11101472
116. Rawla P, Barsouk A, and Hadjinicolaou AV (2019) Immunotherapies and targeted therapies in the treatment of metastatic colorectal cancer Med Sci (Basel) 7(8)
117. Seto T, Sam D, and Pan M (2019) Mechanisms of primary and secondary resistance to immune checkpoint inhibitors in cancer Med Sci (Basel) 7(2)
118. Alissafi T, Hatzioannou A, and Legaki AI, et al (2019) Balancing cancer immunotherapy and immune-related adverse events: The emerging role of regulatory T cells J Autoimmun 104 102310 https://doi.org/10.1016/j.jaut.2019.102310 PMID: 31421963
119. Osipov A, Saung MT, and Zheng L, et al (2019) Small molecule immunomodulation: the tumor microenvironment and overcoming immune escape J Immunother Cancer 7(1) 224 https://doi.org/10.1186/s40425-019-0667-0 PMID: 31439034 PMCID: 6704558
120. Park SM and Youn JI (2019) Role of myeloid-derived suppressor cells in immune checkpoint inhibitor therapy in cancer Arch Pharm Res 42(7) 560–566 https://doi.org/10.1007/s12272-019-01165-6 PMID: 31147902
121. Murciano-Goroff YR, Warner AB, and Wolchok JD (2020) The future of cancer immunotherapy: microenvironment-targeting combinations Cell Res 30(6) 507–519 https://doi.org/10.1038/s41422-020-0337-2 PMID: 32467593 PMCID: 7264181
122. Xiao Z, Mayer AT, and Nobashi TW, et al (2020) ICOS is an indicator of T-cell-mediated response to cancer immunotherapy Cancer Res 80(14) 3023–3032 https://doi.org/10.1158/0008-5472.CAN-19-3265 PMID: 32156777
123. Olino K, Park T, and Ahuja N (2020) Exposing Hidden Targets: Combining epigenetic and immunotherapy to overcome cancer resistance Semin Cancer Biol 65 114–122 https://doi.org/10.1016/j.semcancer.2020.01.001 PMID: 31911188
124. Hong YK, Li Y, Pandit H, Li S, Pulliam Z, Zheng Q, et al. Epigenetic modulation enhances immunotherapy for hepatocellular carcinoma. Cell Immunol. 2019;336:66-74.
125. Keenan TE, Burke KP, and Van Allen EM (2019) Genomic correlates of response to immune checkpoint blockade Nat Med 25(3) 389–402 https://doi.org/10.1038/s41591-019-0382-x PMID: 30842677 PMCID: 6599710
126. Chen X, Yang J, and Wang L, et al (2020) Personalized neoantigen vaccination with synthetic long peptides: recent advances and future perspectives Theranostics 10(13) 6011–6023 https://doi.org/10.7150/thno.38742 PMID: 32483434 PMCID: 7255011
127. Keskin DB, Anandappa AJ, and Sun J, et al (2019) Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial Nature 565(7738) 234–239 https://doi.org/10.1038/s41586-018-0792-9 PMCID: 6546179
128. Gujar S, Bell J, and Diallo JS (2019) SnapShot: cancer immunotherapy with oncolytic viruses Cell 176(5) 1240–1240.e1 https://doi.org/10.1016/j.cell.2019.01.051 PMID: 30794777
129. Shi T, Song X, and Wang Y, et al (2020) Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment Front Immunol 11 683 https://doi.org/10.3389/fimmu.2020.00683 PMID: 32411132 PMCID: 7198760
130. Warner AB and McQuade JL (2019) Modifiable host factors in melanoma: emerging evidence for obesity, diet, exercise, and the microbiome Curr Oncol Rep 21(8) 72 https://doi.org/10.1007/s11912-019-0814-2 PMID: 31263961 PMCID: 7472428
131. Mohiuddin JJ, Chu B, and Facciabene A, et al (2020) Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy J Natl Cancer Inst https://doi.org/10.1093/jnci/djaa057 PMID: 32294209
132. Murciano-Goroff YR, Warner AB, and Wolchok JD (2020) The future of cancer immunotherapy: microenvironment-targeting combinations Cell Res 30(6) 507–519 https://doi.org/10.1038/s41422-020-0337-2 PMID: 32467593 PMCID: 7264181
133. Serrano-Del Valle A, Naval J, and Anel A, et al (2020) Novel forms of immunomodulation for cancer therapy Trends Cancer 6(6) 518–532 https://doi.org/10.1016/j.trecan.2020.02.015 PMID: 32460005
134. Doroshow DB and Herbst RS (2018) Treatment of advanced non-small cell lung cancer in 2018 JAMA Oncol 4(4) 569–570 https://doi.org/10.1001/jamaoncol.2017.5190 PMID: 29494728
135. Hennequin C, Guillerm S, and Quero L (2019) Combination of chemotherapy and radiotherapy: a thirty years evolution Cancer Radiother 23(6–7) 662–665 https://doi.org/10.1016/j.canrad.2019.07.157 PMID: 31473087
136. Opzoomer JW, Sosnowska D, and Anstee JE, et al (2019) Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer Front Immunol 10 1654 https://doi.org/10.3389/fimmu.2019.01654 PMID: 31379850 PMCID: 6652267
137. Luo Q, Zhang L, and Luo C, et al (2019) Emerging strategies in cancer therapy combining chemotherapy with immunotherapy Cancer Lett 454 191–203 https://doi.org/10.1016/j.canlet.2019.04.017 PMID: 30998963
138. Mondini M, Levy A, and Meziani L, et al (2020) Radiotherapy-immunotherapy combinations - perspectives and challenges Mol Oncol 14(7) 1529–1537 https://doi.org/10.1002/1878-0261.12658 PMID: 32112478 PMCID: 7332212
139. Schmid P, Adams S, and Rugo HS, et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer N Engl J Med 379(22) 2108–2121 https://doi.org/10.1056/NEJMoa1809615 PMID: 30345906
140. Kim HJ, Chang JS, and Roh MR, et al (2019) Effect of radiotherapy combined with pembrolizumab on local tumor control in mucosal melanoma patients Front Oncol 9 835 https://doi.org/10.3389/fonc.2019.00835 PMID: 31552171 PMCID: 6738222
141. Mohamad O, Diaz de Leon A, and Schroeder S, et al (2018) Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy Oncoimmunology 7(7) e1440168 https://doi.org/10.1080/2162402X.2018.1440168 PMID: 29900043 PMCID: 5993514
142. Janjigian YY, Maron SB, and Chatila WK, et al (2020) First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial Lancet Oncol 21(6) 821–831 https://doi.org/10.1016/S1470-2045(20)30169-8 PMID: 32437664
143. Kato Y, Tabata K, and Kimura T, et al (2019) Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8 T cells through reduction of tumor-associated macrophage and activation of the interferon pathway PLoS One 14(2) e0212513 https://doi.org/10.1371/journal.pone.0212513 PMID: 30811474 PMCID: 6392299
144. O’Donnell JS, Hoefsmit EP, and Smyth MJ, et al (2019) The promise of neoadjuvant immunotherapy and surgery for cancer treatment Clin Cancer Res 25(19) 5743–5751 https://doi.org/10.1158/1078-0432.CCR-18-2641 PMID: 31040150
145. Said R, Guibert N, and Oxnard GR, et al (2020) Circulating tumor DNA analysis in the era of precision oncology Oncotarget 11(2) 188–211 https://doi.org/10.18632/oncotarget.27418 PMID: 32010431 PMCID: 6968778
146. Snyder A, Morrissey MP, and Hellmann MD (2019) Use of circulating tumor dna for cancer immunotherapy Clin Cancer Res 25(23) 6909–6915 https://doi.org/10.1158/1078-0432.CCR-18-2688 PMID: 31285372
147. Bidard FC, Weigelt B, and Reis-Filho JS (2013) Going with the flow: from circulating tumor cells to DNA. Science translational medicine 5(207) 207ps14
148. Fanelli GN, Naccarato AG, and Scatena C (2020) Recent advances in cancer plasticity: cellular mechanisms, surveillance strategies, and therapeutic optimization Front Oncol 10 569 https://doi.org/10.3389/fonc.2020.00569 PMID: 32391266 PMCID: 7188928
149. Martins F, Sofiya L, and Sykiotis GP, et al (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance Nat Rev Clin Oncol 16(9) 563–580 https://doi.org/10.1038/s41571-019-0218-0 PMID: 31092901
150. Rodriguez-Vida A, Perez-Gracia JL, and Bellmunt J (2018) Immunotherapy combinations and sequences in urothelial cancer: facts and hopes Clin Cancer Res 24(24) 6115–6124 https://doi.org/10.1158/1078-0432.CCR-17-3108 PMID: 29991503
151. Otoshi T, Nagano T, and Tachihara M, et al (2019) Possible biomarkers for cancer immunotherapy Cancers (Basel) 11(7) https://doi.org/10.3390/cancers11070935
152. Yan YF, Zheng YF, and Ming PP, et al (2019) Immune checkpoint inhibitors in non-small-cell lung cancer: current status and future directions Brief Funct Genomics 18(2) 147–156 https://doi.org/10.1093/bfgp/ely029
153. Tsao MS, Kerr KM, and Kockx M, et al (2018) PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project J Thorac Oncol 13(9) 1302–1311 https://doi.org/10.1016/j.jtho.2018.05.013 PMID: 29800747
154. De Meulenaere A, Vermassen T, and Aspeslagh S, et al (2017) TILs in head and neck cancer: ready for clinical implementation and why (not)? Head Neck Pathol 11(3) 354–363 https://doi.org/10.1007/s12105-016-0776-8 PMCID: 5550394
155. Li JX, Huang JM, and Jiang ZB, et al (2019) Current clinical progress of PD-1/PD-L1 immunotherapy and potential combination treatment in non-small cell lung cancer Integr Cancer Ther 18 1534735419890020 https://doi.org/10.1177/1534735419890020 PMID: 31838881 PMCID: 7242804
156. Shergold AL, Millar R, and Nibbs RJB (2019) Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade Pharmacol Res 145 104258 https://doi.org/10.1016/j.phrs.2019.104258 PMID: 31063806
157. Chen L, Cao MF, and Zhang X, et al (2019) The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes Cancer Med 8(17) 7207–7218 https://doi.org/10.1002/cam4.2580 PMID: 31605439 PMCID: 6885882
158. Hamada T, Soong TR, and Masugi Y, et al (2018) TIME (Tumor Immunity in the MicroEnvironment) classification based on tumor CD274 (PD-L1) expression status and tumor-infiltrating lymphocytes in colorectal carcinomas Oncoimmunology 7(7) e1442999 https://doi.org/10.1080/2162402X.2018.1442999 PMCID: 5993482
159. Gettinger S, Choi J, and Hastings K, et al (2017) Impaired HLA class i antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer Cancer Discov 7(12) 1420–1435 https://doi.org/10.1158/2159-8290.CD-17-0593 PMID: 29025772 PMCID: 5718941
160. Tran L and Theodorescu D (2020) Determinants of resistance to checkpoint inhibitors Int J Mol Sci 21(5) https://doi.org/10.3390/ijms21051594
161. Fuchs TL, Sioson L, and Sheen A, et al (2020) Assessment of tumor-infiltrating lymphocytes using International TILs Working Group (ITWG) system is a strong predictor of overall survival in colorectal carcinoma: a study of 1034 patients Am J Surg Pathol 44(4) 536–544 https://doi.org/10.1097/PAS.0000000000001409
162. Lantuejoul S, Damotte D, and Hofman V, et al (2019) Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma J Thorac Dis 11(Suppl 1) S89–S101 https://doi.org/10.21037/jtd.2018.12.103 PMID: 30775032 PMCID: 6353738
163. Galuppini F, Dal Pozzo CA, and Deckert J, et al (2019) Tumor mutation burden: from comprehensive mutational screening to the clinic Cancer Cell Int 19 209 https://doi.org/10.1186/s12935-019-0929-4 PMID: 31406485 PMCID: 6686509
164. Darnell EP, Mooradian MJ, and Baruch EN, et al (2020) Immune-related adverse events (irAEs): diagnosis, management, and clinical pearls Curr Oncol Rep 22(4) 39 https://doi.org/10.1007/s11912-020-0897-9 PMID: 32200442
165. Myers G (2018) Immune-related adverse events of immune checkpoint inhibitors: a brief review Curr Oncol 25(5) 342–347 https://doi.org/10.3747/co.25.4235 PMID: 30464684 PMCID: 6209551
166. Verma V, Sprave T, and Haque W, et al (2018) A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors J Immunother Cancer 6(1) 128 https://doi.org/10.1186/s40425-018-0442-7 PMID: 30470252 PMCID: 6251215
167. Venetis K, Invernizzi M, and Sajjadi E, et al (2020) Cellular immunotherapy in breast cancer: the quest for consistent biomarkers Cancer Treat Rev 90 https://doi.org/10.1016/j.ctrv.2020.102089 PMID: 32889360






