High depletion of breast cancer cells from the peripheral blood with the method of non-specific separation
Petra Payer1, Michael Röder1, Oumar Camara2 and Katharina Pachmann3
1INNOVENT e. V., Prüssingstraße 27 B, D-07745 Jena, Germany
2Hufeland Klinikum GmbH, Rudolph-Weiss-Straße 1-5, D-99947 Bad Langensalza, Germany
3Transfusionsmedizinisches Zentrum Bayreuth, Kurpromenade 2, D-95448 Bayreuth, Germany
Abstract
Circulating epithelial tumour cells (CETCs) play an important role in the formation of metastases in breast cancer patients. The depletion of such CETCs from peripheral blood of breast cancer patients using non-specific separation (without antibodies) of tumour cells from normal blood leucocytes might contribute to reduce the load of the patient’s blood with tumour cells and subsequently reduce the probability of metastasis formation. This method is based on cell type-specific interaction of living cells with Carboxymethyl Dextrane (CMD) coated magnetic nanoparticles. We have developed a mild flow separation method using CMD-coated magnetic nanoparticles (core size ca. 25 nm) along with a low-field gradient magnetic separator and an external separation column (blood bag). The ability of tumour cells to preferentially bind such particles and to separate tumour cells from the white blood cells from blood samples of 25 breast cancer patients (fresh and 24-hour stored blood samples) were tested. The circulating tumour cells were quantified before and after separation by maintrac analysis. We achieved a very high depletion rate of tumour cells to < 3% remaining in the investigated 24 hours stored blood samples and ≤14% in all fresh blood samples concurrent with maintaining 56% ± 4% of vital leukocytes in all fresh blood samples.
Keywords: breast cancer, circulating epithelial tumour cells (CETC), EpCAM-positive cells, magnetic nanoparticles, carboxymethyl dextrane (CMD) coating, non-specific cell separation, mild magnetic separation, external separation column
Correspondence to: Petra Payer
Email: pp@innovent-jena.de
Published: 21/01/2020
Received: 18/06/2019
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer is the most frequent cancer for women in the developed world [1]. Most of the breast cancer patients, who die, do not die as a result of the primary tumour, however, due to later developing metastases. The dissemination of the cancer occurs by tumour cells, which can leave the primary tumour and migrate through the blood and lymphatic systems, as circulating epithelial cells [2]. It is hypothesised that these cells can be detected in the blood circulation already at an early stage of the disease.
The guidelines in breast cancer recommend chemo-, radio- and hormone therapy after surgery with the aim to eliminate occult residual disease such as circulating tumour cells and micro-metastases. Nevertheless, 25% of patients suffer a relapse [3]. This indicates that the adjuvant therapy methods in these cases are insufficient.
Circulating tumour cells may be of high relevance for diagnosis and monitoring of breast cancer. The detection of circulating tumour cells is currently based on different technologies [4–9]. There are at present two main methods: physical separation due to size, deformability or other physical properties of the cells, which can be followed by polymerase chain reaction (PCR) or other molecular diagnostic methods, and immunological assays which utilise monoclonal antibodies against surface antigens of the cells.
A therapeutic method based on these properties for depletion of tumour cells does not exist so far. Methods using ‘unspecific separation’ of tumour cells (without antibodies) from the white blood cells have shown promising results in this respect. The key point of this method is a selective interaction of tumour cells and leukocytes with Carboxymethyl Dextrane (CMD) coated magnetic nanoparticles. The CMD-shell and incubation conditions (time and milieu) play an important role for this cell type-specific interaction. Under defined conditions (incubation time, plasma concentration, etc.) tumour cells have shown a more intense interaction with the CMD-coated magnetic nanoparticles than white blood cells. With the use of magnetic forces it is possible to achieve a depletion of tumour cells from majority of healthy cells.
This non-specific separation method was developed by Clement et al [10]. This research group intensively studied the differential nanoparticle interaction [10–13] and utilised super-paramagnetic nanoparticles and magnetic separation in a high magnetic field gradient (Magnetic Activated Cell Sorting (MACSR), Miltenyi).
Our approach, in contrast, provides a mild separation of cells in order to maintain the viability of the normal remaining blood cells using a new flow separation method that makes use of magnetite nanoparticles with mean size of 25 nm along with a low field gradient and an external separation column (blood bag). Using the breast cancer cell line MCF-7 and leukocytes separately we optimised the conditions for incubation and separation and then artificial mixtures of MCF-7 cells with leucocytes were tested for separation efficiency [14]. Here, the applicability of our approach to remove tumour cells from the peripheral blood was tested in 25 blood samples from breast cancer patients.
Methods and materials
Magnetic nanoparticles
Magnetite-based nanoparticles were prepared by a wet chemical precipitation method using a partial oxidation of Fe(II) salt under a constant pH of 11 at 80 °C. The resulting nanoparticles were characterised by scanning electron microscopy (SEM), X-ray diffractogram and vibrating sample magnetometer (VSM). The particles consist of magnetite with a mean size of 25 nm. The saturation magnetisation of 73 emu/g is sufficiently high for our magnetic separation method.
The biocompatible coating with CMD—essential for this non-specific method —was carried out directly after preparing the nanoparticles. We applied pH 4.5 and 45 °C for this coating process. The use of ultrasound before and after coating was necessary to reduce aggregate formation. The produced magnetofluid was characterised with photon correlations spectroscopy (PCS). The hydrodynamic diameter was approximately 180 nm and the zeta potential was −56 mV. The Fe-content was determined by titration with KMnO4 and Na2S2O3, respectively. The Fe3O4-content was ca. 60 mg/mL (titration) and the CMD-content approxymately 4.4 mg/mmol Fe (photometric).
Blood samples
Peripheral blood anticoagulated with ethylene diamine tetra acetate (EDTA) was drawn from 25 breast cancer patients with informed consent according to the Ethics Committee.
The circulating epithelial tumour cells (CETCs) were quantified before and after separation of analogous to the maintrac method [2], i.e., leukocytes containing tumour cells were prepared by erythrocyte lysis, labelled with fluorescence markers (Anti-Epithelial Cell Adhesion Molecule (EpCAM) and CD45) and analysed by a laser scanning cytometer (LSC CompuCyte, Cambridge MA, USA). We did not distinguish between the dead and living cells (no edition of propidium iodide).
Specific details were as follows: Red blood cells from 1 mL of peripheral blood (anti-coagulated with EDTA) were lysed by adding 9 mL of erythrocytes lysis solution (Qiagen, Hilden, Germany) with an exposure time of 10 minutes at room temperature. The white cell pellet was then spun down at 300×g for 10 minutes and resuspended in 500 μL PE buffer (phosphate-buffered saline (PBS) with 2 mmol/L EDTA). For fluorescence labelling, 5 μL of fluorescein-isothiocyanate- (FITC-) conjugated mouse anti-EpCAM (Miltenyi, Bergisch Gladbach, Germany) and 1 μL of phycoerythrin-(PE-) labelled anti-CD45 were added to 100 μL of cell suspension, mixed well, then incubated for 15 minutes in the dark in the refrigerator and diluted with PE to a volume of 500 μL. 50 μL (100 μL) of this cell suspension were pipetted onto the measuring area on a poly-L-lysine treated slide (Menzel Gläser, Braunschweig, Germany). The settled cells (after 10 minutes) were measured using a LSC (Compucyte Corporation, Cambridge, USA) and displayed in scattergrams and dot plots. Each EpCAM-positive event from the scattergram was visually analysed and confirmed as cells showing a green fluorescence cap in fluorescent light, shown in Figure 1.
With regard to future applications of this method as a therapeutic approach we tested ≥24 hours (day 1) stored blood samples as well as fresh blood samples (day 0). The stored blood samples were prepared —via erythrocyte lysis and fluorescence labelling—as mentioned above. The fresh blood samples could not be labelled in this way due to the masking of surface antigens. Therefore, the cells were treated with TWEEN 20 according to the method described by Hekimian [15] to demask EpCAM. 1 mL of fresh blood sample was incubated with 10 μL TWEEN 20 (Sigma Aldrich) for 5 minutes at room temperature. Thereafter the erythrocyte lysis was carried out and followed by fluorescence labelling and quantification by LSC as described above.
Magnetic separation method
Incubation conditions
The magnetic separation method was performed using 2 mL of peripheral blood from breast cancer patients.
The lysing of the erythrocytes and spinning down the white blood cells was carried out as described above. The leucocyte pellet containing the tumour cells were resuspended in 2 mL PE buffer and 2.5% plasma was added. For magnetic labelling, the cell suspension was incubated with 16 μL magnetofluid (as described above) for 10 minute at 37 °C.
Magnetic separation conditions
The magnetic separator consists of a magnetic arrangement with a blood bag inside (separation column) (Figure 2). The labelled cell suspension was gently pumped in PE buffer medium through the blood bag (3 cm3) using a modified cell pump (ismatec, Wertheim, Germany). The cell suspension, which passed the separator, was designated as negative fraction (effluent). The cell suspension, retained in the blood bag, was designated as positive fraction and was eluded from the blood bag after removing it from magnet and by massaging it. Each fraction was collected in 50-mL falcon tubes with the addition of 10-mL Dulbecco’s Modified Eagle Medium (DMEM) plus 10% fetal calf serum.
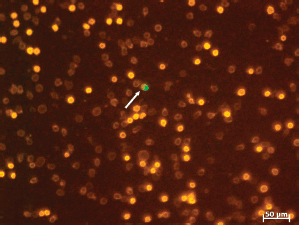
Figure 1. LSC fluorescence image of leucocytes (bright orange: lymphocytes, dim orange: granulocytes) including one tumour cell (arrow: cell with the green cap).
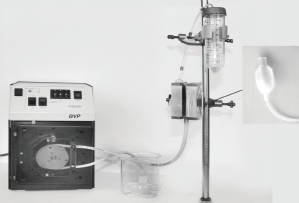
Figure 2. Experimental setup of the magnetic separation device (with cell pump, magnetic arrangement, blood bag and falcon tube for negative fraction).
Analysis of the negative and the positive fraction
The cell fractions were spun down and the cell pellets were resuspended in 1-mL PE. The cell counts of leukocytes were analysed by Cell Scepter (Merck Millipore). The CETCs in the negative fractions were quantified in analogy to the Maintrac method by labelling with fluorescence markers and analysed by LSC as mentioned above. The negative fractions of fresh blood samples had to be treated with tween (analogous to the whole fresh blood) before labelling with fluorescence markers. The reaction was stopped by adding a PE-buffer.
The vitality of leukocytes was controlled with live/dead staining (fluorescein diacetate (FDA)/GelRED). Fluorescein diacetate freely enters the intact cells and is converted by the enzymes to fluorescein intracellularly. The cytoplasm of the vital cells is stained in green. GelRed penetrates the membrane of the dying/dead cells and reacts with nucleic acid of the cell nucleus and fluoresces red. The cells were incubated with the combined staining solution of FDA/GelRED (15 μg/mL FDA and 1:10,000 dilution of GelRED stock solution) at room temperature for 3 minutes and examined under fluorescent light microscopy.
Results
Initial CETCs
Patients were numbered sequentially according to inclusion into the study (Tables 3 and 4). The enumeration of the CETCs pre-separation was correlated to the patient data (Table 1). All patients were without distant metastases.
Low, middle and high concentration of CETCs could be distinguished. Low numbers of epithelial cells (<2000 cells/mL) were observed in patients without lymph nodes involvement, including one patient (sample 15) with nodes had been removed at primary surgery and one patient with estrogen positive tumour after neoadjuvant chemotherapy (sample 25).
Patients with medium cell numbers were mainly patients with invasive ductal carcinomas apart from one with DCIS.
All triple negative (TN) invasive-ductal carcinomas (sample 3 equal 24, 19 and 20) were in the group with high numbers of circulating epithelial cells in contrast to the TN medullary carcinoma (sample 22) which had a low number of circulating epithelial cells. A neoadjuvant therapy obviously leads to a reduction of the initially high numbers in sample 3 (before neoadjuvant therapy) as compared to sample 24 (after neoadjuvant therapy).
Surprisingly, a high number of CETCs was observed in two patients with DCIS (samples 7 and 13).
Table 1. Preseparation of epithelial cell numbers of patients and correlation to tumour stage (≥24 hours blood).
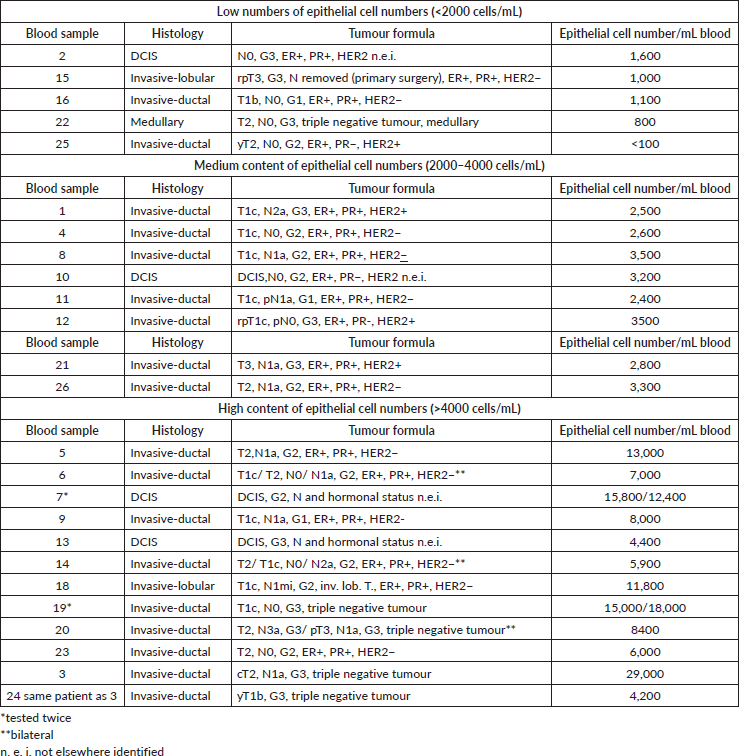
Table 2. Initial epithelial cell numbers of patients and their tumour data (fresh blood).
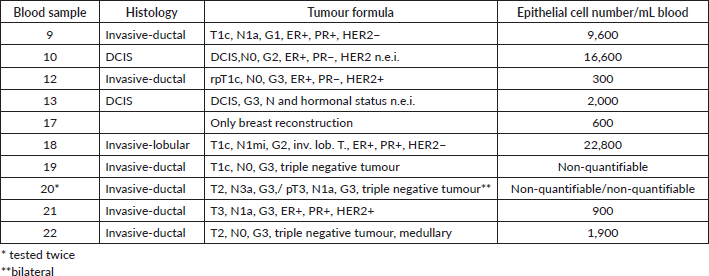
It has been shown previously (Hekimian) that the EpCAM molecule may be masked in freshly drawn blood samples but can be made accessible to the respective antibody after a short treatment of the white blood cells with tween. Ten samples (Table 2) were analysed for the number of epithelial cells after treatment of the freshly drawn samples with tween. Eight samples could be analysed but in two TN tumour tween treatment of the freshly drawn blood samples (19 and 20) resulted in unspecific dying. This made the evaluation of the number of EpCAM positive cells impossible.
Fresh blood compared to 24 hours stored blood samples
After 24 hours the TN fresh blood samples 19 and 20 were quantifiable. These patients, both, had high numbers of CETCs in the 24 hours stored samples without tween treatment indicating that these patients’ cells were especially vulnerable. Sample 20 was tested twice (day after 1st surgery and 2 weeks post 2nd surgery) with the same result.
We found a lower numbers of tumour cells with the storage (24 hours) of the blood samples in the samples 10, 18 and 22. This might, in part be due to short-lived wound healing cells (also EpCAM-positive cells) in the fresh blood samples and these will decrease during storage of the blood samples. In the samples 12, 13 and 21 the results showed an increase of CETCs after storage of the samples. TWEEN-treatment may have been insufficient here and so parts of surface antigens still remained masked.
One patient presenting for breast reconstruction after adjuvant radio-/chemotherapy 3 years ago (sample 17) was only tested in fresh blood sample.
Magnetic separation of ≥24 hours stored blood samples
Magnetic separation was performed in 18 blood samples (16 post-surgery and 2 pre-surgery) from breast cancer patients in different stages of the disease. The blood samples had been kept at room temperature for 24 hours for observation so that EpCAM on the cell surface is partly masked and becomes accessible to the antibody only after at least 24 hours of storage. The timing after surgery was chosen, because surgery is the first inevitable intervention after the diagnosis of a malignant tumour. The interval between surgery and blood collection differed between right before surgery and 5 weeks after surgery (Table 3).
Table 3. Overview of the ≥24 hours stored blood samples.
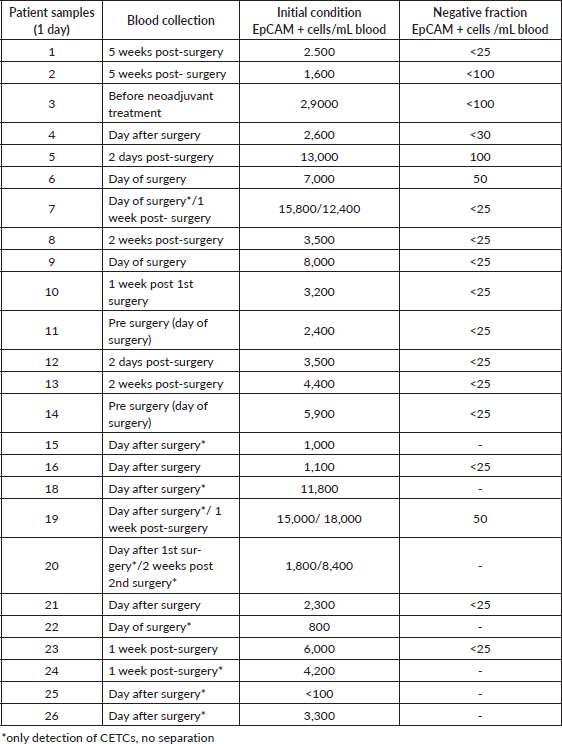
The magnetic separation method of this blood samples was carried out in the following steps: erythrocyte lysis, incubation of the cell suspension with magnetic fluid, tempering at 37 °C during incubation and followed by the magnetic separation as already described.
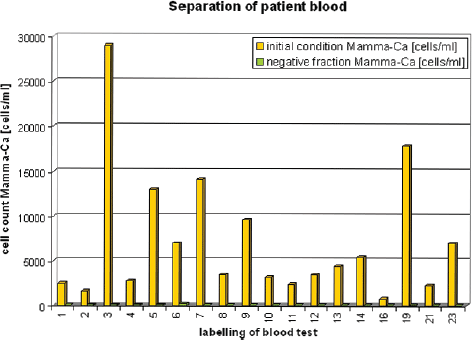
Figure 3. Stored patient blood (≥24 hours) before and after magnetic separation.
The separated cells were collected in the negative and positive fraction. Immediately after the end of the separation, the number of leukocytes in both fractions and the number of CETCs in the negative fraction were determined.
As compared to the pre-separation CETC counts—less than <3% CETCs remained in the negative fraction in all blood samples (Figure 3), with the exception of sample 2 <6% CETCs (no more accurate determination was made). Significant numbers of CETCs were only detected in the negative fractions of samples 5, 6 and 19. All the other results were from <25 to <100 cells/mL (Table 3). Thus, a very high depletion of tumour cells was achieved independent from the initial number of circulating epithelial cells in the blood samples.
Magnetic separation of fresh blood samples
For future development of our method in the direction of therapeutic method, it was necessary to test fresh blood samples, 1–8 hours after the drawing of blood. From ten of the patients fresh blood samples were analysed. The magnetic separation was carried out with eight blood samples. The intervals between surgery and blood collection and the initial number of CETCs cells of these samples are listed in Table 4. It has already been mentioned, that labelling of fresh blood samples for EpCAM-expression may not represent the true numbers present in a sample due to the masking of surface antigens. Therefore, TWEEN-treatment [15] was applied to freshly drawn samples in order to demask EpCAM. The pre-separation numbers of CETCs varied widely.
Magnetic separation of the fresh blood samples was performed in the identical way as for the 24 hours stored samples, however, without the addition of TWEEN. This approach was only necessary for the detection of EpCAM positivity in the pretreatment samples as well as the determination of CETCs in the negative fraction.
As mentioned above in two of the freshly drawn blood samples 19 and 20 the number of CETCs could not be determined. Therefore, they are not shown in Figure 4.
Magnetic separation in the fresh blood samples resulted in ≤14% of tumour cells retained in the negative fraction.
Table 4. Overview of the fresh blood samples.
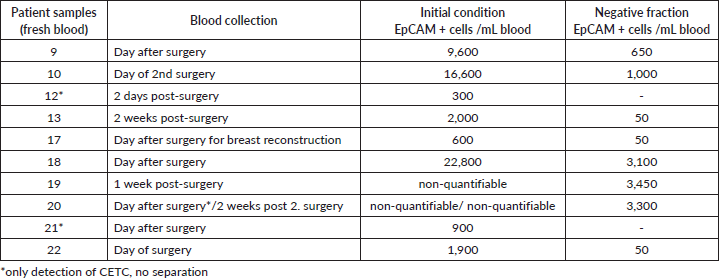
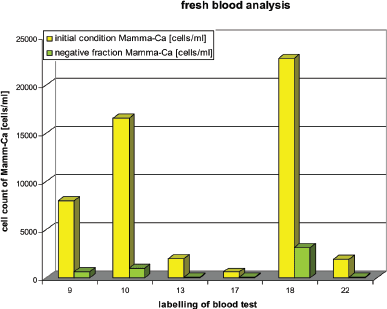
Figure 4. Fresh patient blood before and after using magnetic separation method.
Influence of the separation procedure on leukocytes
The behaviour of the normal blood cells before and after separation was also analysed with respect to quantification and vitality. The negative fraction of the ≥24 hours stored blood samples contained 10% fewer leukocytes in the negative fraction and a corresponding 10% higher loss. In the negative fraction of the fresh blood samples 56% ± 4% leukocytes were retained, whereas in the positive fraction 21% ± 7% leukocytes were retained (Figure 5). Cell loss from the separation process amounted to 23%. This result could be expected because 70% of the white blood cells are granulocytes with a short lifetime. Live/dead staining (FDA/GelRED) to test the vitality of leukocytes in the negative fraction showed a high vitality of leukocytes and only a small amount of dying/dead cells (Figure 6).
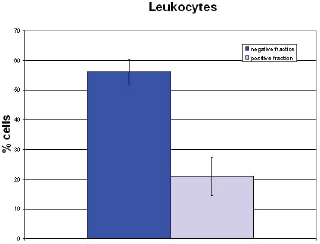
Figure 5. Cell yield of leukocytes in fresh blood samples.

Figure 6. Vital leukocytes (green) after separation.
Annexin/propidium iodide (PI) apoptosis necrosis detection kit (here not shown) showed that the lymphocytes retained a high vitality resulting in a selective enrichment of these cells in the negative fraction with only a small fraction of apoptotic/necrotic lymphocytic cells.
Discussion
EpCAM is expressed on 90% of epithelial cancer cells on the basolateral surface of the cells [15] and can be detected by anti-EpCAM antibodies. Using anti-EpCAM-FITC in analogy to the standardised maintrac method [2] we have determined CETCs in the blood of breast cancer patients.
Maintrac is a fast and sensitive quantitative method for the detection and characterisation of tumour suspected cells in the blood. Different studies have shown that the behaviour of epithelial tumour cells in the breast cancer blood can serve to predict the subsequent course of the disease [16–21]. A decrease in the number of cells during systemic therapy highly significantly correlates with the response to the therapy. On the other hand an increase in the number of circulating tumour cells indicates an increasing risk of formation of metastases and relapse.
Therefore, depletion of EpCAM-positive cells from the peripheral blood of breast cancer patients with a non-specific separation method might be able to reduce the probability of metastasis formation. It needs to be emphasised that the magnetic labelling of the cells took place without antibodies. Antibodies were only used for the quantitative analysis of the cells. We used CMD-coated magnetite-based nanoparticles (mean size Ø 25 nm) for the magnetic labelling which allowed a low-field gradient separation. It is a mild magnetic flow separation method with a blood bag—separation column—inside the permanent magnetic arrangement.
CETCs
Using the maintrac method in most of the 25 breast cancer patients blood samples were drawn around surgery (exception sample 17). In 24 samples CETCs were detected. Only in one sample (sample 25) we did not find any CETCs with our method (<100 cells/mL). In this case, the sampling occurred immediately after the end of neoadjuvant therapy.
It has been shown, that the number of CETCs changes with the interval between surgery and sampling. There is an increase right after surgery and a re-decrease until day 7 after surgery [22]. We could compare repeated sampling in the cases of sample 7, 19 and 20. With the samples 7 and 19 the number of CETCs was in the same range at the day of surgery or following day and 1 week post-surgery. But in the case of sample 20, we found a 4-fold higher number of CETCs after the second surgery as compared with that after the first.
Testing freshly drawn blood showed that the detection of CETCs is only possible with previous TWEEN-treatment before as demonstrated in the samples 1, 2, 10, 13, 17 and 18 where we could not find any CETCs without TWEEN-treatment (not shown).
Repeated testing in the same samples as freshly drawn blood and as 24 hours stored samples (samples 12, 13, 18, 19, 20, 21 and 22) showed a decrease of tumour cells with the storage in samples 18 and 22.
It is noteworthy that we detected a lot of small fully coloured EpCAM-positive cells in the fresh blood sample which were no longer detectable after the 24 hours storage period. They may represent short-lived wound healing cells which are eliminated during the storage time. In the fresh blood samples 19 (Figure 7) and 20 (two tests—following day 1.surgery 2 weeks post 2.surgery) we found unspecific dying with EpCAM precluding correct enumeration. Both samples were from triple receptor negative (TN) breast cancer patients. After storage of blood for 24 hours (e.g. Figure 8) we were able to quantify the CETCs. This interesting aspect must be verified.
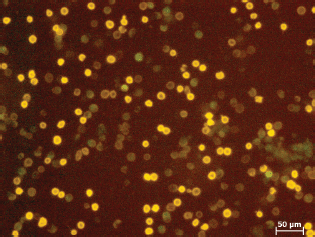
Figure 7. Sample 19 fresh blood.
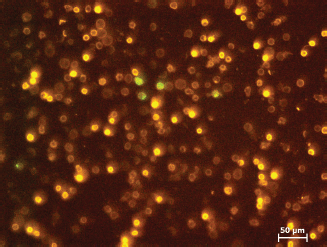
Figure 8. Sample 19 ≥24 hours stored blood.
Magnetic separation method
We achieved a high depletion of tumour cells in all the tested blood samples. This result was independent from the initial number of CETCs and tumour staging and grading. Thus, the absolute number of CETCs seems not to be critical for our separation method.
There were, however, differences in the separation of fresh blood and ≥24 hours (1 day) stored blood samples. In the 1 day stored blood samples we were able to remove >97% of CETCs by using the non-specific separation method. Whereas the depletion of tumour cells in the fresh blood samples was ≥86%. The differences in the separation of fresh blood and ≥1 day stored blood might be explained assuming that not all EpCAM-positive cells necessarily are malignant cells. Benign EpCAM-positive cells may be released into the blood during, e.g., wound healing due to surgery. These cells may be short-lived cells and disappear within 1 day. The endocytosis of magnetic nanoparticles by such cells may be slower than that of the malign cells and as a consequence they cannot be fully removed with this method. In the future, gene analysis of individual cells might be helpful to clarify the nature of such EpCAM-positive cells and future tests with fresh blood, not in relation to surgery, will be needed. Under these conditions, the situation is expected to be similar (number of CETCs) in fresh blood and 1 day stored blood.
In all our tests, we could show that the magnetic separation method developed by us may become a useful tool for the successful depletion of CETCs from the blood of breast cancer patients. Until now, the tests were performed in a low scale. We now are in the process of scaling up the method with a new blood bag (100 cm3) and a new magnetic separator using MCF-7 and the cell line SD-1 in a model system to further develop the method using a blood volume of 60 mL and optimising the separating conditions and continued our tests with cell mixtures of leukocytes (buffy coats) and MCF-7 are ongoing.
The removal of such cells with this unspecific separation method may provide a possibility to reduce the formation of metastases and offer an opportunity to reduce the number of relapses. This method exerts little mechanical stress (cell pump) to the healthy cells. The potential return of the healthy cells (in particular lymphocytes) may be very important for maintaining the immunological competence of the patients. This is also very important for the further health of the patients. The preservation of the immune system plays an exceedingly important role in the control of malignant tumours. This has been shown by the recent therapeutic success of check-point inhibitors.
Conclusion
The developed method of mild magnetic separation is suitable for the successful depletion of CETCs from the blood of breast cancer patients (ex vivo) while maintaining a large proportion of vital healthy cells. This method has a high potential for future therapeutic applications in the adjuvant therapy.
We have scaled up this method and intend to test it in animal studies.
Conflict of interest
The authors have no conflicts of interest.
Acknowledgment
We are grateful to Dr Jürgen Weisser and Birgit Beer for their expert support in working with living cells and Regine Meyer for her helpful support during separation experiments.
Funding
This work was supported by the BMWi-Programme INNO-KOM-OST MF 110033.
References
1. Latest world cancer statistics [http://globocan.iarc.fr] Date accessed: 19/12/14
2. Pachmann K, Clement HJ, and Schneider CP, et al (2005) Standardized quantification of circulating peripheral tumor cells from lung and breast cancer Clin Chem Lab Med 43 617–627 https://doi.org/10.1515/CCLM.2005.107 PMID: 16006258
3. [http://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Brustkrebs/brustkrebs_node.html] Date accessed: 19/12/14
4. Pachmann K, Camara O, and Kohlhase A, et al (2011) Assessing the efficacy of targeted therapy using circulating epithelial tumor cells (CETC): the example of SERM therapy monitoring as a unique tool to individualize therapy J Cancer Res Clin Oncol 137 821–828 https://doi.org/10.1007/s00432-010-0942-4 PMCID: 3074080
5. Paterlini-Brechot P and Benali N L (2007) Circulating tumor cells (CTC) detection: clinical impact and future directions Cancer Lett 253 180–204 https://doi.org/10.1016/j.canlet.2006.12.014 PMID: 17314005
6. Pantel K, Brakenhoff R, and Brandt B (2008) Detection, clinical relevance and specific biological properties of disseminating tumor cells Nat Rev Cancer 8 329–340 https://doi.org/10.1038/nrc2375 PMID: 18404148
7. Hoshino K, Horton AP, and Malik D, et al (2010) Microfluidic chip-based immunomagnetic detection of circulating tumor cell Conference Proceedings of the 8th International Conference on the Scientific and Chemical Applications of Magnetic Carriers (Rostock)
8. Wang S (2010) Silicon-nanowire-array surfaces for high effectively capture circulating tumor cells Conference Proceedings of the BIT's 1th Annual World Congress of Nanomedicine-2010 (Beijing)
9. Hoshino K, Chung CH, and Rajendran K, et al (2014) Single-cell PCR analysis of circulating tumor cells captured by immunomagnetic microchip Conference Proceedings of the 10th International Conference on the Scientific and Chemical Applications of Magnetic Carriers (Dresden)
10. Clement J H, Schwalbe M, and Buske N, et al (2006) Differential interaction of magnetic nanoparticles with tumor cells and peripheral blood cells J Cancer Res Clin Oncol 132 287–292 https://doi.org/10.1007/s00432-006-0076-x PMID: 16432758
11. Schwalbe M, Jörke J, and Buske N, et al (2005) Selective reduction of the interaction of magnetic nanoparticles with leukocytes and tumor cells by human plasma J Magn Magn Mater 293 433–437 https://doi.org/10.1016/j.jmmm.2005.02.037
12. Schwalbe M, Buske N, and Vetterlein M, et al (2006) The carboxymethyl dextran shell is an important modulator of magnetic nanoparticle uptake in human cells Z Phys Chem 220 125–131 https://doi.org/10.1524/zpch.2006.220.1.125
13. Schwalbe M, Pachmann K, and Höffken K, et al (2006) Improvement of separation of tumor cells from peripheral blood cells using magnetic nanoparticles J Phys Condens Matter 18 S2865–S2876 https://doi.org/10.1088/0953-8984/18/38/S22
14. Payer P, Röder M, and Madai E, et al (2010) Synthesis and Clinical Application of Magnetic Nanoparticles Posterbeitrag zur internationalen Tagung “Scientific and Chemical Applications of Magnetic Carriers” (Warnemünde, 2010)
15. Hekimian K, Stein EL, and Pachmann U, et al (2011) Demasking of epithelial cell adhesion molecule (EpCAM) on circulating epithelial tumor cells by Tween 20 treatment in breast cancer patients Clin Chem Lab Med 50 1–8 https://doi.org/10.1515/CCLM.2011.812
16. Pachmann K, Dengler R, and Lobodasch K, et al (2008) An increase in cell number at completion of therapy may develop as an indicator of early relapse J Cancer Res Clin Oncol 134 59–65 https://doi.org/10.1007/s00432-007-0248-3
17. Pachmann K, O Camara, and Kavallaris A, et al (2005) Quantification of the response of circulating epithelial cells to neoadjuvant treatment for breast cancer: a new tool for therapy monitoring Breast Cancer Res 7 R975–R979 https://doi.org/10.1186/bcr1328
18. Camara O, Jörke C, and Hammer U, et al (2008) Monitoring circulating epithelial tumor cells (CETC) to gauge therapy: in patients with disease progression after trastuzumab persisting CETC can be eliminated by combined lapatinib treatment J Cancer Res Clin Oncol https://doi.org/10.1007/s00432-008-0498-8
19. Pachmann K, Camara O, and Kroll T, et al (2011) Efficacy control of therapy using circulating epithelial tumor cells (CETC) as “Liquid Biopsy”: trastuzumab in HER2/ neu-positive breast carcinoma J Cancer Res Clin Oncol 137 1317–1327 https://doi.org/10.1007/s00432-011-1000-6 PMID: 21739182 PMCID: 3155034
20. Hekimian K, Meisezahl S, and Trompelt K, et al (2012) Epithelial cell dissemination and re adhesion analysis of factors contributing to metastasis formation in breast cancer ISRN oncology https://doi.org/10.5402/2012/601810
21. Pachmann K (2012) Die Bedeutung der im Blut zirkulierenden Tumorzellen in der Metastasierungskaskade Deutsche Zeitschrift für Onkologie 42 11–16
22. Camara O, Kavallaris A, and Nöschel H, et al (2006) Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells J Surg Oncol 4 67 https://doi.org/10.1186/1477-7819-4-67






