Repurposing of drugs for triple negative breast cancer: an overview
Andrea Spini1,2, Sandra Donnini3, Pan Pantziarka4, Sergio Crispino4,5 and Marina Ziche1
1Department of Medicine, Surgery and Neuroscience, University of Siena, Siena 53100, Italy
2Service de Pharmacologie Médicale, INSERM U1219, University of Bordeaux, Bordeaux 33000, France
3Department of Life Sciences, University of Siena, Siena 53100, Italy
4Anticancer Fund, Strombeek Bever 1853, Belgium
5ASSO, Siena, Italy
Abstract
Breast cancer (BC) is the most frequent cancer among women in the world and it remains a leading cause of cancer death in women globally. Among BCs, triple negative breast cancer (TNBC) is the most aggressive, and for its histochemical and molecular characteristics is also the one whose therapeutic opportunities are most limited. The REpurposing Drugs in Oncology (ReDO) project investigates the potential use of off patent non-cancer drugs as sources of new cancer therapies. Repurposing of old non-cancer drugs, clinically approved, off patent and with known targets into oncological indications, offers potentially cheaper effective and safe drugs. In line with this project, this article describes a comprehensive overview of preclinical or clinical evidence of drugs included in the ReDO database and/or PubMed for repurposing as anticancer drugs into TNBC therapeutic treatments.
Keywords: triple negative breast cancer, repositioning, non-cancer drug, preclinical studies, clinical studies
Correspondence to: Marina Ziche
Email: marina.ziche@unisi.it
Published: 13/07/2020
Received: 12/02/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer (BC) is the most frequent cancer among women in the world. Triple negative breast cancer (TNBC) is a type of BC that does not express oestrogen receptors, progesterone receptors and epidermal growth factor receptors-2/Neu (HER2) and accounts for the 16% of BCs approximatively [1, 2]. Due to its lack of response to hormone and targeted therapies, the number of therapeutic opportunities is limited [3, 4]. TNBC patients are difficult to treat, with unfavourable prognosis and are generally administered with the standard chemotherapy. At the moment, novel treatment approaches, such as immunotherapy, as well the repurposing of old drugs currently used for indications other than TNBC, is under investigation. In this context, we have previously reviewed the preclinical and clinical anticancer efficacy and safety of beta blockers in TNBC [5].
Drug repurposing is the application of an old drug to a new disease indication: this holds the promise of rapid clinical impact at a lower cost than de novo drug development [6]. In oncology, where new treatments in the last years are becoming more expensive due to the introduction of innovative therapies such as targeted therapies and immunotherapies, there is an increased interest at the use of already clinically approved non-cancer drugs, off patent and with known targets, as possible cancer treatments [7]. One study published by Pantziarka et al [8], point the spotlight on this matter building up a project about drug repurposing in the treatment of cancer. The REpurposing Drugs in Oncology (ReDO) project investigates the potential use of licensed non-cancer medications as sources of new cancer strategies. ReDO project has used a literature-based approach to identify licensed non-cancer drugs with published evidence of anticancer activity. At present, data of 268 drugs have been included in the REDO database (ReDO_DB) [8].
In line with this project, we searched in PubMed for published preclinical or clinical evidence of anticancer activity for all drugs included in the ReDO_DB for TNBC. Specifically, starting from each drug present in ReDO_DB, we searched in PubMed for published preclinical and clinical evidence of anticancer activity for TNBC. The strings were composed by the name of the drugs and specific keywords related to TNBC.
An additional search string was used to investigate potential clinical evidence about drugs not included in ReDO_DB or references not retrieved in the first search. The string was composed by three blocks concerning keywords related to TNBC, repurposing and study type, respectively. Both strings are provided in the supplementary file (Table S1). Observational or clinical trials for which a TNBC cohort was defined were included. The articles that were not written in English were excluded.
Moreover, clinicaltrials.gov [9] was searched for ongoing or completed clinical studies on drug repurposing and TNBC. All searches were performed on March 2019, and the information extracted were the following: 1) preclinical studies: number of studies per drug and pharmacological activity; 2) clinical studies: study type, country, study period, population studies, exclusion criteria, age, follow up, arms, treatments and outcomes; 3) clinicaltrials.gov: number of studies per drug.
The aim of this paper is to give to clinicians and scientists a comprehensive overview about preclinical and clinical studies, including clinical trials, present in literature on the repurposing of old-licensed drugs for TNBC.
We found 188 preclinical studies references (see Supplementary Material), 18 clinical references [10–26] and 16 references on clinical trials.gov on drug repurposing for TNBC [9].
Preclinical studies
Using the PubMed database, we found preclinical evidence on TNBC models (cell lines and xenograft models of TNBC) for 84 out of 268 old drugs (31.3%) present in the ReDO_DB. For 42 of the 84 drugs, only one reference was retrieved (Table S2). Thirteen studies referred to the anti-proliferative, pro-apoptotic and immune-stimulating effects of metformin, thirteen to the cytotoxic and anti-metastatic effects of chloroquine, eleven to the anti-proliferative and anti-invasive effects of simvastatin, eight to the anti-inflammatory and anti-angiogenic effects of acid acetylsalicylic and eight studies to the anti-angiogenic, anti-proliferative and anti-apoptotic effects of zoledronic acid. Main indications for drugs with preclinical evidence of efficacy on TNBC model were various and heterogeneous including epilepsy, analgesia, hypertension, diabetes, insomnia and other.
Clinical studies
Table 1 shows all 17 clinical references collected (the article of Spera et al analyses two different retrospective studies on beta blockers efficacy and safety on TNBC [13], and the articles of Hagasewa et al [15] and Ishikawa et al [16] analysed the same cohort of patients). Clinical evidence on twelve licensed drugs was found, and of these drugs, eleven out of 268 (4.1%) were included in ReDO_DB. Eleven studies out of 18 were retrospective studies [10–13, 17, 19, 20, 22, 25, 26], six were phase II and [14–16, 18, 21, 23] one was a phase I clinical trial [24] (see Figure 1 for more details). Retrospective studies ranged from 1995 to 2016, and six out of eleven studies analysed a USA cohort of patients [10, 12, 19, 20, 25, 26]. Eight studies were performed using medical records [10, 12, 17, 19, 20, 22, 25, 26], one was based on disease registries [11] and two reported the results of previous clinical trials [13]. Of the 18 clinical studies collected, four analysed the efficacy of beta blockers (BB) [11–13], five of non-steroidal anti-inflammatory drugs (NSAIDs) [17–21], two of zoledronic acid [15, 16], one of metformin [10], one of tetramolybdate [14], one of itraconazole [22], one of esomeprazole [23], one of mifepristone [24] and two of statins [25, 26]. Outcomes retrieved from clinical studies were grouped, whenever possible, in pharmacological categories and summarised in Table 2.
Table 1. Characteristics of clinical studies about repurposing of old drugs for TNBC treatment.
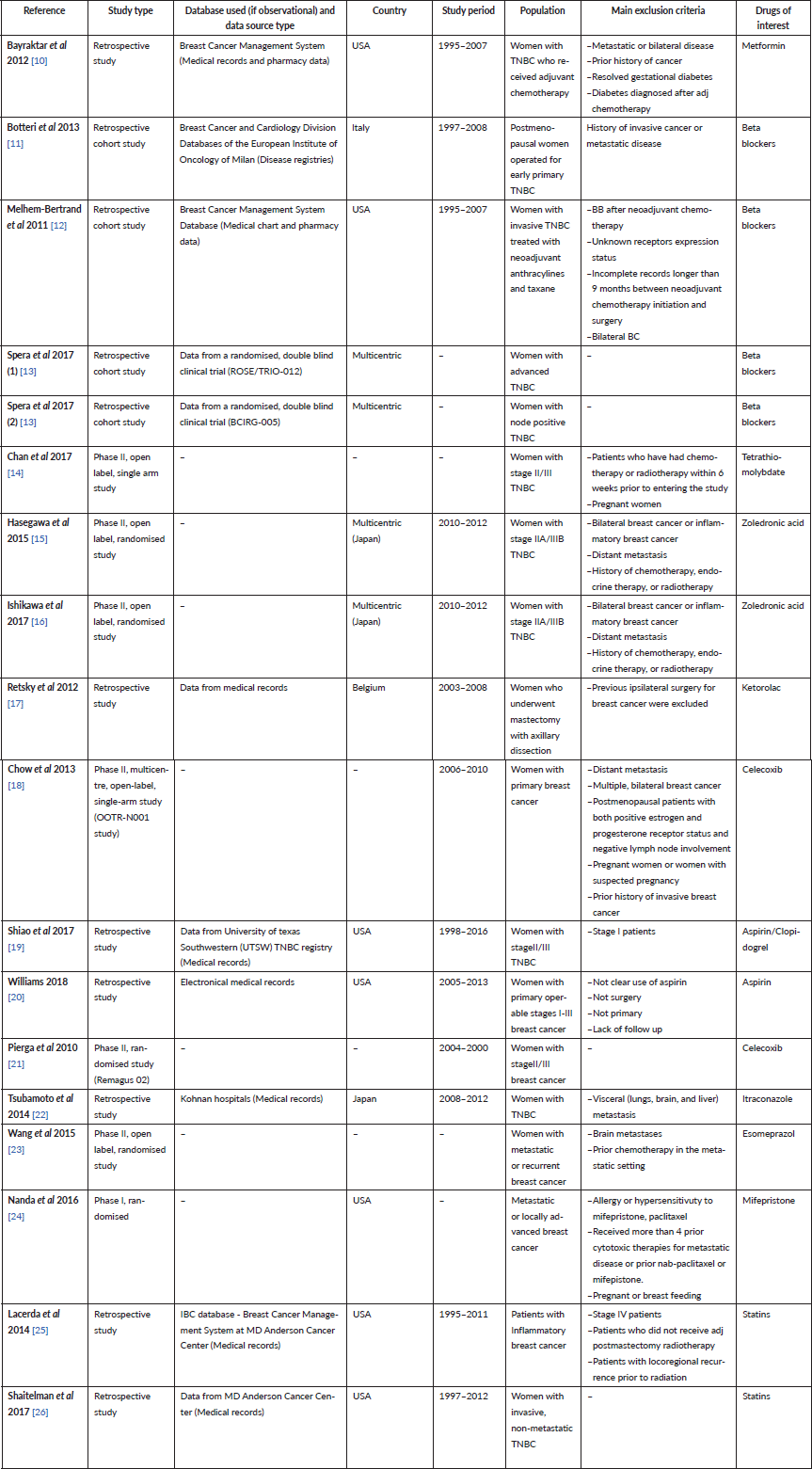

Figure 1. Type of studies per drug. This shows the number of clinical trials (only phase 1 and 2 studies were found) and observational studies conducted per drug/pharmacological classes.
Beta blockers (BBs)
BBs were evaluated on postmenopausal women with operated early primary TNBC, on women with invasive TNBC (receiving neoadjuvant chemotherapy), and on women with advanced or nodal positive TNBC. Study populations ranged from 35 patients to 1,417 patients. In the study of Melhem-Bertrandt et al [12], using medical chart and pharmacy data from the Breast Cancer Management System Database in the USA, women with invasive TNBC receiving neoadjuvant chemotherapy plus BBs were compared to patients receiving only neoadjuvant chemotherapy between 1995 and 2007. Hazard ratio of recurrence free survival for women administered with chemotherapy plus BBs was 0.30 (95% CI, 0.10–0.87; p = 0.027) and hazard ratio of overall survival was 0.35 (95% CI, 0.12–1.00; p = 0.05) [12]. Also, in the retrospective study of Botteri et al [11] using Breast Cancer and Cardiology Division Databases in Italy and analysing 800 postmenopausal women diagnosed and operated for early primary TNBC between 1997 and 2008, BB users showed significant benefit when compared to not BB users. Breast cancer related events where lower in BB users (13.6% versus 27.9%; p = 0.02) and hazard ratio of metastasis and BC death were significant (0.32: 95% CI 0.12–0.90; p = 0.031; 0.42: 95% CI 0.18–0.97; p = 0.042, respectively). The study of Spera et al [13], using data of a randomised, double blind clinical trial (ROSE/TRIO-012), showed significant benefit in women with advanced TNBC using BBs when compared to not users about progression free survival (Hazard ratio = 0.52; 95% CI, 0.34–0.80; p = 0.002) but not in overall survival (Hazard ratio = 0.87; 95% CI 0.58–1.31; p = 0.504). The second study presented by Spera et al [13] using also data from another randomised, double blind clinical trial (BCIRG-005) about women with node positive TNBC did not show any significant benefit of relapse free survival and overall survival (Hazard ratio = 0.69; 95% CI, 0.35–1.34; p = 0.269; 0.73; 95% CI, 0.35–1.48; p = 0.38, respectively).
Table 2. Outcomes for each clinical study.

Metformin
The retrospective study of Bayraktar et al [10] using medical chart and pharmacy data from the Breast Cancer Management System Database compared women who received adjuvant chemotherapy with or without metformin in the USA between 1995 and 2007. In total, 1,448 patients (63 diabetic patients receiving metformin, 67 diabetic patients not receiving metformin and 1318 not diabetic patients). The 5 years survival estimates for distant metastasis free survival were 73% in the metformin group, 66% in the non-metformin group and 60% in the non-diabetic group (p = 0.23). Overall survival was 67% in the metformin group, 69% in the non-metformin group and 66% in the non-diabetic group (p = 0.58). Recurrence free survival was 65% in the metformin group, 64% in the non-metformin group and 54% in the non-diabetic group (0.38). Also, after adjustments, no significant survival outcomes were obtained.
Tetramolybdate
The primary endpoint of phase II open label single arm study of Chan et al [14] was to assess the change in VEGFR2 endothelial progenitor cells in women treated with tetrathiomolybdate. The study, performed on 36 women with stage II/III TNBC during adjuvant setting, showed that two year event free survival was 90%.
Zoledronic acid
The articles of Hasegawa et al [15] and Ishikawa [16] referred to the same phase II, open label, randomised study but analysed different outcomes in the same cohort of patients (34 women with stage IIA/IIIB TNBC) treated with zoledronic acid plus chemotherapy versus chemotherapy in neoadjuvant setting. Pathological complete response was not significant (p = 0.112) when comparing neoadjuvant chemotherapy plus zoledronic acid (6/17 (35.3%) CI: 12.6–58.0) with chemotherapy alone (2/17 (11.8%) CI: 0.0–27.1). Also for the 3 years disease free survival, neoadjuvant chemotherapy plus zoledronic acid showed no significant benefit compared to the neoadjuvant treatment alone (p = 0.077) despite the fact that the percentage of patients in treatment with zoledronic acid was higher compared to the other arm (94.1% versus 70.6%).
NSAIDs
Celecoxib was analysed in two studies: the first, a phase II randomised study of Pierga et al [21] performed between 2004 and 2007, analysed 23 women with stage II/III TNBC comparing chemotherapy alone with chemotherapy plus celecoxib. The authors stated that celecoxib did not improve pathological complete response rates, but no specific comparison on this outcome were shown in the article for TNBC patients. The second study, a phase II multicentre open-label single arm study of Chow et al [18], analysed women with primary breast cancer. Unfortunately, only two patients with primary TNBC were included and authors could not show any result about this cohort.
Aspirin was analysed in two retrospective studies. The first retrospective study of Shiao et al [19] that collected medical records from University of Texas Southwestern TNBC registry, analysed a cohort of 222 women with stage II/III TNBC in the USA between 2005 abd 2013. Sixty-five women were treated with anti-platelet therapy (as aspirin or clopidogrel) and 157 with no anti-platelet therapy. A percentage of patients in both arms (6.3% and 7.1%, respectively) did not receive chemotherapy. Five years disease free survival and 5 years distant metastasis hazard ratios was significantly improved in favour of the first arm (anti-platelet 80.4%, no anti-platelet 62.3%, HR: 0.503 (0.261–0.970); p = 0.04; anti-platelet 8.8%, no anti-platelet 31.9%, HR: 0.310 (0.132–0.729); p = 0.007, respectively). Five years overall survival hazard ratio was not significant between the two arms (HR: 0.652 (0.343–1.239); p = 0.192). The second retrospective study of Williams et al [20] performed in USA used electronic medical records of 147 women with primary operable stages I-III TNBC (114 never used aspirin, 19 before diagnosis, and 14 after diagnosis) to analyse overall survival and disease-free survival between 2005 and 2013. Results of this study indicated that aspirin may have an impact on the pathogenesis of TNBC but do not seem to affect breast cancer survival when used after cancer diagnosis (results were presented only for the total cohort of breast cancer patients and not for TNBC subtype).
Finally, Retsky et al [17] showed the updated results of a retrospective study performed in Belgium using medical records between 2003 and 2008 [27], in which ketorolac plus chemotherapy was compared to chemotherapy alone in women who underwent mastectomy with axillary dissection. No information about the cohort (as for the number of patients with TNBC, age, etc…) was reported. Also, for the results the authors said that the group receiving chemotherapy plus ketorolac showed a ‘far superior disease free survival in the first few years after surgery’ but no data were shown in particular about TNBC.
Itraconazole
The article of Tsubamoto et al [22] reported the results of a retrospective study that used medical records of the Kohan hospital in Japan between 2008 and 2012 to analye response rate, median progression-free survival and median overall survival of thirteen patients. TNBC patients who progressed after prior chemotherapy were treated with chemotherapy in combination with itraconazole. No comparison was made. The authors showed that response rate was 62% ([CI], 35%–88%), progression free survival was 10.8 months (95%CI, 7.6–15.3) and overall survival was 20.4 months (95%CI: 13.1–41.4 months).
Esomeprazole
The phase II, open label, randomised study of Wang et al [23] analysed a cohort of 15 women with metastatic or recurrent TNBC (seven receiving only chemotherapy, two esomeprazole low dose and six esomeprazole high dose). The authors showed that the time to progression of patients receiving esomeprazole when compared to chemotherapy was significantly higher (10.7 versus 5.8 months; p = 0.011).
Mifepristone
In the Phase I, randomised study of Nanda and colleagues performed in USA, four women with metastatic or locally advanced TNBC were analysed (those patients were allocated to mifepristone plus paclitaxel or placebo). Unfortunately, no information about patients allocation, nor any outcome information could be retrieved from this article [24].
Statins
The retrospective study of Shaitelman et al [26] used medical records from the MD Anderson Cancer Centre to investigate if women with stage I–III TNBC receiving statins at any time from diagnosis. The authors showed that patients receiving statins did not get any advantage compared to the non-statin users group (0.82 (0.57–1.16); 0.70 (0.47–1.03) relative risk of recurrence and breast cancer death, respectively); when a multivariate analysis was performed (taking in consideration cholesterol and triglyceride values, stage and chemotherapy, the authors showed that statin use was predictive for OS (HR: 0.10, p = 0.026, 95% CI: 0.01–0.76).
The retrospective study of Lacerda et al [25] using Breast Cancer Management database at MD Anderson Cancer Centre in USA between 1995 and 2011, analysed the risk of loco-regional recurrence at 3 years associated to the use of statins, in patients with inflammatory breast cancer who received adjuvant post-mastectomy radiotherapy. 102 patients underwent post-mastectomy radiation (86 patients) or post-mastectomy radiation plus statins (16 patients). Unfortunately no information about the outcome in TNBC patients was shown.
Clinicaltrials.gov
Searching the web site of clinicaltrials.gov (clinicaltrials.gov), we found only 17 drugs out of 286 presented in the ReDo_DB with ongoing or completed clinical trials for TNBC. Table 3 shows the list of trials and the recruitment status for each drug. As shown in Table 3, most part of the drugs present only one or few studies published on this website. In total, three studies are recruiting for the assessment of atorvastatin, two for metformin, two for mifepristone, and three for zoledronic acid.
Future directions
This review presents an overview of all the evidences about the repurposing of old, licensed, non-cancer-drugs in the treatment of TNBC, starting from preclinical evidence and going through current clinical trials. ReDO is an ambitious project aiming to investigate the repurposing of non-cancer-drugs in oncology, and ReDO_DB is a powerful tool that need to be dynamically implemented with recent findings, by adding to the database new drugs for which there are preclinical evidence, and by giving visitors a specific PubMed search string for each tumour and tumour subtypes. The ReDO approach is based on published literature and does not aim to identify new active compounds against cancer. Thus, the database does not include potential repurposing candidates identified through in silico modelling or other computational pharmacological approaches that, despite the interest for the research [28–31], unless validated by preclinical studies, represent only future hypothetical repurposed drugs and far from the aim of the ReDO project. The project, in particular, aims to drive scientist attention to investigate already approved non cancer-drugs in the oncology setting. Using this ReDO_DB, we found out that despite a lot of preclinical evidence was produced for drugs included in the database for the treatment of TNBC, only few of them were tested in clinical trials. Moreover, in clinical trials only few of the studies used a large sample of cases and gave explicit results on the repurposing of old drugs for TNBC. Some of the studies did not report any result for TNBC cohort when this is a part of a bigger BC cohort.
Beta Blockers (BBs) seem to be the more promising drugs in the repurposing for the treatment of TNBC. Three articles showed significant benefits of these drugs in women with advanced TNBC and in early primary TNBC patients treated with the combination of chemotherapy plus BBs [11–13]. Unfortunately, in clinicaltrials.gov we found no studies that specifically attempt to evaluate BBs within clinical trials for TNBC patients. One triple blinded phase II randomised trial evaluated the use of pre-operative propranolol (seven days before surgery) compared to placebo in 60 women with early stage surgically-resectable breast cancer. [32]. The authors showed that the treatment with propranolol reduced intra-tumoral mesenchymal transition and promoted immune cell infiltration reducing biomarkers associated with metastatic potential. Unfortunately, authors did not present results stratified for breast cancer sub-type.
While BBs demonstrated to be beneficial in the treatment of TNBC, metformin, a promising molecule in preclinical studies, did not show any efficacy in the treatment of women with TNBC. Bayraktar et al [10] showed that metformin does not improve survival outcomes in a population of TNBC women when compared to not users. Of note, two studies on the use of metformin in clinicaltrials.gov on TNBC patients are ongoing.
The articles of Shiao et al [19] and Williams et al [20] showed conflicting results on aspirin. While the first study showed a significant survival benefit in women with stage II/III by the use of aspirin, Williams et al [20] did not show this benefit in the breast cancer population examined (women with operable stage I-III TNBC).
Despite many studies trying to evaluate the use of statins in breast cancer treatment [33–36], in the literature search on PubMed, we retrieved only two retrospective studies on their use in the TNBC cohort. The article of Shaitelman et al [26] reported a non-significant improvement of OS for patients in the statin group (with the exception of the multivariate analysis), while the second study of Lacerda et al [25] did not show any results for TNBC patients.
Table 3. Ongoing trials found in Clinicaltrials.gov.

Other authors showed significant results on the survival of TNBC patients treated with esomeprazole. Recently, one phase II study on activity of omeprazole on patients with operable TNBC independent of baseline Fatty acid synthase (FASN) expression was presented at the ASCO meeting. [37] In vitro, proton pump inhibitors inhibit FASN activity and induce apoptosis in breast cancer cell lines. In this study, omeprazole in combination with anthracycline-taxane (AC-T) was administered to 42 patients until surgery, and pathologic complete response (pCR) was investigated. FASN positivity significantly decreased with omeprazole from 0.53 (SD = 0.25) at baseline to 0.38 (SD = 0.30; p = 0.02), and the drug was well tolerated with no known grade 3 or 4 toxicities. Furthermore, the pCR rate was 71.4% (95% CI: 51.3–86.8) in FASN patients and 71.8 % (95% CI: 55.1–85.0) in all enrolled patients, demonstrating that the omeprazole in addition to neoadjuvant AC-T yields a promising pCR rate without adding toxicity.
For those drugs collected in ReDO_DB with favourable preclinical evidence or whose retrospective clinical trials were not so large to provide strong evidence, large retrospective cohort studies are needed to evaluate effectiveness. Further, as for BBs that have proven by retrospective studies to be effective in the treatment of TNBC patients, randomised clinical trials might be important to confirm the evidence of the repurposing.
Final remarks
Drug repurposing is a highly interesting novel strategy for the oncology community and ReDO_DB is a powerful tool that can give authors the opportunity to investigate weather non-anticancer drugs might be effective in cancer treatment. Some precision medicine studies, based on omics data, have included repurposed drugs and have reported interesting case reports of responses from patients [38, 39], however no one on TNBC. Due to the low number of therapeutic opportunities approved for TNBC, repurposing of old drugs seems a valuable approach for this particular type of cancer.
From the literature retrieved, BBs seemed to be the more promising drugs for the repurposing, while evidence about other drugs as NSAIDs still need to be assessed or proven for the treatment of TNBC.
Conflicts of interest
The authors declare that they have no conflict of interest
Authors' contributions
MZ and SC conceived the study. AS extracted the data. SD supervised the data extraction. MZ, SD, SC, AS, and PP contributed to the interpretation and discussion of study results. AS and SD drafted the manuscripts. All authors revised and approved the final version of the paper.
Funding
This study was supported by Fondazione Decima Regio ‘Olga e Raimondo Curri’, Via Cimarra 44-B, Roma.
References
1. Perou CM (2010) Molecular stratification of ripple-negative breast cancers Oncologist 15(S5) 39–48 https://doi.org/10.1634/theoncologist.2010-S5-39
2. Brouckaert O, Wildiers H, and Floris G, et al (2012) Update on triple-negative breast cancer: prognosis and management strategies Int J Women’s Health 4 511–520. PMID: 23071421 PMCID: 3469230
3. Gucalp A and Traina TA (2011) Triple-negative breast cancer: adjuvant therapeutic options Chemother Res Pract 2011 696208
4. Dent R, Trudeau M, and Pritchard KI, et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence Clin Cancer Res 13(15) 4429–4434 https://doi.org/10.1158/1078-0432.CCR-06-3045 PMID: 17671126
5. Spini A, Roberto G, and Gini R, et al (2019) Evidence of β-blockers drug repurposing for the treatment of triple negative breast cancer: a systematic review Neoplasma 66(6) 963–970 https://doi.org/10.4149/neo_2019_190110N34 PMID: 31607128
6. Pushpakom S, Iorio F, and Eyers PA, et al (2019) Drug repurposing: progress, challenges and recommendations Nat Rev Drug Discov 18(1) 41–58 https://doi.org/10.1038/nrd.2018.168
7. Goldstein DA, Stemmer SM, and Gordon N (2016) The cost and value of cancer drugs—are new innovations outpacing our ability to pay? Isr J Health Policy Res 5 40 https://doi.org/10.1186/s13584-016-0097-0
8. Pantziarka P, Verbaanderd C, and Sukhatme V, et al (2018) ReDO_DB: the repurposing drugs in oncology database Ecancermedicalscience 12 886 https://doi.org/10.3332/ecancer.2018.886
9. ClinicalTrials.gov [https://clinicaltrials.gov/] Date accessed: 31/03/19
10. Bayraktar S, Hernadez-Aya LF, and Lei X, et al. (2012) Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer Cancer 118(5) 1202–1211 https://doi.org/10.1002/cncr.26439
11. Botteri E, Munzione E, and Rotmensz N, et al (2013) Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast cancer research and treatment Breast Cancer Res Treat 140(3) 567–575 https://doi.org/10.1007/s10549-013-2654-3 PMID: 23912960
12. Melhem-Bertrandt A, Chavez-Macgregor M, and Lei X, et al (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer J Clin Oncol 29(19) 2645–2652 https://doi.org/10.1200/JCO.2010.33.4441 PMID: 21632501 PMCID: 3139371
13. Spera G, Fresco R, and Fung H, et al (2017) Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study Ann Oncol 28(8) 1836–1841 https://doi.org/10.1093/annonc/mdx264 PMID: 28520849
14. Chan N, Willis A, and Kornhauser N, et al (2017) Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases Clin Cancer Res 23(3) 666–676 https://doi.org/10.1158/1078-0432.CCR-16-1326
15. Hasegawa Y, Tanino H, and Horiguchi J, et al (2015) Randomized controlled trial of zoledronic acid plus chemotherapy versus chemotherapy alone as neoadjuvant treatment of HER2-negative primary breast cancer (JONIE study) PLoS One 10(12) e0143643 https://doi.org/10.1371/journal.pone.0143643 PMID: 26633806 PMCID: 4669153
16. Ishikawa T, Akazawa K, and Hasegawa Y, et al (2017) Survival outcomes of neoadjuvant chemotherapy with zoledronic acid for HER2-negative breast cancer J Surg Res 220 46–51 https://doi.org/10.1016/j.jss.2017.05.066 PMID: 29180210
17. Retsky M, Rogers R, and Demicheli R, et al (2012) NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup Breast Cancer Res Treat 134(2) 881–888 https://doi.org/10.1007/s10549-012-2094-5 PMID: 22622810
18. Chow LWC, Tung SY, and Ng T-Y, et al (2013) Concurrent celecoxib with 5-fluorouracil/epirubicin/cyclophosphamide followed by docetaxel for stages II - III invasive breast cancer: the OOTR-N001 study Expert Opin Investig Drugs 22(3) 299–307 https://doi.org/10.1517/13543784.2013.766715 PMID: 23394482
19. Shiao J, Thomas KM, and Rahimi AS, et al (2017) Aspirin/antiplatelet agent use improves disease-free survival and reduces the risk of distant metastases in Stage II and III triple-negative breast cancer patients Breast Cancer Res Treat 161(3) 463–471 https://doi.org/10.1007/s10549-016-4081-8
20. Williams AD, Li YR, and So A, et al (2018) The impact of aspirin use on breast cancer subtype and clinical course J Surg Res 230 71–79 https://doi.org/10.1016/j.jss.2018.04.040 PMID: 30100043
21. Pierga J-Y, Delaloge S, and Espié M, et al (2010) A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients Breast Cancer Res Treat 122(2) 429–437 https://doi.org/10.1007/s10549-010-0939-3 PMID: 20480225
22. Tsubamoto H, Sonoda T, and Inoue K. (2014) Impact of itraconazole on the survival of heavily pre-treated patients with triple-negative breast cancer Anticancer Res 34(7) 3839–3844 PMID: 24982411
23. Wang B-Y, Zhang J, and Wang J-L, et al (2015) Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer J Exp Clin Cancer Res 34 85 https://doi.org/10.1186/s13046-015-0194-x PMID: 26297142 PMCID: 4546346
24. Nanda R, Stringer-Reasor EM, and Saha P, et al (2016) A randomized phase I trial of nanoparticle albumin-bound paclitaxel with or without mifepristone for advanced breast cancer Springerplus 5(1) 947 https://doi.org/10.1186/s40064-016-2457-1 PMID: 27386391 PMCID: 4929099
25. Lacerda L, Reddy JP, and Liu D, et al (2014) Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation Stem Cells Transl Med 3(7) 849–856 https://doi.org/10.5966/sctm.2013-0204 PMID: 24833589 PMCID: 4073823
26. Shaitelman SF, Stauder MC, and Allen P, et al (2017) Impact of statin use on outcomes in triple negative breast cancer J Cancer 8(11) 2026–2032 https://doi.org/10.7150/jca.18743 PMID: 28819403 PMCID: 5559964
27. Forget P, Berlière M, and van Maanen A, et al (2013) Perioperative ketorolac in high risk breast cancer patients. Rationale, feasibility and methodology of a prospective randomized placebo-controlled trial Med Hypotheses 81(4) 707–712 https://doi.org/10.1016/j.mehy.2013.07.033 PMID: 23937996
28. Vitali F, Cohen LD, and Demartini A, et al (2016) A network-based data integration approach to support drug repurposing and multi-target therapies in triple negative breast cancer PLoS One 11(9) e0162407 https://doi.org/10.1371/journal.pone.0162407 PMID: 27632168 PMCID: 5025072
29. Turanli B, Karagoz K, and Gulfidan G, et al (2018) A network-based cancer drug discovery: from integrated multi-omics approaches to precision medicine Curr Pharm Des 24(32) 3778–3790 https://doi.org/10.2174/1381612824666181106095959 PMID: 30398107
30. Ocana A and Pandiella A (2017) Targeting oncogenic vulnerabilities in triple negative breast cancer: biological bases and ongoing clinical studies Oncotarget 18 8(13):22218–34 https://doi.org/10.18632/oncotarget.14731
31. Bianchini G, Balko JM, and Mayer IA, et al (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease Nat Rev Clin Oncol 13(11) 674–690 https://doi.org/10.1038/nrclinonc.2016.66 PMID: 27184417 PMCID: 5461122
32. Hiller JG, Cole SW, and Crone EM, et al (2020) Pre-operative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a Phase II randomized trial Clin Cancer Res 26(8) 1803–1811 https://doi.org/10.1158/1078-0432.CCR-19-2641
33. Ahern TP, Pedersen L, and Tarp M, et al (2011) Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study J Natl Cancer Inst 103(19) 1461–1468 https://doi.org/10.1093/jnci/djr291 PMID: 21813413 PMCID: 3186780
34. Boudreau DM, Yu O, and Chubak J, et al (2014) Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer Breast Cancer Res Treat 144(2) 405–416 https://doi.org/10.1007/s10549-014-2870-5 PMID: 24557337 PMCID: 3988288
35. Kwan ML, Habel LA, and Flick ED, et al (2008) Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors Breast Cancer Res Treat 109(3) 573–579 https://doi.org/10.1007/s10549-007-9683-8
36. Murtola TJ, Visvanathan K, and Artama M, et al Statin use and breast cancer survival: a nationwide cohort study from Finland PLoS One 9(10) ell0231
37. Sardesai SD, Thomas A, and Gallagher C, et al (2020) Inhibiting fatty acid synthase in operable triple negative breast cancer J Clin Oncol 38(suppl; abstr 584) https://doi.org/10.1200/JCO.2020.38.15_suppl.584
38. Brown RE, Buryanek J, and McGuire MF (2017) Metformin and melatonin in adrenocortical carcinoma: morphoproteomics and biomedical analytics provide proof of concept in a case study Ann Clin Lab Sci 47(4) 457–465 PMID: 28801373
39. Jones MR, Schrader KA, and Shen Y, et al (2016) Response to angiotensin blockade with irbesartan in a patient with metastatic colorectal cancer Ann Oncol 27(5) 801–806 https://doi.org/10.1093/annonc/mdw060 PMID: 27022066 PMCID: 4843189
Supplementary tables
Table S1. Search strings.

Table S2. Preclinical references for repurposing of drugs for TNBC by ReDO DB.







