Precision oncology in Latin America: current situation, challenges and perspectives
Ali Calderón-Aparicio and Andrea Orue
Tumor Cell Biology Laboratory, Instituto Venezolano de Investigaciones Científicas IVIC, Centro de Microbiología, Caracas 1020A, Venezuela
Abstract
Background: Anti-cancer cytotoxic treatments like platinum-derived compounds often show low therapeutic efficacy, high-risk side effects and resistance. Hence, targeted treatments designed to attack only tumour cells avoiding these harmful side effects are highly needed in clinical practice. Due to this, precision oncology has arisen as an approach to specifically target alterations present only in cancer cells, minimising side effects for patients. It involves the use of molecular biomarkers present in each kind of tumour for diagnosis, prognosis and treatment. Since these biomarkers are specific for each cancer type, physicians use them to stratify, diagnose or take the best therapeutic options for each patient depending on the features of the specific tumour.
Aim: This review aims to describe the current situation, limitations, advantages and perspectives about precision oncology in Latin America.
Main body: For many years, many biomarkers have been used in a clinical setting in developed countries. However, in Latin American countries, their broad application has not been affordable partially due to financial and technical limitations associated with precarious health systems and poor access of low-income populations to quality health care. Furthermore, the genetic mixture in Latin American populations could generate differences in treatment responses from one population to another (pharmacoethnicity) and this should be evaluated before establishing precision therapy in particular populations. Some research groups in the region have done a lot of work in this field and these data should be taken as a starting point to establish networks oriented to finding clinically useful cancer biomarkers in Latin American populations.
Conclusion: Latin America must create policies allowing excluded populations to gain access to health systems and next generation anti-cancer drugs, i.e. high-cost targeted therapies to improve survival. Also, cancer clinical research must be oriented to establish cancer biomarkers adapted to specific populations with different ethnicity, allowing the improvement of patient outcomes.
Keywords: precision oncology, biomarkers, cancer, targeted therapy, access to health care, Latin America
Correspondence to: Andrea Orue
Email: andreaorue@gmail.com
Published: 03/04/2019
Received: 27/12/2018
Publication costs for this article were supported by the ecancer Global Foundation.
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Cancer is considered to be a collection of diseases, with their own characteristics derived from specific genetic expression and mutations located in the tissue and cells where it originates [1]. Cancer chemotherapy has always used generic cytotoxic drugs that aimed to inhibit rapid cellular proliferation, a characteristic hallmark of malignant cells. These chemotherapies, although effective at controlling malignant proliferation by inhibiting cellular division, have little precision for specific tumours and often produce high-risk side effects, i.e. development of resistance and immune suppression. Currently, anti-tumour therapies are selected and combined based on their efficacy for particular cancers [2], stratifying cancer treatments based on the tumour-specific features and supporting the concept of personalised medicine of cancer [3, 4]. The National Cancer Institute has defined personalised medicine ‘as a form of medicine that uses information about patient’s genes-proteins signature and environment to prevent, diagnose and treat diseases’ [5].
Often, the terms personalised medicine and precision medicine are used interchangeably. Traditionally, precision medicine referred to specific targeting of molecular abnormalities for diagnosis and stratification of patients who may respond to specific drugs, while personalised medicine has been referred to as the most individualised form of precision therapy, tailored uniquely for each patient [2] (Figure 1). However, there were concerns that the word ‘personalised’ could be misinterpreted to imply that treatments and preventions are being developed uniquely for each person. On the contrary, precision medicine focuses on identifying which approaches will be effective for one group of patients with similar features based on genetic, environmental and lifestyle factors which they share. Therefore, the term ‘precision medicine’ is preferred to ‘personalised medicine’ [6, 7]. In this context, precision oncology refers to the use of patient-specific molecular signatures to perform diagnosis, prognosis, treatment and prevention of cancer [3]. Physicians use these tumour-specific biomarkers to carry out diagnosis, prognosis and delivery of the most recommended treatment decisions to the right patient at the correct dose and time [8].
Many biomarkers predicting the effectiveness of particular treatments have been described for some types of cancer. These biomarkers have been extensively used in clinical practice in developed countries. However, in Latin American countries, the scenario is different, principally due to limited financial and technological resources. In this review, we aim to summarise some of the most promising biomarkers for treating and preventing cancers with high incidence and address the current situation and advances regarding their use in targeted therapies in Latin America.
Methods
Literature review
Searches of PubMed with MeSH were carried out between October 2018 and January 2019 using terms specific to precision oncology, personalised medicine, precision medicine, biomarkers, cancer, molecular targeted therapy, Latin America and access to health care, and cross-checked with the name of each country of Latin America. Electronic database searches were restricted to English and Spanish. Articles published between 2000 and 2019 were included. To calculate the number of papers studying biomarkers in Latin America, only reports from the region were included (Flowchart 1). Two independent researchers analysed the information and made the conclusions.
Main body
Biomarkers and precision oncology
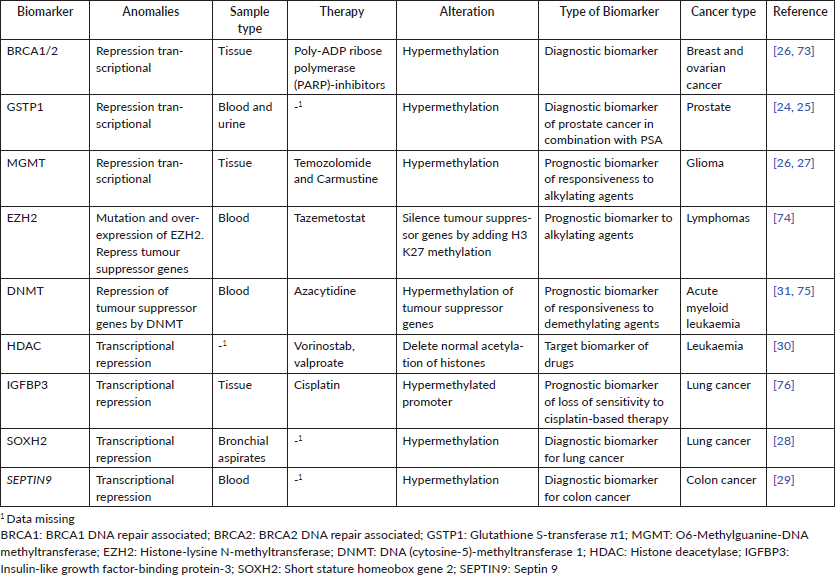
Cancer biology has revealed that each tumour accumulates a unique set of alterations, allowing it to escape from checkpoints that maintain cellular homeostasis [9]. Initially, cancer chemotherapy frequently used cytotoxic drugs that aimed to inhibit proliferative highly cells. However, this clinical approach has little precision and produces high-risk side effects [2]. Therefore, current cancer therapies have moved onto targeting specific features of tumours, improving effectiveness and survival. The high tumour-inter- and intra-heterogeneity bound to the different mutations in each tumour [10] provides a wealth of biomarkers which are used in clinic precision oncology for cancer risk assessment, determination of prognosis and selection of treatment [11, 12] (Tables 1 and 2, biomarkers more widely used in clinic worldwide). This approach has enormously increased during the last years, and diverse consortia have been created for managing all data generated [13], boosting the number of potential targets and associated biomarkers for use in clinical practice [14].
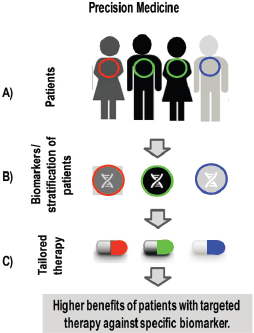
Figure 1. Steps for applying precision medicine in clinic. A) Different patients carry different biomarkers in the same type of cancer. B) Diagnosis of biomarkers allows to stratify patients depending on its specific alteration. C) Targeted-therapies are given to each individual patient to get higher benefits compared to standard therapy.
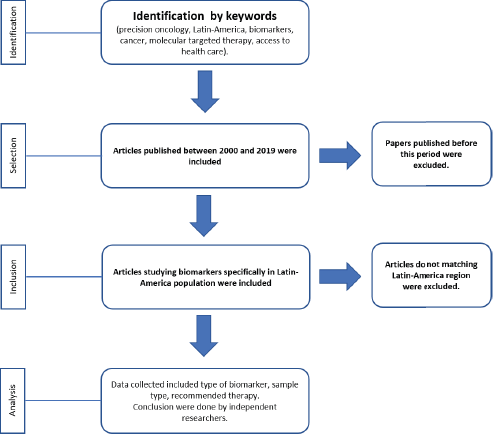
Flowchart 1. Process for selection of papers working on biomarkers for precision oncology in Latin-America.
Table 1. Relevant genetic biomarkers for precision oncology used in clinics worldwide.
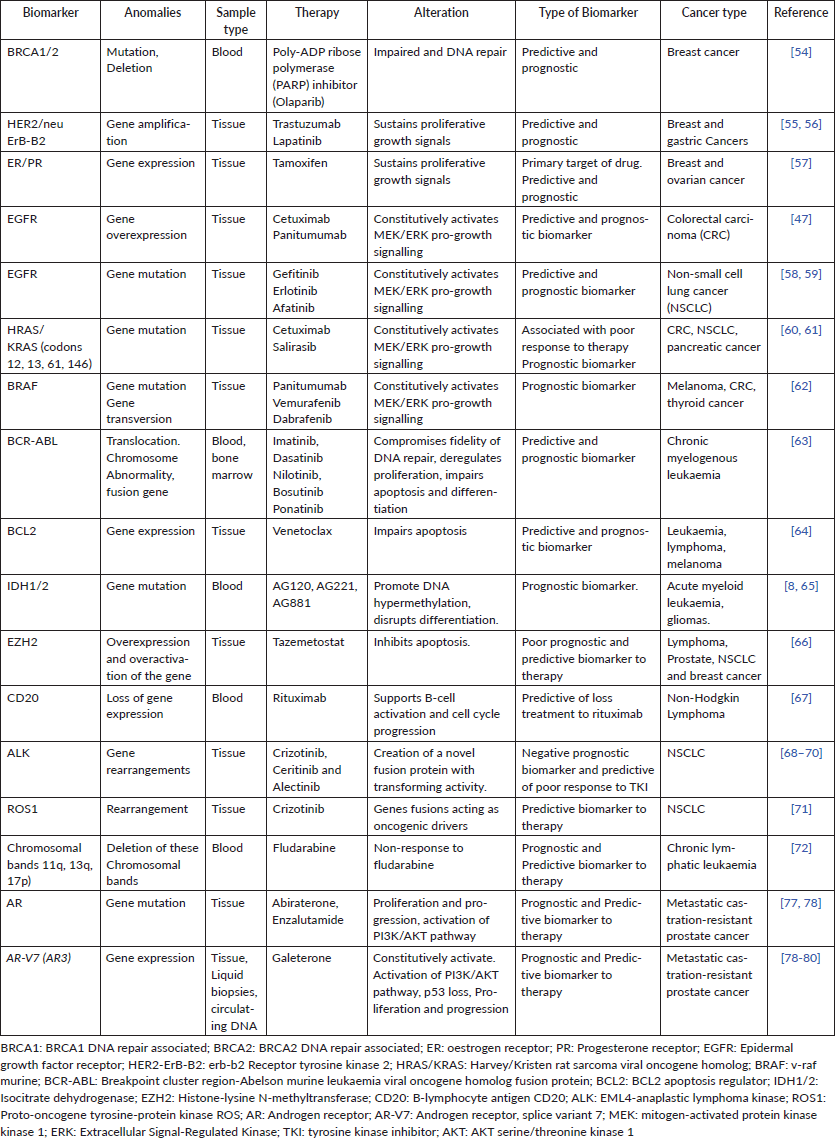
Table 2. Epigenetic biomarkers with potential for precision oncology in clinic worldwide.
Types of biomarkers
A biomarker is defined as an objectively measured and evaluated indicator of normal/pathogenic processes or pharmacologic responses to a therapeutic intervention. An ideal cancer biomarker may be useful for different purposes, easily and inexpensively measurable and should identify early-stage disease [15]. Biomarkers are used in precision oncology for the treatment of several types of cancer, i.e. leukaemia, colon cancer, breast cancer, lung cancer and melanoma [16]. These can be divided into groups: (a) Diagnostic biomarkers: used to identify and characterise the disease [17], (b) Predictive biomarkers: allowing to optimise therapeutic decisions by providing information about the likelihood of response of patients to specific treatments, i.e. alterations in Epidermal growth factor receptor (EGFR), Kristen rat sarcoma viral oncogene homolog (KRAS) and v-raf murine are critical biological determinants of therapeutic response in colon cancer [18], (c) Prognostic biomarkers: enable the monitoring of advances of anticancer therapies, the assessment of the stage of tumour and its potential malignancy, as well as the prognosis of disease remission [19] and (d) Prevention biomarkers: used to guide individual therapy by identifying patients with different outcome risks (i.e. recurrence of disease) [19]. In Table 1, we summarise some of the most widely used genetic biomarkers for precision oncology in haematological malignancies and solid tumours and its specific alteration.
Biomarkers in cancer epigenetic
Beside genetic markers, epigenetic markers have also been used for precision oncology. Epigenetic refers to modifications in the genome that do not involve changes in the nucleotide sequence [2]. These changes can be exploited in precision oncology. Different kinds of epigenetic modifications lead to the initial transformation from normal to tumour tissue [20, 21] (Table 2). In general, cancer cells display a global hypomethylation of genome accompanied by focused hypermethylation in dinucleotides CG (cytosine nucleotide followed by a guanine nucleotide), known as CpG islands, in promoters of tumour suppressor genes which become transcriptionally repressed. Conversely, global hypomethylation occurs at the same time in cancer cells and it has been linked with the expression of proto-oncogenes, genomic instability and malignant transformation [22]. Other important epigenetic changes in cancer cells rely on the expression of noncoding RNAs and alterations in patterns of histone methylation and acetylation [21] which alter the transcriptional activity of tumour-development related genes, contributing to cancer progression.
For example, the hypermethylation of Glutathione S-transferase π1 is accurately detected in ~50% of prostate cancer patients [23] and it has been proposed as a diagnostic marker jointly with prostate-specific antigen (PSA) [24]. O6-Methylguanine-DNA methyltransferase, an enzyme that promotes the elimination of alkyl-groups from the O6 position of guanine, is the most clinically advanced epigenetic marker in gliomas, where it shows a high grade of hypermethylated CpG sites compared to non-neoplastic tissue, predicting responsiveness to therapy with clinically used alkylating agents as temozolomide and carmustine [25, 26]. The genes Short stature homeobox gene 2 and SEPTIN9 have also been shown to be hypermethylated in lung and colon cancer, respectively, and their hypermethylation status has been used as a complementary assay for tumour diagnosis in patients through a blood test approved by the Food and Drug Administration [27, 28]. 5-azacytidne and Vorinostab, targeted to inhibit DNA (cytosine-5)-methyltransferase 1 and histone deacetylases, are well-known drugs affecting the chromatin structure and inducing antitumour effects by up-regulating tumour suppressor genes in leukaemia [29, 30]. Thus, the use of epigenetic biomarkers is growing rapidly; even though more studies are necessary to find new targets and gauge their effectiveness in a clinical setting.
Barriers and economical limitations for applying precision oncology in Latin America
The cancer incidence in Latin America is in general lower than in developed countries, but the mortality is significantly higher. The cancer mortality-to-incidence ratio for Latin America is 0.59, compared with 0.35 in the US [31]. This is in part due to diagnosis at later stages of the disease, as well as barriers for accessing the health system or low quality of health systems in Latin America [32]. Projections to the year 2030 show that cancer cases will increase by 35% in South America and 42% in Mexico [33].
Furthermore, the access of most low-income populations to next generation anti-cancer targeted drugs is limited, mainly due to economic conditions. As well as this, health-care systems in the region are characterised by a lack of coverage for populations excluded from social security or other public financing mechanisms [34] despite that fact that access to healthcare is recognised as a constitutional right in most Latin-American countries [35]. According to The World Health Organization (WHO), 50% of the Latin American population does not have access to high-cost drugs [36]. WHO states that high-cost antineoplastic targeted therapies are essential medicines which should be available in adequate amounts and appropriate dosage forms, at accessible prices [37] and in 2016, three high-cost antineoplastic targeted therapies (imatinib, rituximab and trastuzumab) have been added to the WHO’s Model List of Essential Medicines [33, 37]. Unfortunately, healthcare coverage is not the rule in Latin American countries, and even in those regions where the entitlement to oncology services is guaranteed by law, it is not accompanied by the necessary resources [38]. It means that governments must finance these drugs, allowing people to use them according to the recommended protocols.
Regrettably, anticancer drugs have a higher price in many low- and middle-income countries when compared with higher income nations [39]. A study comparing prices of eight high-cost cancer-targeted therapies found that the United Kingdom paid less for these drugs than Argentina, Brazil, Paraguay and Uruguay. For example, these drugs in the United Kingdom cost 29%–75% less than they cost in Argentina [40]. Another study related to the cost-effectiveness of trastuzumab in Latin America found that, as currently priced, it is not cost-effective in Latin America using the WHO threshold, and to become cost-effective, the price needs to drop between 69.6% and 94.9% [40].
Although some countries in the region have programmes to provide high-cost medicines, e.g. Universal Assurance Program for Explicit Guarantees (AUGE, Chile), Costa Rican Social Security Fund [37], Badan (Drug Bank, Venezuela) and Venezuelan Institute of Social Security (IVSS, Venezuela) nevertheless, these programmes should be continually reviewed, strongly strengthened and continuously updated to guarantee the treatment of the population. In the case of the program IVSS from Venezuela, currently there is a shortage of drugs because of the financial limitations in the country, so the reactivation and development of strategies to improve the distribution of antineoplastic therapies among the population is urgently needed.
On average, the percentage of Latin America’s Gross Domestic Product devoted to health is 7.7% compared with 18% in the United States (ranging from less than 5% in Venezuela and Peru to more than 10% in Costa Rica and Cuba). The overall mean expenditure per new cancer patient in Latin-America is US $7.92 compared with US $183 and US $460 spent by the United Kingdom and the United States, respectively. The overall cost of cancer care has been calculated to represent 0.12% of gross national income per capita in South-America versus 0.51% and 1.02% in the United Kingdom and the United States, respectively [36]. These statistics highlight the striking inequity and shortage of resources for cancer care and control in the region [33]. Thus, programmes allowing access of cancer patients to high-cost drugs for precision oncology should be developed to improve survival in Latin America.
Challenges for implementation of precision oncology in Latin America: much to be done
Characterising the frequency of alterations of cancer-predisposing genes in different types of tumours in Latin America is a first step towards providing useful biomarkers and a precision approach for this disease. The overall cancer profiles regarding Latin American countries are abruptly different because of the genetic mixture between several ethnic groups and different lifestyles [31, 41]. This genetic mixture is reflected in differences in treatment responses between different populations (pharmacoethnicity) [42]. Therefore, from the point of view of public health, diagnostic-therapeutic strategies should be adapted to each population and take into consideration the relationship between ethnicity and types of biomarkers in each population [32]. In some Latin American countries, a wide spectrum of mutations and polymorphisms has been identified in several oncogenes. However, it is necessary to perform a deeper analysis in different Latin American populations to pursue the best biomarkers and therapeutic options for each population. Moreover, oncologists, physicians and all care providers involved in cancer screening, diagnosis and treatment need up-to-date training on how to integrate genomic and molecular data into clinical practice. This represents a great challenge, principally due to large disparities in the level of research among Latin American countries, mainly attributed to funding.
In general, Latin America has a low level of investment in research and development compared to developed countries [43]. Excluding Brazil, Chile provides the most public funds for clinical studies in South America, followed by Argentina, with Bolivia, Paraguay and Uruguay providing very little [44]. Figure 2 shows the differences in the number of studies related to some biomarkers used for precision oncology in Latin America. Here, Brazil appears to be the highest contributor for all the markers evaluated. This correlates with the level of expenditure for clinical research between countries in the region since Brazil has the highest budget for research in Latin America. This highlights the importance of the level of funding for clinical research to establish adequate biomarkers for precision oncology in the region.
Furthermore, 65% of all clinical trials in Latin America are sponsored by the pharmaceutical industry (e.g. Mexico, Argentina) [44], which means that Latin American clinical research is highly dependent on funding from these pharmaceutical consortia. Furthermore, public and private hospitals are not adapted to perform clinical research, and the time length for regulatory approval for clinical trial applications is one of the longest in the world, which limits the interest of pharmaceutical companies to conduct clinical trials in Latin America [43].
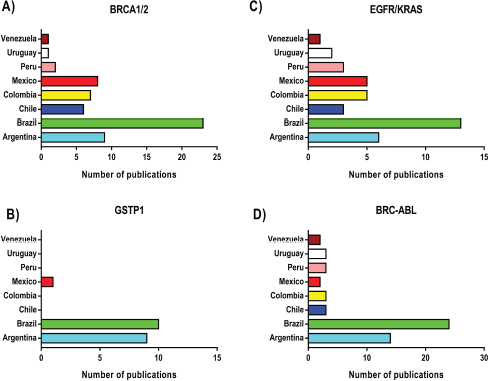
Figure 2. Number of publications development in Latin-America based on use of Biomarkers. These publications correspond to the number of studies published in journals indexed in the PubMed database, between the periods 2000 and 2019. As shown, Brazil does the highest numbers of studies in the field.
Another challenge to implementing precision oncology is to overcome the lack of specialised oncologists and diagnoses in rural areas or remote from big cities. The low number of cancer specialists results in an overwhelming workload, as well as a lack of time or interest in clinical research. Besides, there is a lack of educational programmes to motivate health professionals to participate in cancer clinical research, and often these practitioners do not have the necessary training to do this research. In a similar way, the precarious socioeconomic conditions, such as low income or lack of health insurance coverage, limit the access of certain ethnic/racial groups to counselling and genetic testing. For example, the indigenous populations in Latin America have poor health outcomes compared with their non-indigenous counterparts [45].
These issues greatly restrict the development of cancer research in Latin American countries. They highlight the need to develop policies to get patients access to low-cost cancer treatments and to cancer specialists’ care in public centres. These financial constraints are the major challenges for governments and their health systems [33].
Various groups have been working to strengthen the clinical research in the region, such as the Latin American Cooperative Oncology Group, US-Latin-America Cancer Research Network (LACRN), Latin American Federation of Cancer Societies and Latin American Consortium for Lung Cancer Research (CLICaP). CLICaP has developed studies in lung cancer in which more than nine countries in the region participated, detecting genomics differences between populations for mutations in oncogenes: EGFR, KRAS, EML4-anaplastic lymphoma kinase, Proto-oncogene tyrosine-protein kinase ROS, B-cell lymphoma 2 (BCL-2)-like 11 (BIM)-deletion and their differences in response to targeted agents [46–48]. For example, an important heterogeneity has been observed in EGFR and KRAS mutations between Latin American populations [45, 46]. The mutation frequency in EGFR was 26.4% but it highly varies according to the population. Notably, the EGFR mutation frequency was found to be higher in Peru and lower in Argentina, which could be attributed to differences in ethnicity. Traditionally, Peru has been a destination of Asian migration, where the EGFR mutations occur in 30%–50%, contrary to the Argentinean population which has a significant European ancestry, whose mutation rate is 8%–13% [46]. Besides this, the frequency of KRAS mutations was lower (14%) than for EGFR because both mutations are mutually exclusive [46].
The South American Office for Research and Treatment of Cancer in Southern Brazil has been working with semi-purified plant extracts isolated from South American medicinal plants for potential use as anticancer treatments [36]. As well as this, LACRN, whose aim is to strengthen collaborative research efforts among the participating countries (Argentina, Brazil, Chile, Mexico and Uruguay), is advancing into translational cancer research, including research institutions, hospitals and clinical scientific investigators [49].
Moreover, the Ibero-American network of Pharmacogenetics and Pharmacogenomics seeks to promote precision medicine and research networks in Latin America and the Iberian Peninsula, leading to the inclusion of Latin American populations to analyse their ethnicity, genotype and/or metabolic phenotype in response to therapy (i.e. the MESTIFAR project) [50]. All these efforts should be taken as a starting point to begin establishing precision oncology practices in the clinic.
Perspectives of precision oncology in Latin America
Currently, the access to up-to-date treatments of cancer in Latin America is complicated by the series of barriers previously mentioned, and the approach to precision oncology is challenging and mainly limited to private practice. In this context, the implementation of not-for-profit programmes for precision oncology is necessary, which should be granted by governments. These programmes should include certain key features: in-depth genomic analysis of the patient’s tumour, interpretation of genomic test results and programmes of access to high-cost drugs for low-income patients to help them to get any molecularly targeted therapy that is prescribed. These programmes should also focus on developing strategies to create networks evolving translational research [51].
As previously mentioned, the incorporation of molecular tests in the coverage of private and public insurance is necessary. A good example is the Oncosalud program, the largest pre-paid system of Peru, which offers free testing with Oncotype DX of breast tumours for their affiliates [52].
On the other hand, the use of liquid biopsies (samples obtained from biological fluids, such as blood, cerebrospinal fluid, semen and others with the aim to detect and evaluate circulating tumour cells, circulating-free DNA, exosomes and other molecules) can aid appropriated patient stratification for targeted therapy and provide important prognostic information. These types of samples have had a great impact on early diagnosis of Non-small cell lung cancer and breast cancer, as well as in other malignant tumours. Unfortunately, its incorporation in clinical practice in Latin America is currently limited [52].
Finally, the education of physicians and healthcare workers must be continually improved to get them prepared for the age of genomic medicine, introducing them to computational methods of genomic analysis in oncology precision [53] in order to obtain highly trained healthcare professionals for precision oncology.
Conclusion
Latin America is making important efforts in cancer research despite the great deficiencies in funding and disparities in research between countries. Improvements in cancer treatment, including the development of less toxic targeted therapies, improve the quality of life of patients. Unfortunately, these developments are accompanied by increases in cancer treatment costs, making access to these treatments difficult for low-income populations. Additionally, the success of precision therapies requires accurate diagnosis and specialised oncologists. Unfortunately, the lack of specialised doctors in remote or rural areas far from cities restricts access to healthcare for a significant number of people. Therefore, the application of policies that guarantee precise diagnosis, specialised oncology care in public centres and access to treatments at low-costs that allow the administration of cancer therapy in a large proportion of the population are all imperative for the region. Also, Latin America needs an increase of specialised professionals for cancer patient care and updated equipment for cancer treatments. Furthermore, the strengthening and funding of Latin American cancer research centres and networks will also provide good opportunities for the development of cancer research in our countries adapted to the specific features of our populations. Thus, the survival and prognosis of cancer patients in our region could be much improved.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial and nonfinancial relationships that could be construed as a potential conflict of interest.
Funding statement
This work was financed in part by the Venezuelan Institute for Scientific Research, project Biomarkers and Cancer. The funders did not have a role in the preparation of the manuscript.
Acknowledgments
The authors would like to thank Manuel Rieber, PhD for helpful discussions; Diana Rosentul, PhD and Humberto de Vitto, PhD for revision of the manuscript and Alejandro Cornejo for the assistance in graphic design.
References
1. Le Tourneau C, Delord JP, and Goncalves A, et al (2015) Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial Lancet Oncol 16 1324–1334 https://doi.org/10.1016/S1470-2045(15)00188-6 PMID: 26342236
2. Coyle K, Boudreau J, and Marcato P (2017) Genetic mutations and epigenetic modifications: driving cancer and informing precision medicine BioMed Res Inter 2017 9620870 https://doi.org/10.1155/2017/9620870
3. Ciriello G, Miller ML, and Aksoy BA, et al (2013) Emerging landscape of oncogenic signatures across human cancers Nat Genet 45 1127–1133 https://doi.org/10.1038/ng.2762 PMID: 24071851 PMCID: 4320046
4. West HJ (2016) Can we define and reach precise goals for precision medicine in cancer care? J Clin Oncol 34 3595–3596 https://doi.org/10.1200/JCO.2016.68.8226 PMID: 27480152
5. Kalia M (2015) Biomarkers for personalized oncology: recent advances and future challenges Metabolism 64 16–21 https://doi.org/10.1016/j.metabol.2014.10.027
6. National Research Council (US). Committee on a framework for developing a new taxonomy of disease (2011) Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease (Washington, DC: The National Academies Press)
7. Yates LR, Seoane J, and Le Tourneau C, et al (2017) The European society for medical oncology (ESMO) precision medicine glossary Ann Oncol 29 30–35 https://doi.org/10.1093/annonc/mdx707 PMID: 29140430
8. Schwartzberg L, Kim ES, and Liu D, et al (2017) Precision oncology: who, how, what, when, and when not? Am Soc Clin Oncol Educ Book 37 160–169 https://doi.org/10.14694/EDBK_174176 PMID: 28561651
9. Mansur MR, Abraham BJ, and Anders L, et al (2014) Oncogene regulation. An oncogenic super enhancer formed through somatic mutation of a noncoding intergenic element Science 346 1373–1372 https://doi.org/10.1126/science.1259037
10. Allison KH and Sledge GW (2014) Heterogeneity and cancer Oncol 28 772–778
11. Bettaieb A, Paul C, and Plenchette S, et al (2017) Precision medicine in breast cancer: reality or utopia? J Transl Med 15 139 https://doi.org/10.1186/s12967-017-1239-z PMID: 28623955 PMCID: 5474301
12. Pasche B and Absher D (2011) Whole-genome sequencing: a step closer to personalized medicine JAMA 305 1596–1597 https://doi.org/10.1001/jama.2011.484 PMID: 21505140
13. Boehm JS and Hahn WC (2011) Towards systematic functional characterization of cancer genomes Nat Rev Genet 12 487–498 https://doi.org/10.1038/nrg3013 PMID: 21681210
14. Moran S, Martinez-Cardús A, and Boussios S, et al (2017) Precision medicine based on epigenomics: the paradigm of carcinoma of unknown primary Nat Rev Oncol 14 682–694 https://doi.org/10.1038/nrclinonc.2017.97
15. Thomas M and Marcato P (2018) Epigenetic modifications as biomarkers of tumor development, therapy response, and recurrence across the cancer care continuum Cancers 1 10–14
16. Grullich C and Von Kalle C (2012) Recent developments and future perspectives of personalized oncology Onkologie 35 4–7 https://doi.org/10.1159/000334825 PMID: 22286581
17. Blay JY, Lacombe D, and Meunier F, et al (2012) Personalized medicine in oncology: questions for the next 20 years Lancet 13 448–449 https://doi.org/10.1016/S1470-2045(12)70156-0
18. Nalejska E, Maczynska E, and Lewandowska MA (2014) Prognostic and predictive biomarkers: tools in personalized oncology Mol Diagn Ther 18 273–284 https://doi.org/10.1007/s40291-013-0077-9 PMID: 24385403 PMCID: 4031398
19. Kalia M (2013) Personalized oncology: recent advances and future challenges Metabolism Clin Exp 62 11–14 https://doi.org/10.1016/j.metabol.2012.08.016
20. Chung C and Ma H (2017) Driving toward precision medicine for acute leukemias: are we there yet? Pharmacotherapy 37 1052–1072 https://doi.org/10.1002/phar.1977 PMID: 28654205
21. Esteller M (2007) Cancer epigenomics: DNA methylomes and histone-modification maps Nat Rev Genet 8 286–298 https://doi.org/10.1038/nrg2005 PMID: 17339880
22. Mari-Alexandre J, Diaz-Lagares A, and Villalba M, et al (2017) Translating cancer epigenomics into the clinic: focus on lung cancer Transl Res 189 76–92 https://doi.org/10.1016/j.trsl.2017.05.008 PMID: 28644958
23. Hoque M, Topaloglu O, and Begum S, et al (2005) Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects J Clin Oncol 23 6569–6575 https://doi.org/10.1200/JCO.2005.07.009 PMID: 16170165
24. Sunami E, Shinozaki M, and Higano C, et al (2009) Multimarker circulating DNA assay for assessing blood of prostate cancer patients Clin Chem 55 559–567 https://doi.org/10.1373/clinchem.2008.108498 PMID: 19131636
25. Esteller M, Garcia-Foncillas J, and Andion E, et al (2000) Inactivation of the DNA repair gene MGMT and the clinical response of gliomas to alkylating agents N Engl J Med 343 1350–1354 https://doi.org/10.1056/NEJM200011093431901 PMID: 11070098
26. Xie H, Tubbs R, and Yang B (2015) Detection of MGMT promoter methylation in glioblastoma using pyrosequencing Int J Clin Exp Pathol 8 636–642 PMID: 25755756 PMCID: 4348884
27. Ilse P, Biesterfeld S, and Pomjanski N, et al (2014) Analysis of SHOX2 methylation as an aid to cytology in lung cancer diagnosis Cancer Genomics Proteomics 11 251–258 PMID: 25331797
28. Semaan A, van Ellen A, and Meller S, et al (2016) SEPT9 and SHOX2 DNA methylation status and its utility in the diagnosis of colonic adenomas and colorectal adenocarcinomas Clin Epigenetics 8 100 https://doi.org/10.1186/s13148-016-0267-5 PMCID: 5028994
29. Mann BS, Johnson JR, and Cohen MH, et al (2007) FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma Oncologist 12 1247–1252 https://doi.org/10.1634/theoncologist.12-10-1247 PMID: 17962618
30. Musolino C, Sant’Antonio E, and Penna G, et al (2010) Epigenetic therapy in myelodysplastic syndromes Eur J Haematol 84 463–473 https://doi.org/10.1111/j.1600-0609.2010.01433.x PMID: 20192987
31. Forman D and Sierra M (2016) Cancer in central and South America: introduction Cancer Epidemiol 44 3–10 https://doi.org/10.1016/j.canep.2016.04.008
32. Lopez-Cortez A, Guerrero S, and Redal MA, et al (2017) State of art of cancer pharmacogenomics in Latin American populations Int J Mol Sci 18 639 https://doi.org/10.3390/ijms18060639
33. Ruiz R, Strasser-Weippl K, and Touya D, et al (2017) Improving access to high-cost cancer drugs in Latin America: much to be done Cancer 123 1313–1323 https://doi.org/10.1002/cncr.30549 PMID: 28182258
34. Goss P, Lee B, and Badovinac-Crnjevic T, et al (2013) Planning cancer control in Latin America and the Caribbean Lancet Oncol 14 391–436 https://doi.org/10.1016/S1470-2045(13)70048-2 PMID: 23628188
35. Marın G and Polach MA (2011) Medicamentos de alto costo: análisis y propuestas para los países del Mercosur [in Spanish] Rev Panam Salud Pública 30 167–176
36. Pan American Health Organization [https://www.paho.org/hq/index.php?option=com_content&view=article&id=2149%3A2008-el-acceso-medicamentos-alto-costo-americas&catid=1266%3Amedicines-access-innovation&Itemid=1178&lang=en] Date accessed: 30/6/18
37. World Health Organization WHO Model list of essential medicines [http://www.who.int/medicines/publications/essentialmedicines/en/.2017] Date accessed: 29/12/17
38. Barrios C, Reinert T, and Werutsky G (2019) Access to high-cost drugs for advanced breast cancer in Latin America, particularly trastuzumab Ecancermedicalscience 13 898 https://doi.org/10.3332/ecancer.2019.898 PMID: 30792815 PMCID: 6372298
39. Morel CM, McGuire A, and Mossialos E (2011) The level of income appears to have no consistent bearing on pharmaceutical prices across countries Health Affairs 30 1545–1552 https://doi.org/10.1377/hlthaff.2010.0317 PMID: 21821572
40. Pichon-Riviere A, Garay OU, and Augustovski F, et al (2015) Implications of global pricing policies on access to innovative drugs: the case of trastuzumab in seven Latin American countries Int J Technol Assess Health Care 31 2–11 https://doi.org/10.1017/S0266462315000094 PMID: 25989703
41. Avena S, Via M, and Ziv E, et al (2012) Heterogeneity in genetic admixture across different regions of Argentina PLoS One 7 e34695 https://doi.org/10.1371/journal.pone.0034695 PMID: 22506044 PMCID: 3323559
42. O’Donnell PH and Dolan ME (2009) Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy Clin Cancer Res 15 4806–4814 https://doi.org/10.1158/1078-0432.CCR-09-0344
43. Rolfo C, Caglevic C, and Bretel D, et al (2016) Cancer clinical research in Latin America: current situation and opportunities expert opinion from the first ESMO workshop on clinical trials, Lima ESMO 1(4) e000055 https://doi.org/10.1136/esmoopen-2016-000055
44. Montenegro RA and Stephens C (2006) Indigenous health in Latin America and the Caribbean Lancet 367 1859–1869 https://doi.org/10.1016/S0140-6736(06)68808-9 PMID: 16753489
45. Maceira D, Paraje G, and Aramayo F, et al (2010) Financiamiento público de la investigación en salud en cinco países de América Latina Rev Panam Salud Publica 27 442–451 PMID: 20721444
46. Arrieta O, Cardona AF, and Federico Bramuglia G, et al (2011) Genotyping non-small cell lung cancer (NSCLC) in Latin America J Thorac Oncol 6 1955–1959 https://doi.org/10.1097/JTO.0b013e31822f655f PMID: 22005474
47. Arrieta O, Cardona AF, and Martín C, et al (2015) Updated frequency of EGFR and KRAS mutations in nonsmall-cell Lung cancer in Latin America: the Latin-American consortium for the investigation of Lung cancer (CLICaP) J Thorac Oncol 10 838–843 https://doi.org/10.1097/JTO.0000000000000481 PMID: 25634006
48. Cardona AF, Rojas L, and Wills B, et al (2016) BIM deletion polymorphisms in Hispanic patients with non-small cell lung cancer carriers of EGFR mutations Oncotarget 7 68933–68942 https://doi.org/10.18632/oncotarget.12112 PMID: 27926478 PMCID: 5356601
49. Llera AS, Podhajcer OL, and Breitenbach MM, et al (2015) Translational cancer research comes of age in Latin America Sci Transl Med 7(319) 319–350
50. Sosa-Macías M, Teran E, and Waters W, et al (2016) Pharmacogenetics and ethnicity: relevance for clinical implementation, clinical trials, pharmacovigilance and drug regulation in Latin America Pharmacogenomics 17 2016–2153 https://doi.org/10.2217/pgs-2016-0153
51. Nadauld L, Ford J, and Pritchard D, et al (2018) Strategies for clinical implementation: precision oncology at three distinct institutions Health affairs 37 (5) 751–756 https://doi.org/10.1377/hlthaff.2017.1575 PMID: 29733728
52. Pinto J, Saravia C, and Flores C, et al (2019) Precision medicine for locally advanced breast cancer: frontiers and challenges in Latin America ecancermedicalscience 13 896 https://doi.org/10.3332/ecancer.2019.896 PMID: 30792813 PMCID: 6372295
53. Mc Grath S and Ghersi D (2016) Building towards precision medicine: empowering medical professionals for the next revolution BMC Med Genomics 9(1) 23 https://doi.org/10.1186/s12920-016-0183-8
54. Lee JM, Hays JL, and Annunziata CM, et al (2014) Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses J Natl Cancer Inst https://doi.org/10.1093/jnci/dju089
55. Bedard PL, de Azambuja E, and Cardoso F (2009) Beyond trastuzumab: overcoming resistance to targeted HER-2 therapy in breast cancer Curr Cancer Drug Targets 9 148–162 https://doi.org/10.2174/156800909787581024 PMID: 19275756
56. Jácome A, Coutinho A, and Lima E, et al (2016) Personalized medicine in gastric cancer: where are we and where are we going? World J Gastroenterol 22(3) 1160–1171 https://doi.org/10.3748/wjg.v22.i3.1160 PMID: 26811654 PMCID: 4716027
57. Ravdin PM, Green S, and Dorr TM, et al (1992) Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study J Clin Oncol 10 1284–1291 https://doi.org/10.1200/JCO.1992.10.8.1284 PMID: 1634918
58. Mitsudomi T, Morita S, and Yatabe Y, et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial Lancet Oncol 11 121–128 https://doi.org/10.1016/S1470-2045(09)70364-X
59. Rosell R, Carcereny E, and Gervais R, et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial Lancet Oncol 13 239–246 https://doi.org/10.1016/S1470-2045(11)70393-X PMID: 22285168
60. Naidoo J and Drilon A (2016) KRAS-mutant Lung cancers in the Era of Targeted Therapy Adv Exp Med Biol 893 155–178 https://doi.org/10.1007/978-3-319-24223-1_8
61. Gong J, Cho M, and Fakih M (2016) RAS and BRAF in metastatic colorectal cancer management J Gastrointest Oncol 7 687–704 https://doi.org/10.21037/jgo.2016.06.12 PMID: 27747083 PMCID: 5056249
62. Banavath HN, Sharma OP, and Kumar MS, et al (2014) Identification of novel tyrosine kinase inhibitors for drug resistant T315I mutant BCR-ABL: a virtual screening and molecular dynamics simulations study Sci Rep 4 6948 https://doi.org/10.1038/srep06948 PMID: 25382104 PMCID: 4225644
63. Stone A, Cowley MJ, and Valdes-Mora F, et al (2013) BCL-2 hypermethylation is a potential biomarker of sensitivity to antimitotic chemotherapy in endocrine-resistant breast cancer Mol Cancer Ther 12 1874–1885 https://doi.org/10.1158/1535-7163.MCT-13-0012 PMID: 23861345
64. Dang L, White D, and Gross S, et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate Nature 462 739–744 https://doi.org/10.1038/nature08617 PMID: 19935646 PMCID: 2818760
65. Mukasa A, Takayanagi S, and Saito K, et al (2012) Significance of IDH mutations varies with tumor histology, grade, and genetics in Japanese glioma patients Cancer Sci 103 587–592 https://doi.org/10.1111/j.1349-7006.2011.02175.x
66. Takawa M, Masuda K, and Kunizaki M, et al (2011) Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker Cancer Sci 102 1298–1305
67. Katchi T and Liu D (2017) Diagnosis and treatment of CD20 negative B cell lymphomas Biomark Res 5 5 https://doi.org/10.1186/s40364-017-0088-5 PMID: 28191314 PMCID: 5297138
68. Hayashi H, Okamoto I, and Kimura H, et al (2012) Clinical outcomes of thoracic radiotherapy for locally advanced NSCLC with EGFR mutations or EML4-ALK rearrangement Antican Res 32 4533–4538
69. Yang P, Kulig K, and Boland JM, et al (2012) Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma J Thorac Oncol 7 90–97 https://doi.org/10.1097/JTO.0b013e31823c5c32
70. Koh Y, Kim DW, and Kim TM, et al (2011) Clinicopathologic characteristics and outcomes of patients with anaplastic lymphoma kinase-positive advanced pulmonary adenocarcinoma: suggestion for an effective screening strategy for these tumors J Thorac Oncol 6 905–912 https://doi.org/10.1097/JTO.0b013e3182111461 PMID: 21358343
71. Davies KD, Le AT, and Theodoro MF, et al (2012) Identifying and targeting ROS1 gene fusions in non-small cell lung cancer Clin Cancer Res 18 4570–4579 https://doi.org/10.1158/1078-0432.CCR-12-0550 PMID: 22919003 PMCID: 3703205
72. Döhner H, Stilgenbauer S, and Benner A, et al (2000) Genomic aberrations and survival in chronic lymphocytic leukemia N Engl J Med 343 1910–1916 https://doi.org/10.1056/NEJM200012283432602
73. Vos S, Moelans CB, and van Diest PJ (2017) BRCA promoter methylation in sporadic versus BRCA germline mutation-related breast cancers Breast Cancer Res 31 64 https://doi.org/10.1186/s13058-017-0856-z
74. McCabe M, Ott H, and Gangi J, et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations Nature 492 108–112 https://doi.org/10.1038/nature11606 PMID: 23051747
75. Aggerholm A, Holm MS, and Guldberg P, et al (2006) Promoter hypermethylation of p15INK4B, HIC1, CDH1, and ER is frequent in myelodysplastic syndrome and predicts poor prognosis in early-stage patients Eur J Haematol 76 23–32 https://doi.org/10.1111/j.1600-0609.2005.00559.x
76. Ibanez de Caceres I, Cortes-Sempere M, and Moratilla C, et al (2010) IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer Oncogene 29 1681–1690 https://doi.org/10.1038/onc.2009.454
77. Balk SP (2002) Androgen receptor as a target in androgen-independent prostate cancer Urology 60 132–138 https://doi.org/10.1016/S0090-4295(02)01593-5 PMID: 12231070
78. Ryan CJ and Tindall DJ (2011) Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically J Clin Oncol 29 3651–3658 https://doi.org/10.1200/JCO.2011.35.2005 PMID: 21859989
79. Nakazawa M, Antonarakis ES, and Luo J (2014) Androgen receptor splice variants in the era of enzalutamide and abiraterone Horm Cancer 5 265–273 https://doi.org/10.1007/s12672-014-0190-1 PMID: 25048254 PMCID: 4167475
80. Antonarakis ES, Lu C, and Wang H, et al (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer N Engl J Med 371 1028–1038 https://doi.org/10.1056/NEJMoa1315815 PMID: 25184630 PMCID: 4201502






