Intraoperative radiotherapy: review of techniques and results
Avinash Pilar, Meetakshi Gupta, Sarbani Ghosh Laskar and Siddhartha Laskar
Department of Radiation Oncology, Tata Memorial Hospital, Dr Ernest Borges’ Marg, Parel, Mumbai, MS, India 400012
Correspondence to: Siddhartha Laskar. Email: laskars2000@yahoo.com
Abstract
Intraoperative radiotherapy (IORT) is a technique that involves precise delivery of a large dose of ionising radiation to the tumour or tumour bed during surgery. Direct visualisation of the tumour bed and ability to space out the normal tissues from the tumour bed allows maximisation of the dose to the tumour while minimising the dose to normal tissues. This results in an improved therapeutic ratio with IORT. Although it was introduced in the 1960s, it has seen a resurgence of popularity with the introduction of self-shielding mobile linear accelerators and low-kV IORT devices, which by eliminating the logistical issues of transport of the patient during surgery for radiotherapy or building a shielded operating room, has enabled its wider use in the community.
Electrons, low-kV X-rays and HDR brachytherapy are all different methods of IORT in current clinical use. Each method has its own unique set of advantages and disadvantages, its own set of indications where one may be better suited than the other, and each requires a specific kind of expertise.
IORT has demonstrated its efficacy in a wide variety of intra-abdominal tumours, recurrent colorectal cancers, recurrent gynaecological cancers, and soft-tissue tumours. Recently, it has emerged as an attractive treatment option for selected, early-stage breast cancer, owing to the ability to complete the entire course of radiotherapy during surgery. IORT has been used in a multitude of roles across these sites, for dose escalation (retroperitoneal sarcoma), EBRT dose de-escalation (paediatric tumours), as sole radiation modality (early breast cancers) and as a re-irradiation modality (recurrent rectal and gynaecological cancers).
This article aims to provide a review of the rationale, techniques, and outcomes for IORT across different sites relevant to current clinical practice.
Keywords: IORT, techniques, indications, outcomes, complications
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 29/06/2017; Received: 18/01/2017
Background
Intraoperative radiation therapy (IORT) constitutes delivery of radiation to the tumour/tumour bed while the area is exposed during surgery. IORT is capable of delivering high doses of radiation, precisely to the tumour bed with minimal exposure to the surrounding healthy tissues.
Abe et al. from the University of Kyoto, Japan, were the first to introduce IORT in the early 1960s reporting its use in various intra-abdominal tumours [1–3].
IORT is typically used in combination with other modalities like maximal surgical resection, external beam radiotherapy (EBRT) or chemotherapy as a part of the multidisciplinary approach.
Efficacy of IORT has been reported in a wide variety of sites like locally advanced and recurrent rectal cancer, retroperitoneal sarcoma, pancreatic cancer, early breast cancer, and selected gynaecologic and genitourinary malignancies.
Rationale for the use of IORT
Traditionally, surgery is followed by EBRT in most solid tumours for the elimination of any microscopic residual disease and reducing the risk of local recurrence. However, EBRT in the post-operative setting has the following drawbacks:
• The usual delay between the surgical removal of the tumour and EBRT may allow repopulation of the tumour cells.
• Difficulty in tumour bed localisation or use of larger margins, which may increase normal tissue morbidity.
Most solid tumours exhibit a dose–response relationship, the likelihood of local control improving with increasing dose; however, there are limitations to the doses that can be delivered even with conformal EBRT techniques due to the presence of dose-limiting structures adjacent to the tumour/tumour bed. Especially, in the setting of gross residual disease, doses with EBRT may never be sufficient to achieve adequate local control without causing significant morbidity.
IORT allows
• Precise localisation of the tumour bed and targeted delivery of high-dose radiation to the tumour bed.
• Minimal exposure of the dose-limiting normal tissues that are displaced away from the tumour bed and shielded from radiation.
• Opportunities for dose escalation beyond that which can be achieved with EBRT.
• Opportunities for re-irradiation especially in recurrent cancers where further irradiation with EBRT may not be possible.
Thus, IORT can deliver higher total effective dose to the tumour bed, facilitate dose escalation without significantly increasing normal tissue complications and improve therapeutic ratio compared with EBRT.
IORT may be used alone or in combination with conventionally fractionated EBRT. Most centres use it in combination with EBRT, as it seems to provide the best therapeutic ratio (decreased risk of late normal tissue damage due to the use of fractionation for some part of the dose).
Methods of IORT
Several methods have been used to deliver IORT. Electron beams (electron IORT/IOERT), X-rays (kV IORT) and High-dose-rate brachytherapy (HDR IORT) are some of the commonly used methods for the delivery of IORT in current clinical practice.
Electron IORT
Introduction of electron IORT (IOERT) marked the beginning of the IORT era in the early 1960s [3, 4]. Using variable electron energies depth dose distribution could be controlled to provide uniform dose to target area. However, patients needed to be transported from the operating room (O.R) to the radiation department during surgery, posing logistical issues related to transportation and sterilisation [2, 5]. These problems were overcome with the use of dedicated IOERT facilities, which were quite expensive because of added costs of shielding the O.R and dedicated linear accelerator requirements, limiting their use to few centres in the United States and Europe. The advent of miniaturised, self-shielded, mobile linear accelerators [6] (Novac7, Hitesys SPA, Aprillia, Italy; 7–10 MeV and the Mobetron, IntraOp Medical Corporation, Sunnyvale, CA, USA; 4–12 MeV) in the 1990s, has brought about resurgence of IORT and allowed its use in many centres across the world while reducing the costs. Greater depth of penetration and dose homogeneity relative to HDR-IORT or kV IORT is possible with these devices. They come with applicators of different shapes and sizes, for the treatment of various sites and can deliver the treatment in a matter of minutes [7]. However, these applicators are rigid, thus challenging to use in difficult sites (pelvis and narrow cavities) and can treat a maximum diameter of 15 cm only, larger volumes requiring multiple, closely placed fields. Abutment of fields to treat a wider area is made possible by the use of rectangular applicators or D-shaped applicators called ‘Squircle’.
HDR IORT
HDR brachytherapy offers distinct dosimetric advantages due to its steep dose fall off and has the ability to deliver high doses to the tumour bed while reducing doses to nearby critical structures, these characteristics of HDR brachytherapy make it well suited for the purpose of IORT. Since many centres already own a HDR after loading machine, which can be transported to the OR for IORT, it reduces the cost of dedicated system; however, like IOERT, a shielded O.R or a shielded room in the O.R complex becomes necessary for HDR IORT. HDR IORT in most centres is delivered using surface applicators like Harrison–Anderson–Mick (HAM) applicator [8, 9] or superflab [10, 11] applicators and prescribed at 0.5–1 cm depth. These applicators are flexible, can treat relatively uneven surfaces and come in larger sizes for larger surfaces. Disadvantages of HDR IORT are reduced depth of penetration and prolonged treatment time relative to IOERT.
KV IORT
With increasing use of IOERT in the 1980s, orthovoltage X-rays were attempted for use in IORT to reduce the shielding costs of the OR. However, poor uniformity, higher bone doses and prolonged treatment time quickly reduced the interest in their use. Recently, low-kV (20–50 kV) mobile IORT devices like Intrabeam, (Carl Zeiss AG, Germany) and Axxent Electronic Brachytherapy System (Xoft Inc., Fremont, California) are gaining popularity for use in IORT. They have steep dose gradients and do not require special shielding requirements. They come with spherical applicators and have a very limited depth of penetration of 0.5–1 cm. They are therefore best suited for spherically shaped target volumes as in breast cancer.
With a strong oncological rationale at its heart, IORT in its various forms has been tested throughout the evolution of radiotherapy and has weathered the tests of time and technology showing periodic resurgences with the advent of newer technology. The following section will focus on recently published results to describe the current role of IORT across various sites.
Search strategy and selection criteria
A literature search was performed through the PubMed database by using the following terms: ‘intraoperative radiotherapy/IORT’, ‘head and neck cancer’, ‘breast cancer’, ‘colorectal/rectal/colon cancers’, ‘pancreas/pancreatic cancers’, ‘gastric/stomach cancer’, ‘soft-tissue sarcoma/sarcoma’, ‘paediatric/childhood cancers’, ‘gynaecological cancer’, ‘uterine/endometrial cancer’, ‘cervical/cervix cancer’, ‘renal/kidney cancer’, “bladder cancer”, and “prostate cancer”. IORT was defined as single large dose delivered intraoperatively during surgery, articles of perioperative brachytherapy with continuous low-dose rate or pulsed dose rate or HDR with multiple small fractions, delivered over subsequent days post-surgery were not included in this review. Search was limited to articles published between 1995 and 2017. Reviews, case reports and data presented, only as an abstract at conferences were excluded. Whenever updated data from the same institute was available, earlier articles with smaller numbers were not included. For the purpose of uniformity, in the respective sections, reports combining the results of primary with recurrent colorectal cancers, extremity sarcomas with retroperitoneal sarcomas and metastatic pancreatic cancers with locally advanced pancreatic cancers together were not included in the review. A total of 123 articles were finally included in the review.
Clinical results with IORT
Head and neck cancers
Despite the use of multidisciplinary treatment protocols locoregional recurrences occur in more than 30% of locoregionally advanced head and neck cancers [12–15]. Outcomes are poor even after surgical salvage with high rates of local failure. Re-irradiation, in this setting, has shown to improve local control [16]. However, persistent late sequelae from previous course of radiotherapy (RT) may hamper the chances of effective re-irradiation with EBRT. IORT is an attractive tool in this setting.
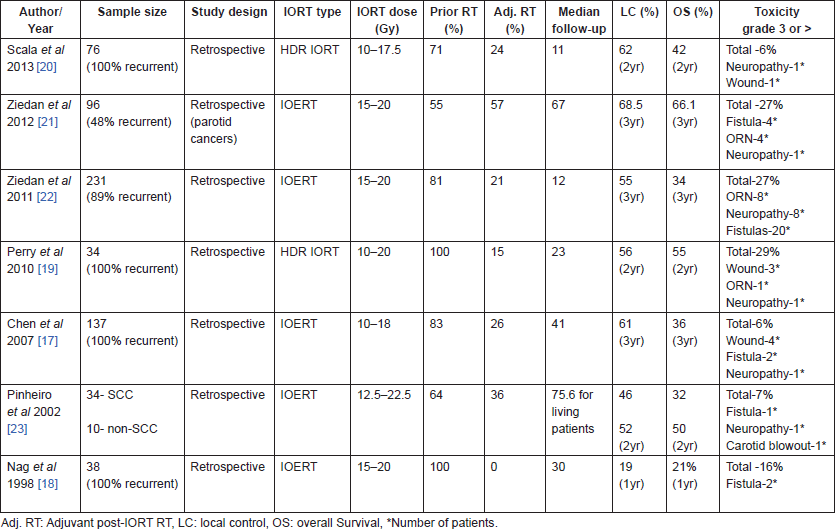
Many retrospective series [17–22] have demonstrated the efficacy of IORT in recurrent head and neck cancer after gross total resection (Table 1). Both IOERT and HDR IORT have been used to deliver IORT in recurrent head and neck cancer. Patients selected for IORT mainly consisted of recurrent or persistent cancers, who have been previously irradiated and delivery of sufficient doses of EBRT was not possible at the time of recurrence. Most studies have shown effective local control with acceptable complications [17, 19–22]. Resection status at salvage was the most important factor determining local control [17, 19, 21]. Microscopically residual tumours did better with IORT [23], gross residual disease however did not [20, 23]. Adjuvant EBRT after IORT appears to further improve local control, however the small sample size of these studies precludes any definite conclusions [20, 23]. Wound complications, osteoradionecrosis (ORN), fistulae, and neuropathy are the most common complications [17–22] after IOERT; however, these are rare with doses less than 20 Gy [22] and no different than that of re-irradiation with EBRT [16]. Carotid artery blow out is a rare but a fatal complication that may occur after IORT. Attempts should be made whenever possible to shield or space out the major vessels and nerves from the treatment field.
Table 1. Studies of IORT in recurrent head and neck cancer after gross total resection.
Breast cancer
The majority of breast cancer recurrences after breast conservation surgery and whole breast irradiation (WBI) occur in the tumour bed, questioning the need for WBI. This has led to widespread adoption of accelerated partial breast irradiation (APBI) in women with early breast cancer without adverse features. IORT has seen a growing interest in early breast cancer as a modality of delivering APBI in a single fraction.
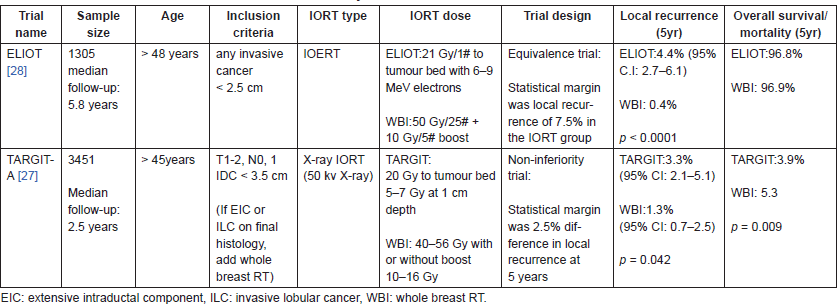
Several phase-II trials [24, 25] and prospective series [6, 26] have shown excellent early tumour control, survival, and cosmetic outcomes. Two large phase-III studies TARGIT-A (targeted intraoperative radiotherapy) [27] and ELIOT (intraoperative radiotherapy with electrons) [28], have evaluated the role of IORT as single-dose, partial breast irradiation treatment compared to standard, conventionally fractionated WBI for highly selected patients with relatively low-risk early-stage invasive breast cancer.
Table 2 summarises the relevant differences in the two trials with the 5-year results. Both the Eliot and TARGIT trials demonstrated significantly higher recurrence rates compared to WBI; however, the results were reported to be within the predefined statistical margin for equivalence/non-inferiority. Also, in both the trials, fewer skin side effects were seen in the IORT group compared to those in the WBI group.
TARGIT-A trial also reported significantly lower non-breast cancer deaths in the TARGIT group (p = 0.0086). This difference was attributed to fewer radiotherapy-related cardiovascular deaths in the TARGIT group; however, radiotherapy-related cardiovascular deaths may not become apparent so early in the follow-up period and these differences could have resulted due to imbalance in the treatment arms [29–32]. The TARGIT-A trial has also come in for criticism related to its statistical assumptions [33–35]. Though the trial seems to show a non-inferiority in 5-year local recurrence rates, median follow-up of all randomised patients is just 29 months which is too early to make assumptions regarding local recurrence rate at 5 years and also the authors seem to have misinterpreted the non-inferiority criterion, which require the upper confidence interval (CI) be less than the predefined non inferiority level of 2.5% [33–35].
To summarise the trials of single-dose IORT, both ELIOT trial (IOERT) and TARGIT trial (kV-IORT) demonstrated a higher recurrence rate compared to WBI, although within the equivalence margin [36]. TARGIT-A (KV-IORT) also requires a longer follow-up before drawing definite conclusions and adopting it for widespread use in place of WBI [35, 36]. It is prudent to use these techniques in a highly selected group of low-risk early-breast cancer to achieve acceptable results. Leonardi et al [37, 38] used the American Society for Therapeutic Radiation Oncology (ASTRO) consensus statement [39] and the Groupe Européen de Curiethérapie–European Society for Therapeutic Radiology and Oncology (GEC–ESTRO) recommendations [40] for APBI patient selection, to stratify 1822 patients treated with ELIOT outside the trial into different risk groups, 16% of women met ASTRO suitable criteria and 31% were good candidates as per GEC-ESTRO recommendations, local recurrence rates were 1.5% and 1.9%, respectively. Thirty-six per cent of women who had favourable biology disease with a luminal-A subtype also showed a very low local recurrence rate of 1.7% irrespective of the risk group. Therefore, ASTRO suitable, GEC ESTRO good and luminal-A subtype identify a subset of women, who may be safely treated with single-dose IORT with acceptable results [36–38, 41].
Table 2. Randomised control trials of IORT versus WBI in early-breast cancer.
Though it may not be time yet for IORT to replace WBI in early-breast cancer, IORT has been investigated as a strategy for boost in limited-stage breast cancer prior to WBI. Compared to post-operative boost, IORT boost allows precise delivery to a smaller target volume separated from skin, rather than to a volume distended or distorted by seroma, thus improving accuracy and cosmesis. Also, a single-shot boost treatment significantly reduces the duration of adjuvant RT. In a pooled analysis by the International Society of Intraoperative Radiotherapy (ISIORT), IOERT has been demonstrated to be an effective boost strategy with excellent local control rates [42]. A total of 1109 unselected patients belonging to any of the risk groups were treated with IOERT boost (median 10 Gy) followed by WBI (50–54 Gy). At a median follow-up of 6 years, only 16 local recurrences were observed resulting in a local control rate of 99.2%. Grade was the only significant predictor of local recurrence, while none of the age groups demonstrated a higher recurrence rates. Efficacy of KV-IORT as a modality for intraoperative boost has been demonstrated in two large prospective series, an IORT boost of 18–20 Gy was followed by WBI, a local recurrence rate of 1.73% was observed in the study by Vaidya et al [43], while a 3% recurrence rate was seen in the study by Blank et al [44]. The TARGIT-B trial (NCT01792726), which compares EBRT boost versus an IORT boost, in patients at high risk for local recurrence who are receiving breast-conserving treatment, with standard postoperative EBRT has been launched and may provide definite answers.
IORT boost has emerged as an attractive option for boost in combination with oncoplastic surgery [45]. Oncoplastic reconstruction techniques allow for a wider resection margin while maintaining the cosmetic outcome; however, an externally delivered boost, in such cases, has higher chance of partially missing the target volume due to the tissue displacement techniques used for reconstruction. IORT allows for a precise delivery of the radiation boost directly to the tumour bed during surgery and can be followed by oncoplastic reconstruction thus maintaining the oncological safety and improving cosmetic outcome, with other added advantages like avoiding seroma formation and reducing the duration of EBRT. The Breast Centre of the University Hospital of Cologne [45, 46] has recently reported the aesthetic outcomes of X-ray IORT boost (20 Gy) combined with oncoplastic surgery in 149 patients treated since 2011, with excellent cosmetic outcomes in over 90% and seroma formation rates of 2% at 4 weeks.
Colorectal cancers
Locally advanced rectal cancer is best managed with aggressive multimodality treatment involving chemoradiotherapy and radical resection. Most locally advanced (T3) tumours do well with this multimodality approach and local recurrences are seen in only 5–10% of patients. However, in 15% of T4 (unresectable) tumours R0 resections may not be possible [47, 48] and 10% of complete resections still develop local recurrences [47, 48] after full course chemoradiotherapy. Resection status is the most important determinant of local control and survival; incomplete resections yield few long-term survivors. There may be a case for dose escalation in locally advanced/unresectable rectal cancers with incomplete resections or at high risk of local recurrence (close margins); however, gastrointestinal tolerance limits the radiation dose delivered by EBRT. With IORT, higher doses can be delivered directly to the tumour bed without significantly increasing doses to nearby structures. This high dose may be capable of sterilising the margins even after microscopic/macroscopic residual disease.
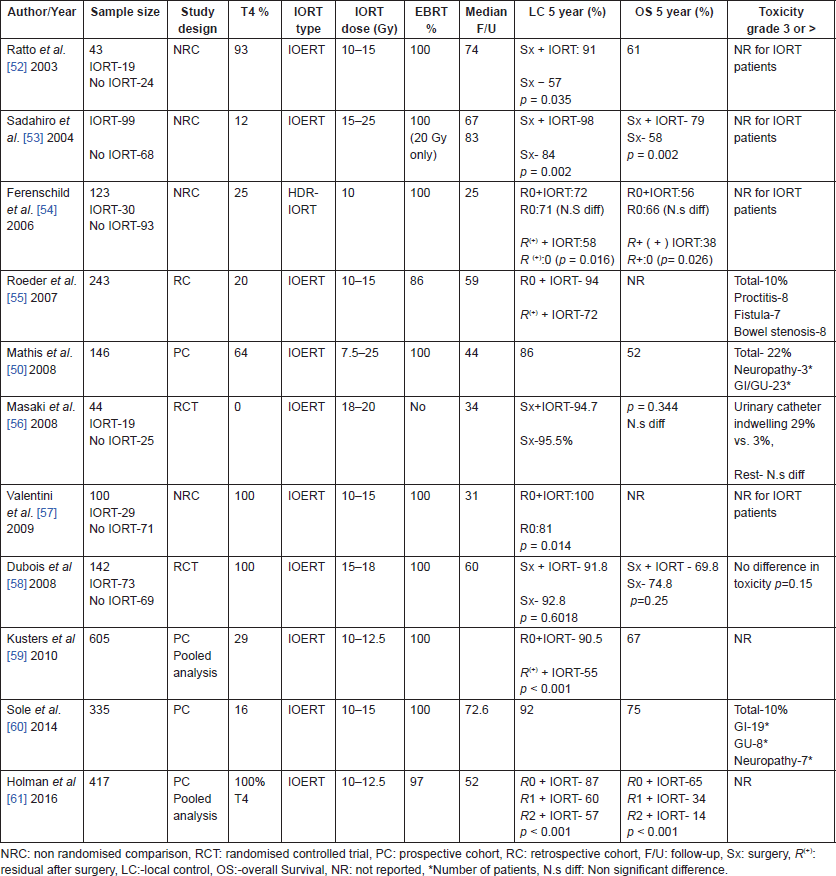
There is increasing evidence (Table 3) to suggest that inclusion of IORT in the multi-modal treatment of locally advanced rectal cancer can lead to improved local control and survival [11, 49–51] especially in the setting of R resection. IORT in locally advanced rectal cancer is commonly delivered as an intraoperative boost and used in combination with pre-operative or post-operative radiotherapy with or without chemotherapy [11, 49]. Most studies have utilised IOERT and others have utilised HDR IORT for delivery of IORT in rectal cancer.
Table 3 summarises various non-randomised and randomised studies of IORT in locally advanced rectal cancers. The initial non-randomised comparisons [52–54, 57, 62] showed conflicting results with IORT after complete resection (R0), while some studies showed equivalent local control [54] in IORT and non IORT group, others showed a significant benefit in local control with IORT [52, 57, 62]. One thing which is certain was that IORT provided significant benefit in local control and survival in patients with R resection [54, 55, 59, 61]. The only two randomised studies [56, 58] comparing the addition of IORT to standard treatment failed to show any benefit with addition of IORT in terms of local control or survival. The study by Dubois et al. [58] had a large proportion of T3 tumours (89%), complete resection in most patients would have likely minimised the benefits of IORT, while on the other hand, the study by Masaki et al. [56] was limited by small sample size and inclusion of T1/T2 patients.
Management of locally recurrent rectal (LRRC) cancers presents unique challenges. Prior irradiation in these patients limits the scope for further treatment of these patients with EBRT and is generally associated with poorer survival. IORT with its ability to limit the dose to critical structures serves as a reasonable technique for re-irradiation in LRRC. With the addition of IORT to gross total resection and EBRT, various initial series [63, 64] reported a 5-year survival of over20% even without chemotherapy.
Table 3. Studies of IORT in locally advanced colorectal cancer after gross total resection.
Non-randomised studies of IORT in LRRC (Table 4) have shown a significant improvement in local control with IORT and many series have also shown a survival advantage. Recent series [65–70] have also employed Re-EBRT and chemotherapy along with IORT in patients previously treated with pelvic radiotherapy and were able to achieve survival in the range of 30–40%, using these aggressive strategies. Important factors affecting outcomes in most of these studies was completeness of surgical resection [65, 68–70] and addition of IORT boost [63, 66, 67]. EBRT during recurrent setting appears to improve the outcomes further and should be considered whenever feasible [68, 70, 71].
The complication rates in these IORT studies are variable and could range anywhere between 5% and 60%. Wound complications, gastrointestinal problems, ureteric obstruction and neuropathy are some of the frequently encountered morbidities. Wound complications were most common and in some series was quite high, upwards of 40% [50, 62, 72, 73]. Gastrointestinal fistulae and ureteric damage have an incidence ranging from 2% to 12% [50, 62, 64, 72, 73]. Plexopathy and neuropathy are late toxicities of pelvic IORT and have shown a dose-dependent relationship after IORT [50, 62, 64, 65, 72, 73].
A meta-analysis [74] of studies of IORT in locally advanced and recurrent rectal cancers together, has shown a significant benefit with addition of IORT on local control, disease-free survival and overall survival. Meta-analyses of complications did not demonstrate a significant increase in urologic or gastrointestinal complications; however, a greater number of wound complications did occur [74].
Soft-tissue sarcomas
Surgery constitutes the main treatment modality for soft-tissue sarcomas; however, surgery alone cannot provide acceptable local control rates without hampering the functionality of the limb/organ in cases of large and high grade sarcomas, thus making radiation therapy an integral component of function preserving surgery. Radiation therapy used either preoperatively or postoperatively provides acceptable local control rates after an adequate surgery with negative margins. However, in cases of advanced tumours where negative margin is not possible without mutilating surgery (retroperitoneal sarcoma) or in case of recurrent tumours, optimum doses of EBRT cannot be delivered to provide acceptable local control.
IORT has been used in such tumours to escalate doses beyond that of conventional EBRT in an attempt to improve local control rates. In extremity sarcomas, IORT has also been used to replace external boost, reducing the dose and volumes treated with EBRT, so that tolerance of normal structures like joint space, bone, and skin can be respected.
Table 5 summarises studies of IORT in extremity soft-tissue sarcomas, these studies were heterogeneous with varying proportion of recurrent tumours and incomplete resections. Use of IORT in these unfavourable patients aimed at preserving the limb while maintaining acceptable local control. IORT was mostly used in combination with function preserving surgery and moderate doses of EBRT. Recent series [82–85] of IORT demonstrate excellent LC rates and functional outcomes, comparable to the series of EBRT alone, despite including higher proportion of tumours with unfavourable factors. Dose of IORT was dependant on resection status, volume and dose of EBRT. While R disease fared equally well as R0 disease in the series by Call et al and Kretzler et al [79, 82], studies by Niewald et al and Kretzler et al [79, 81] reported equivalent outcomes in recurrent as well as primary disease. However, in some of the larger series, [82–85] resection status and recurrent disease were the most important factors determining local control. Limb preservation was achievable in most patients even with recurrent disease. The complications of neuropathy, contracture, and lymphedema were low, wound complications were the most common complications, and were not much different from that with EBRT [79–82].
Soft-tissue sarcomas in the retro peritoneum are difficult to remove with adequate margins due to their large size, advanced stage, and difficult location with multiple critical organs in close vicinity. Therefore, surgery is often combined with radiotherapy in order to improve the local control rate. However, the proximity of normal organs, such as viscera and neurovascular structures, has made the delivery of therapeutic doses of postoperative EBRT problematic, with higher rates of gastrointestinal complications, including disabling chronic enteritis and fistulae. These difficulties have led to adoption of IORT in the treatment regimen for retroperitoneal sarcoma since the late 1980s.
A randomised trial at the NCI [86], at a median follow-up of 8 years, showed a significantly better local control with IOERT and low-dose post-operative EBRT compared to high-dose post-operative EBRT alone (60% vs. 20%, p < 0.05). The IOERT arm experienced significantly more peripheral neuropathy attributed in part to use of concurrent radio-sensitisers (60% vs. 5%, p < 0.05), while the EBRT only arm had significantly higher GI complications. Experience from other series, summarised in Table 6, has also shown encouraging results with a favourable toxicity profile.
Table 4. studies of IORT in locally recurrent colorectal cancers.
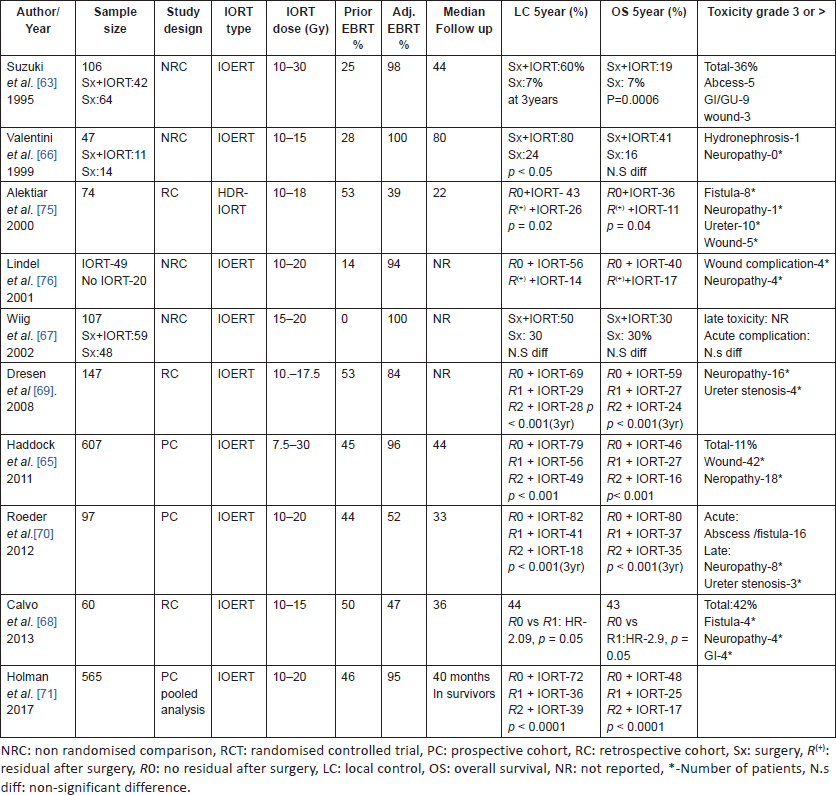
Table 5. Studies of IORT in extremity soft tissue sarcoma in combination with function preserving surgery and moderate doses of EBRT (40-50Gy).
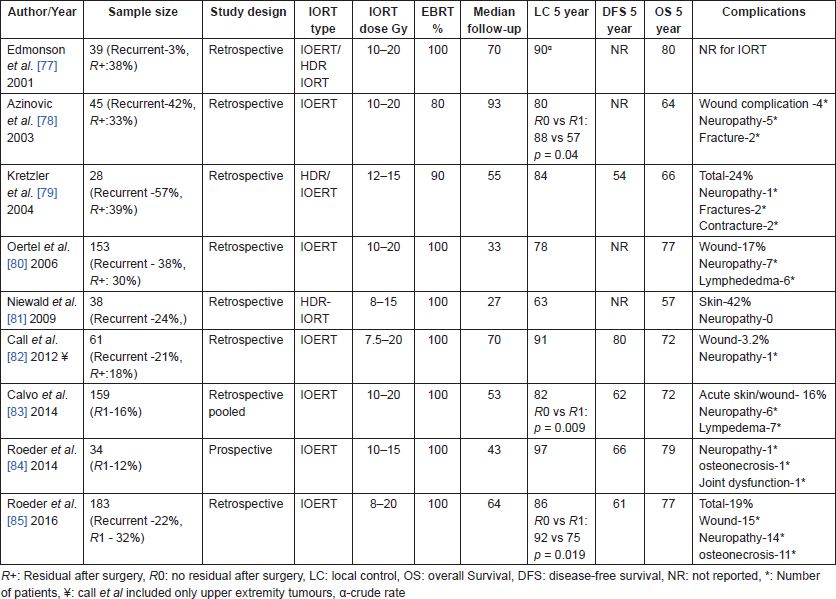
Table 6. Studies of IORT in Retroperitoneal sarcoma.
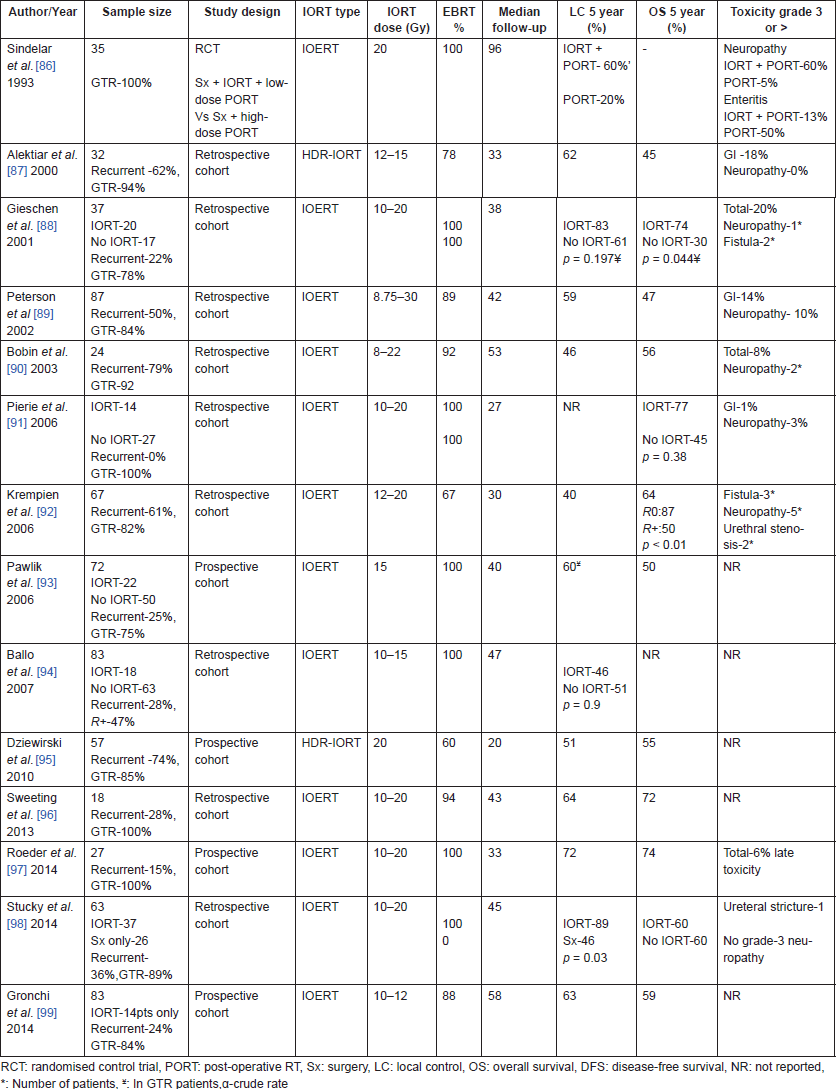
IORT in combination with pre-operative or post-operative RT has shown encouraging results [87–94, 97, 99]. While initial reports [87, 89, 90, 92] had higher proportion of patients receiving post-operative RT, recent series [88, 91, 93, 97–99] mostly use pre-operative RT because of the smaller volumes that are required with reduced rates of complications. Combination of pre-operative RT, gross total resection and IORT has demonstrated improved local control [91, 98] as well as survival [88, 91] compared to the non-IORT regimens in some of the recent non-randomised comparisons. Resection status and recurrent disease were the most important determinants for local control [89, 92, 94, 97]. GI toxicities, neuropathy and ureteric stenosis are the most common complications with reported rates of 10–35%. They may be dose-dependent, high single dose resulting in greater risk of complications [65, 86, 100].
Most studies of IORT shown in Tables 5 and 6 had a large proportion of recurrent tumours, emphasising the fact that IORT plays a pivotal role in the management of these locally recurrent sarcomas. In a multi-centric, long-term outcomes analysis by the Spanish Cooperative Initiative [101] for Intraoperative electron radiotherapy, 103 patients were investigated to analyse long-term outcomes of locally recurrent soft-tissue sarcoma (LR-STS) patients treated with a multidisciplinary approach. The 5-year IORT in-field control, disease-free survival (DFS), and overall survival were 73%, 43%, and 52%, respectively. Not combining EBRT with surgical resection and IOERT in patients with LR-STS was associated with a significantly increased probability of LR and IOERT in-field relapse. They concluded that low rate of severe toxic events suggests that a multimodality approach with re-resection and IOERT is feasible without prohibitive long-term side effects.
Paediatric tumours
Most paediatric tumours are radiosensitive and radiotherapy constitutes an integral component in their management schema, more so for the unresectable and recurrent tumours, where outcomes remain dismal with chemotherapy alone. However, the use of radiotherapy, especially EBRT in the paediatric population is fraught with late effects like retarded bone and soft tissue growth, abnormal organ development and the risk of second malignancies due to the sensitive nature of these maturing tissues. Thus, there is a narrow therapeutic window within which local control and late effects, which needs to be balanced. The goal of IORT for paediatric tumours is to improve the therapeutic ratio by increasing local control while limiting these late toxicities.
Table 7 summarises various studies of IORT in paediatric tumours, though the numbers are small, IORT has been used across a wide variety of sites and histologies, as a sole radiation modality for radio-sensitive tumours like neuroblastoma [102] or in combination with EBRT for dose escalation to improve local control in sarcomas [103] or for dose de-escalation in RMS with low-dose EBRT. Oertel [103], Goodman [104] and Sole [105] et al included quite a number of recurrent tumours. Use of IORT in combination with surgery and EBRT provided excellent local control across most studies with acceptable toxicity.
In a study by Sole [105] et al, after a median follow-up of 72 months (range, 4–10 months), 10-year LC, disease-free survival, and OS was 74%, 57%, and 68%, respectively. In multivariate analysis after adjustment for other covariates, disease status (p = 0.04 and p = 0.05) and resection margin status (p < 0.01 and p = 0.04) remained significantly associated with LC and OS.
IOERT can be considered as an effective option as a part of multimodality regimen for paediatric solid malignancies, especially for patients with recurrent tumours and abdominopelvic malignancies.
Gynaecological cancers
Recurrent gynaecological malignancies are associated with poor survival due to lack of effective salvage options. Survival rates of locally recurrent cervical cancer after prior radiation therapy are dismal. Most recurrences especially those involving the pelvic sidewall are not resectable and when resection is possible (as in central recurrences), extensive procedures like pelvic exenteration are required, which are associated with a high rate of complications and operative mortality of over 10% [108–111]. Introduction of IORT has widened the scope of patients who may be offered surgery and patients who have been previously treated with non-surgical modalities can be offered radical resection when combined with IORT. In resectable recurrences, IORT given after gross total resection can improve local control rates.
IORT has been used to treat locally advanced primary cervical cancers also; however, these series [112] are small and most of the experience comes from recurrent cancers (Table 8). IORT has shown to improve local control and thus survival in locally recurrent cancers [113–121] of the uterine cervix and endometrium, limited locoregional recurrences from endometrial cancers doing much better than recurrences from cervical cancers [119, 122–124]. The benefit of IORT is seen much more in patients with microscopic residual disease than in those with gross residual disease [113–115, 121]. Patient selection based on resection status and volume of recurrence are the most important factors determining outcome after IORT. Previously, irradiated patients when adjusted for resection status and volume of recurrence appear to fare as well as previously un-irradiated patients [115] and addition of EBRT to IORT regimen further improves the control rates [117, 119, 120, 123]. IORT does not seem to increase the rate of acute complications following surgery. Neuropathy and gastrointestinal toxicity are the most common IORT-related toxicities and occur in 5–30% of patients.
Genitourinary cancers
Bladder cancer
Although multiple reports of perioperative brachytherapy in bladder cancer are available with encouraging results, there is limited data on IORT in bladder cancer, with only one small retrospective series in recurrent bladder cancer meeting our search and selection criteria. Recurrent bladder tumours after a cystectomy are associated with dismal survival rates, owing to the fact that adequate surgery is often not feasible and salvage with high doses of EBRT is difficult due to the tolerance of adjacent organs. IORT is used to deliver high doses to the tumour in an effort to improve local control. Hallemeier et al [128] reported the use of IOERT in 17 patients after maximal resection of disease. Pre- or post-operative EBRT was used in 94% of patients. Encouraging 2-year local control and survival was seen, completely resected tumours were associated with a significant improvement in survival compared to gross residual disease.
Renal cancer
Radical surgery forms the mainstay of treatment in patients with renal cell carcinoma (RCC). However, in patients with recurrent and advanced tumours, achieving complete resection with wide margins may be difficult due to proximity to the critical structures and this effects not only the local control but survival as well [129]. Adjuvant EBRT in this setting may improve local control; however, the doses achievable with EBRT is limited due to low tolerance of the surrounding structures like stomach, small bowel, contralateral kidney, liver, and spinal cord. IORT offers an attractive treatment option to escalate doses to the tumour bed, especially in cases with positive resection margins. Studies evaluating the role of IORT in the management of locally advanced and recurrent RCC are summarised in Table 9 [129–134].
Table 7. Studies of IORT in various paediatric tumours.
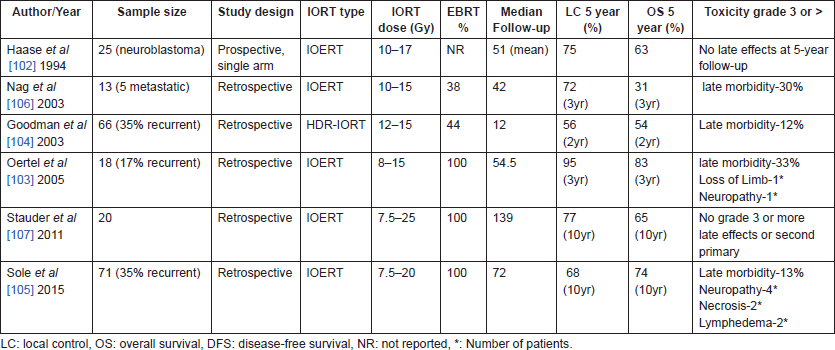
Table 8. Studies of IORT in recurrent gynaecological malignancies after gross total resection.
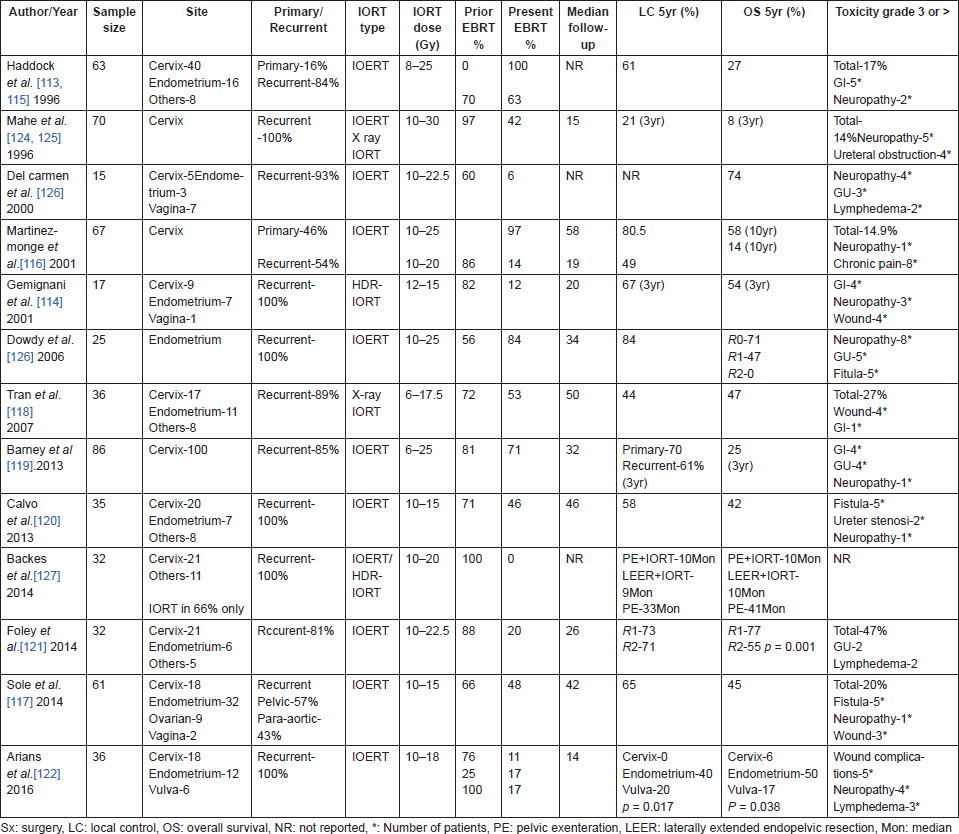
Table 9. Studies of IORT in bladder and renal cancers.
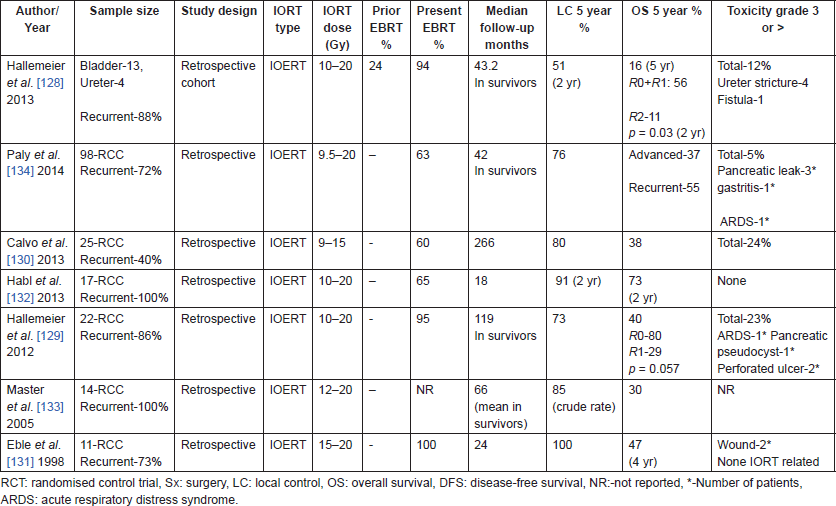
Habl et al [132] reported outcomes with IOERT after complete surgical resection in a cohort of 17 patients with locally recurrent RCC. Although R0 resection could be achieved in only one-third of the patients, most patients failed distally, with only two local recurrences. None of the patients suffered from any acute or late radiation toxicities. One of the largest series of IOERT in RCC has been reported by Paly et al in a multi-institutional cohort of 98 patients. Twenty-eight per cent patients had advanced disease at presentation and 72% had recurrent disease. More than 50% had residual disease after resection. Sixty-two per cent received additional pre-operative or post-operative EBRT. An excellent local control of 72% at 5 years was demonstrated with grade 3 toxicity in 5% of patients. Higher IORT dose was associated with improved survival (p < 0.001). Thus, studies of IORT in RCC, though retrospective in nature demonstrate a consistently high local control rate in recurrent/advanced RCC with acceptable toxicity rates.
Prostate cancer
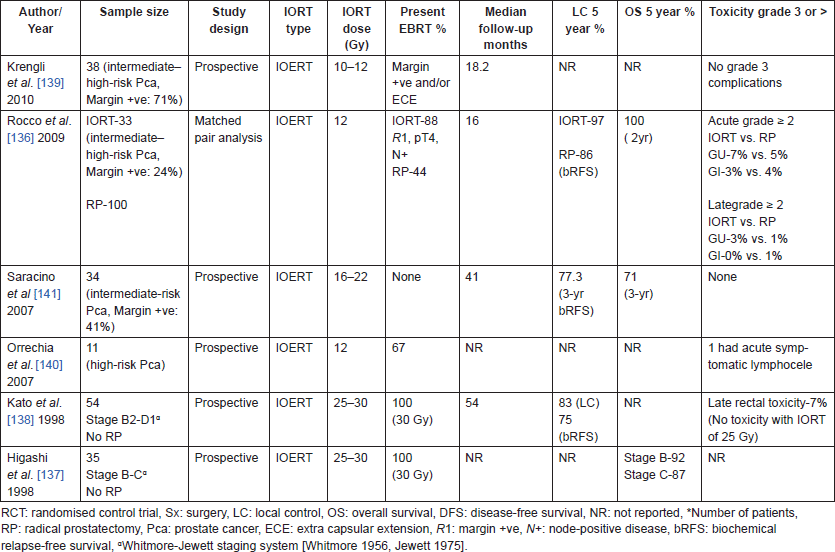
Locally advanced/high-risk prostate cancer is associated with significant risk of relapse when treated with radical prostatectomy alone, risk being the highest when the margins are positive. Adjuvant radiotherapy in this setting reduces the risk of relapse significantly [135]. IORT has been explored in high-risk prostate cancers in combination with radical prostatectomy and post-operative EBRT to improve local control via dose escalation. IORT has the added radiobiological advantage of high single dose of radiation, which improves the therapeutic gain due to low α/β of prostate. It also helps limit doses to the rectum and has been shown to have low gastrointestinal (GI) morbidity even in combination with EBRT [136]. Several small prospective series (Table 10 [136–141]) have evaluated the feasibility of this multi-modality approach in patients with non-metastatic, node-negative disease with probability of LN involvement being less than 15%. Encouraging local control and acceptable toxicity has been demonstrated even though significant proportion of patients had margin positive disease in these series [136, 139, 141]; however, long-term results are awaited.
Table 10. IORT studies for prostate cancers.
Upper gastro-intestinal tumours
Gastric cancers
Curative resection is the mainstay of treatment for gastric cancer; however, high incidence of locoregional and systemic failures, makes outcomes dismal, especially in cases with gastric serosal involvement and/or nodal involvement [142, 143]. Attempts to improve locoregional control and survival include addition of adjuvant radiotherapy/chemoradiation [144], perioperative chemotherapy [145] and extensive surgeries including D2/D3 resections [146, 147]. Despite significant improvements in disease control and survival with adjuvant chemoradiotherapy, local and regional recurrences remain high at 19% and 65%, respectively, after tri-modality therapy [144]. Therefore, there may be a case for dose escalation with IORT in advanced gastric carcinomas (especially serosal/nodal involvement) to improve local/regional control. IORT in gastric cancer involves boosting the tumour bed, remaining lymphatic networks, and nodal basins to control residual microscopic disease and improve locoregional control.
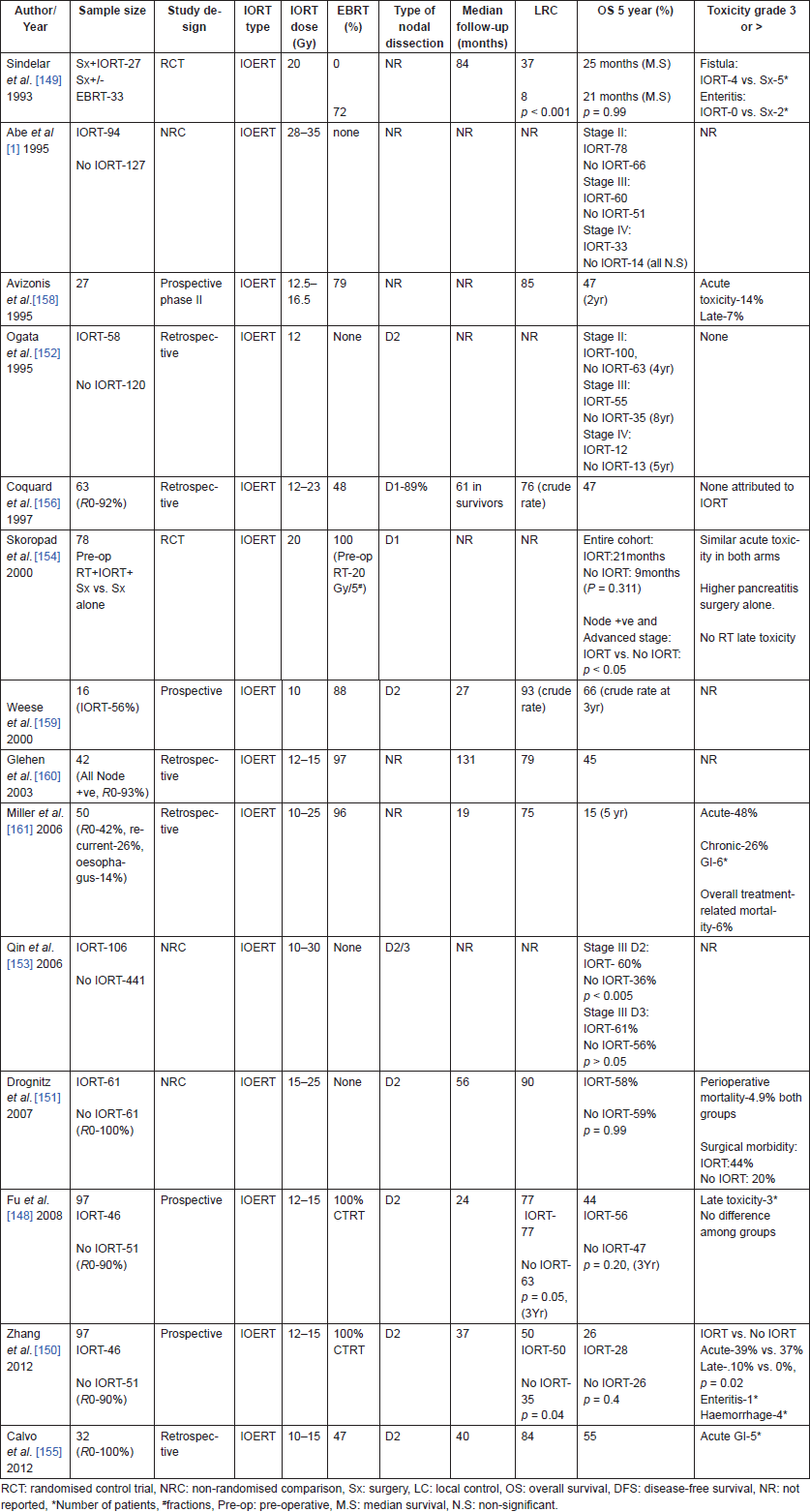
Role of IORT in gastric cancer after curative resection has been evaluated in multiple studies (retrospective, prospective, and randomised control), which have shown an improvement in locoregional control [148, 150] and survival with IORT, especially in patients with stage-II/stage-III and node-positive disease (Table 11) [1, 148, 150–154]. While initial studies of IORT involved less aggressive surgeries (D1) and infrequent use of adjuvant radiotherapy recent studies [148, 150, 153] have demonstrated a consistent benefit with IORT, even in combination with D2 resections and post-operative CTRT. Extended resections like D3 may reduce the benefit with IORT [153], however, IORT combined with a limited lymph node dissection (D1) may be associated with survival similar to extended dissection (D2/3), with lesser post-operative mortality [156]. While most studies did not show an increase in complications with the use of IORT, Drognitz et al [151] have demonstrated a significant increase in surgical complications with the use of IORT (44% vs. 20%, p < 0.05). They also did not show a benefit with addition of IORT to surgical resection. Complication rates need to be carefully weighed against improvement in locoregional control to maximise benefits with IORT [157].
Table 11. IORT studies for gastric cancers.
Pancreatic cancer
Pancreatic cancer is associated with dismal survival rates even in completely resected patients. Significant proportion of patients either develop locoregional recurrence or systemic metastases. Multi-modality treatment approaches combining chemotherapy and radiotherapy in addition to surgery have resulted in some improvement in locoregional control and survival [162–164]. Attempts at radiotherapy dose escalation with EBRT have been limited due to the location of tumour. IORT can result in delivery of higher doses to the tumour bed and may improve local control and survival in resected pancreatic cancers. In unresectable tumours, IORT alone or in combination with EBRT can provide some local control along with effective palliation of symptoms.
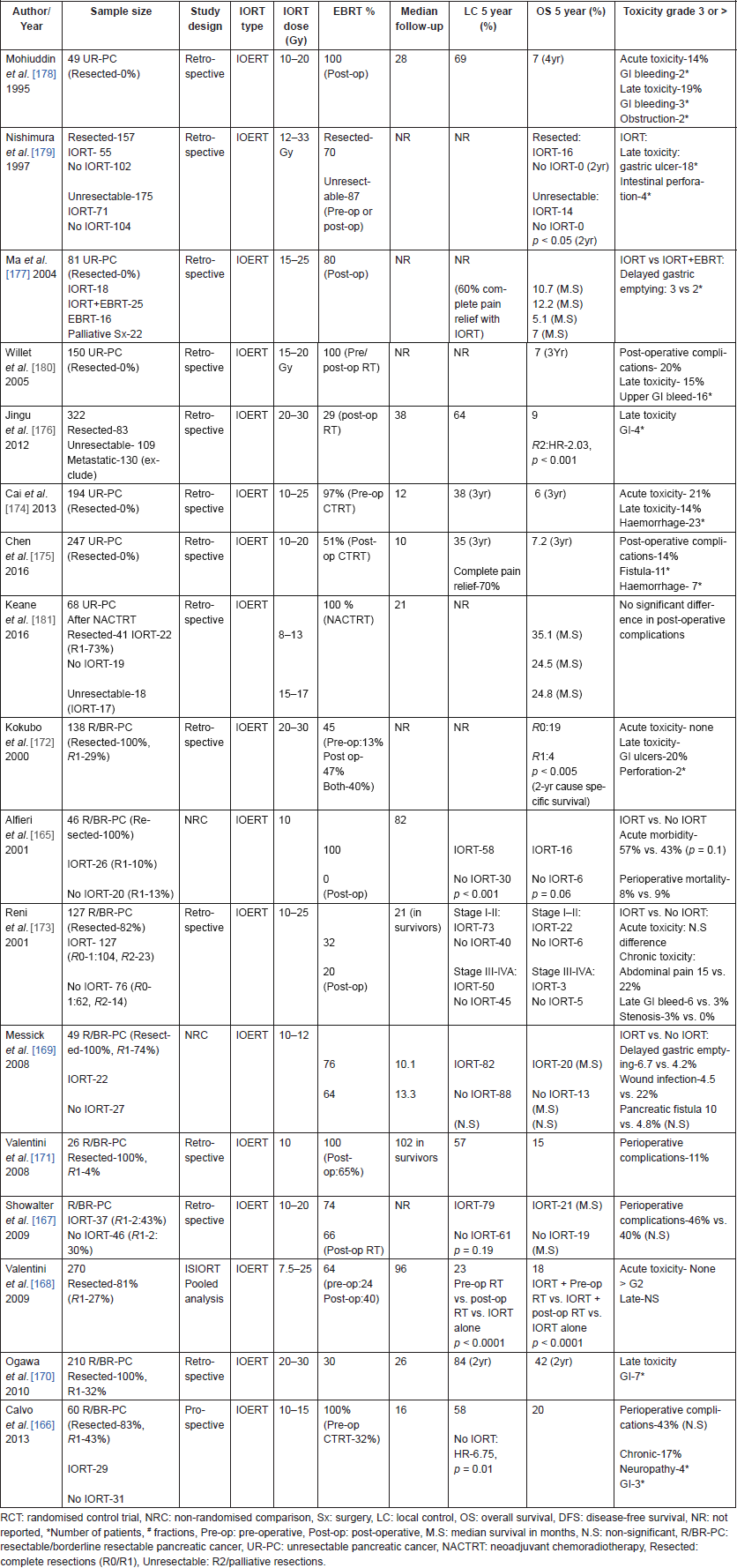
Studies of IORT in resectable pancreatic cancers are summarised in Table 12 [165–171], though heterogeneous in proportion of R1 resections and use of adjuvant EBRT and/or chemotherapy, they have been consistent in showing an improvement in locoregional control [165–168]. Some studies have also shown an improvement in survival [165, 168, 172, 173]. Addition of IORT to standard treatment did not result in any increase in perioperative morbidity or late toxicity rates [165, 167–169, 173]. Stage [172, 173], R0 resection [166], chemotherapy [170], and pre-operative treatment [168] were other important determinants of survival in these studies. A systematic review also agreed with observations from these non-randomised studies and suggested a survival benefit with IORT in resected pancreatic patients.
Studies of IORT in unresectable pancreatic cancer (Table 12 [174–180]) on the other hand, have failed to demonstrate a survival benefit with the addition of IORT, though an improved local control was seen [174–176, 178, 181]. IORT also resulted in significant pain relief and palliation of symptoms [175, 177–179] with no additional morbidity or toxicity [175, 177, 181]. Tumour size [17, 174, 176, 180], metastasis [179], and chemotherapy [17, 174, 176] were predictors of survival in these studies of unresectable pancreatic cancer. Most of these studies included patients treated before the year 2000 and utilised post-operative radiotherapy and chemotherapy with older regimes. In the current era, pre-operative chemotherapy (± radiotherapy) with novel systemic agents (like FOLFIRINOX and nab-Paclitaxel) has shown to improve resectablity rates and survival in unresectable pancreatic cancers [182, 183]. Keane et al [181], evaluated the role of IORT in combination with intensive neoadjuvant chemoradiotherapy regimens and demonstrated encouraging survival rates in patients with close/positive margins and unresectable disease with no increase in toxicity. Further studies are required to better define the role of IORT in the management of pancreatic cancers, in the current era especially with the advent of novel systemic agents.
Table 12. Studies of IORT in the management of pancreatic cancers.
Conclusion
Intraoperative radiation therapy is an attractive treatment option for patients with colorectal, gynaecological, intra-abdominal, head and neck, and most recently, breast cancers. IORT has been used in a multitude of roles across these sites, for dose escalation, EBRT dose de-escalation, as sole radiation modality in early-breast cancers and as a Re-irradiation modality in recurrent cancers. IORT serves its role best in combination with gross total resection and moderate doses of EBRT. Utility of IORT has been tested in the setting of a randomised control trial in early breast, retroperitoneum, gastric and colorectal cancers, the results of which support the use of IORT as a management option in these settings. However, appropriate technique and patient selection is the key to success with IORT. IORT has the potential to improve outcomes in recurrent cancers of the pelvis, head and neck and colorectum and can be considered as a supplement to gross total resection. In paediatric tumours, IORT serves to decrease late toxicities associated with EBRT. In appropriately selected patients, complication rates associated with IORT are low.
References
1. Abe M, Shibamoto Y, and Ono K, et al (1991) Intraoperative radiation therapy for carcinoma of the stomach and pancreas Front Radiat Ther Oncol 25 258–69 https://doi.org/10.1159/000429597 PMID: 1908417
2. Abe M, Takahashi M (1981) Intraoperative radiotherapy: the Japanese experience Int J Radiat Oncol Biol Phys 7(7) 863–8 https://doi.org/10.1016/0360-3016(81)90001-8 PMID: 7198109
3. Abe MFM, Yaniano K, et al (1971) Intraoperative irradiation in abdominal and cerebral tumours Acta Radiol 10 408–16
4. Intraoperative radiation therapy Abe M, Takahashi M, editors (1991) Proceedings of the third international symposium on intraoperative radiation therapy
5. Goldson A (1981) Past, present and prospects of intraoperative radiotherapy (IOR) Semin Oncol
6. Veronesi U OR, Luini A, et al (2001) A preliminary report of intraoperative radiotherapy (IORT) in limited-stage breast cancers that are conservatively treated Eur J Cancer 2001(37) 2178–83 https://doi.org/10.1016/S0959-8049(01)00285-4
7. Vaeth JMea (1996) Intraoperative radiation therapy in the treatment of cancer Front Radiat Ther Oncol 31 65–7
8. Harrison LB EW, Anderson LL (1995) High dose rate intraoperative radiation therapy for colorectal cancer I Oncol 9 679–83
9. Harrison LB EW, Anderson LL (1995) High-dose rate intraoperative radiation therapy for colorectal cancer: II Oncol 9 737–41.
10. Bratengeier K KT (2002) Homogeneous Ir-192 afterloading-flab irradiation of plane surfaces Z Med Phys 12(230–7) https://doi.org/10.1016/S0939-3889(15)70477-0
11. Huber FT SR, Zimmerman F, et al (1996) Locally advanced rectal cancer: resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy Dis Colon Rectum 39 (774–9) https://doi.org/10.1007/BF02054443 PMID: 8674370</a>
12. Bernier J, Cooper JS, and Pajak TF, et al (2005) Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck 27(10) 843–50 https://doi.org/10.1002/hed.20279 PMID: 16161069
13. Bernier J, Domenge C, and Ozsahin M, et al (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer N Engl J Med 350(19) 1945–52 https://doi.org/10.1056/NEJMoa032641 PMID: 15128894
14. Cooper JS, Zhang Q, and Pajak TF, et al (2012) Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck Int J Radiat Oncol Biol Phys 84(5) 1198–205 https://doi.org/10.1016/j.ijrobp.2012.05.008 PMID: 22749632 PMCID: 3465463
15. Pignon JP, Bourhis J, and Domenge C, et al (2000) Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta-analyses of updated individual data MACH-NC collaborative group Meta-analysis of chemotherapy on head and neck cancer Lancet 355(9208) 949–55 https://doi.org/10.1016/S0140-6736(00)90011-4 PMID: 10768432
16. Janot F, de Raucourt D, and Benhamou E, et al (2008) Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma J Clin Oncol 26(34) 5518–23 https://doi.org/10.1200/JCO.2007.15.0102 PMID: 18936479
17. Chen AM, Bucci MK, and Singer MI, et al (2007) Intraoperative radiation therapy for recurrent head-and-neck cancer: the UCSF experience Int J Radiat Oncol Biol Phys 67(1) 122–9 https://doi.org/10.1016/j.ijrobp.2006.08.038
18. Nag S, Schuller DE, and Martinez-Monge R, et al (1998) Intraoperative electron beam radiotherapy for previously irradiated advanced head and neck malignancies Int J Radiat Oncol Biol Phys 42(5) 1085–9 https://doi.org/10.1016/S0360-3016(98)00289-2 PMID: 9869233
19. Perry DJ, Chan K, and Wolden S, et al (2010) High-dose-rate intraoperative radiation therapy for recurrent head-and-neck cancer Int J Radiat Oncol Biol Phys 76(4) 1140–6 https://doi.org/10.1016/j.ijrobp.2009.03.025
20. Scala LM, Hu K, and Urken ML, et al (2013) Intraoperative high-dose-rate radiotherapy in the management of locoregionally recurrent head and neck cancer Head Neck 35(4) 485–92 https://doi.org/10.1002/hed.23007 PMID: 23460243
21. Zeidan YH, Shiue K, and Weed D, et al (2012) Intraoperative radiotherapy for parotid cancer: a single-institution experience Int J Radiat Oncol Biol Phys 82(5) 1831–6 https://doi.org/10.1016/j.ijrobp.2011.02.033
22. Zeidan YH, Yeh A, and Weed D, et al (2011) Intraoperative radiation therapy for advanced cervical metastasis: a single institution experience Radiat Oncol 6 72 https://doi.org/10.1186/1748-717X-6-72 PMID: 21676211 PMCID: 3141525
23. Pinheiro AD, Foote RL, and McCaffrey TV, et al (2003) Intraoperative radiotherapy for head and neck and skull base cancer Head Neck 25(3) 217–25 https://doi.org/10.1002/hed.10203 PMID: 12599289
24. Vaidya JS BM, Tobias JS, et al (2001) Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer Ann Oncol 12 https://doi.org/10.1023/A:1011609401132 PMID: 11583188
25. Vaidya JS BM, Tobias JS, et al (2006) Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost Int J Radiat Oncol Biol Phys 66 1335–38 https://doi.org/10.1016/j.ijrobp.2006.07.1378 PMID: 17084562
26. Veronesi U OR, Luini A, et al (2010) Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons Breast Cancer Res Treat 124 141–51 https://doi.org/10.1007/s10549-010-1115-5 PMID: 20711810
27. Vaidya JS, Wenz F, and Bulsara M, et al (2014) Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial Lancet 383(9917) 603–13 https://doi.org/10.1016/S0140-6736(13)61950-9
28. Veronesi U, Orecchia R, and Maisonneuve P, et al (2013) Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial Lancet Oncol 14(13) 1269–77 https://doi.org/10.1016/S1470-2045(13)70497-2 PMID: 24225155
29. Cuzick J SH, Peto R, et al (1987) Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer Cancer Treat Rep 71 15–29 PMID: 2856861
30. Harness JK, Silverstein MJ, and Wazer DE, et al (2014) Radiotherapy for breast cancer, the TARGIT-A trial Lancet 383(9930) 1718–9 https://doi.org/10.1016/S0140-6736(14)60829-1 PMID: 24835612
31. Mackenzie P, Fyles A, Chung C (2014) Radiotherapy for breast cancer, the TARGIT-A trial Lancet 383(9930) 1717 https://doi.org/10.1016/S0140-6736(14)60827-8 PMID: 24835610
32. Yarnold J, Offersen BV, and Olivotto I, et al (2014) Radiotherapy for breast cancer, the TARGIT-A trial Lancet 383(9930) 1717–8 https://doi.org/10.1016/S0140-6736(14)60828-X PMID: 24835611
33. Cuzick J (2014) Radiotherapy for breast cancer, the TARGIT-A trial Lancet 383(9930) 1716 https://doi.org/10.1016/S0140-6736(14)60825-4 PMID: 24835608
34. Haviland JS, A’Hern R, and Bentzen SM, et al (2014) Radiotherapy for breast cancer, the TARGIT-A trial Lancet 383(9930) 1716–7 https://doi.org/10.1016/S0140-6736(14)60826-6 PMID: 24835609
35. Silverstein MJ, Fastner G, and Maluta S, et al (2014) Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials Part 2–TARGIT Ann Surg Oncol 21(12) 3793–9 https://doi.org/10.1245/s10434-014-3999-5 PMID: 25138079 PMCID: 4189006
36. Silverstein MJ, Fastner G, and Maluta S, et al (2014) Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials Part 1–ELIOT Ann Surg Oncol 21(12) 3787–92 https://doi.org/10.1245/s10434-014-3998-6 PMID: 25160734 PMCID: 4189005
37. Leonardi MC, Maisonneuve P, and Mastropasqua MG, et al (2013) Accelerated partial breast irradiation with intraoperative electrons: using GEC-ESTRO recommendations as guidance for patient selection Radiother Oncol 106(1) 21–7 https://doi.org/10.1016/j.radonc.2012.10.018
38. Leonardi MC, Maisonneuve P, and Mastropasqua MG, et al (2012) How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology Int J Radiat Oncol Biol Phys 83(3) 806–13 https://doi.org/10.1016/j.ijrobp.2011.08.014 PMID: 22245196
39. Smith BD, Arthur DW, and Buchholz TA, et al (2009) Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys 74(4) 987–1001 https://doi.org/10.1016/j.ijrobp.2009.02.031 PMID: 19545784
40. Polgar C, Van Limbergen E, and Potter R, et al (2010) Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol 94(3) 264–73 https://doi.org/10.1016/j.radonc.2010.01.014 PMID: 20181402
41. Maluta S, Dall’Oglio S, and Goer DA, et al (2014) Intraoperative electron radiotherapy (IOERT) as an alternative to standard whole breast irradiation: only for low-risk subgroups? Breast care 9(2) 102–6 https://doi.org/10.1159/000362392 PMID: 24944552 PMCID: 4038312
42. Fastner G, Sedlmayer F, and et al (2013) IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis Radiother Oncol 108(2) 279–86 https://doi.org/10.1016/j.radonc.2013.05.031 PMID: 23830467
43. Vaidya JS, Baum M, and Tobias JS, et al (2011) Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery Int J Radiat Oncol Biol Phys 81(4) 1091–7 https://doi.org/10.1016/j.ijrobp.2010.07.1996
44. Blank E, Kraus-Tiefenbacher U, and Welzel G, et al (2010) Single-center long-term follow-up after intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage x-rays Ann Surg Oncol 17 Suppl 3 352–8 https://doi.org/10.1245/s10434-010-1265-z PMID: 20853058
45. Malter W, Puppe J, and Rogee K, et al (2012) Single center experiences with intraoperative radiotherapy as a boost during oncoplastic breast-conserving surgery Eur J Cancer 48 S219 https://doi.org/10.1016/S0959-8049(12)70663-9
46. Malter W, Kirn V, and Mallmann P, et al (2014) Oncoplastic breast reconstruction after IORT Transla Cancer Res 3(1) 74–82
47. Braendengen M, Tveit KM, and Berglund A, et al (2008) Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer J Clin Oncol 26(22) 3687–94 https://doi.org/10.1200/JCO.2007.15.3858 PMID: 18669453
48. Frykholm GJ, Pahlman L, Glimelius B (2001) Combined chemo- and radiotherapy vs. radiotherapy alone in the treatment of primary, nonresectable adenocarcinoma of the rectum Int J Radiat Oncol Biol Phys 50(2) 427–34 https://doi.org/10.1016/S0360-3016(01)01479-1 PMID: 11380230
49. Calvo FA, Gomez-Espi M, and Diaz-Gonzalez JA, et al (2002) Intraoperative presacral electron boost following preoperative chemoradiation in T3-4Nx rectal cancer: initial local effects and clinical outcome analysis Radiother Oncol 62(2) 201–6 https://doi.org/10.1016/S0167-8140(01)00477-7 PMID: 11937247
50. Mathis KL, Nelson H, and Pemberton JH, et al (2008) Unresectable colorectal cancer can be cured with multimodality therapy Ann Surg 248(4) 592–8 PMID: 18936572
51. Minsky BD, Cohen AM, and Enker WE, et al Radiation therapy for unresectable rectal cancer Int J Radiat Oncol Biol Phys 21(5) 1283–9 PMID: 1938525
52. Ratto C, Valentini V, and Morganti AG, et al (2003) Combined-modality therapy in locally advanced primary rectal cancer Dis Colon Rectum 46(1) 59–67 https://doi.org/10.1007/s10350-004-6497-1 PMID: 12544523
53. Sadahiro S, Suzuki T, and Ishikawa K, et al (2004) Preoperative radio/chemo-radiotherapy in combination with intraoperative radiotherapy for T3-4Nx rectal cancer Eur J Surg Oncol 30(7) 750–8 https://doi.org/10.1016/j.ejso.2004.04.012 PMID: 15296989
54. Ferenschild FT, Vermaas M, and Nuyttens JJ, et al (2006) Value of intraoperative radiotherapy in locally advanced rectal cancer Dis Colon Rectum 49(9) 1257–65 https://doi.org/10.1007/s10350-006-0651-x PMID: 16912909
55. Roeder F, Treiber M, and Oertel S, et al (2007) Patterns of failure and local control after intraoperative electron boost radiotherapy to the presacral space in combination with total mesorectal excision in patients with locally advanced rectal cancer Int J Radiat Oncol Biol Phys 67(5) 1381–8 https://doi.org/10.1016/j.ijrobp.2006.11.039 PMID: 17275208
56. Masaki T, Takayama M, and Matsuoka H, et al (2008) Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer Langenbecks Arch Surg 393(2) 173–80 https://doi.org/10.1007/s00423-007-0260-8 PMID: 18172677
57. Valentini V, Coco C, and Rizzo G, et al (2009) Outcomes of clinical T4M0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: a prospective evaluation of a single institutional experience Surgery 145(5) 486–94 https://doi.org/10.1016/j.surg.2009.01.007 PMID: 19375606
58. Dubois JB, Bussieres E, and Richaud P, et al (2011) Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study Radiother Oncol 98(3) 298–303 https://doi.org/10.1016/j.radonc.2011.01.017 PMID: 21339010
59. Kusters M, Valentini V, and Calvo FA, et al (2010) Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastases Ann Oncol 21(6) 1279–84 https://doi.org/10.1093/annonc/mdp501
60. Sole CV, Calvo FA, and Serrano J, et al (2014) Post-chemoradiation intraoperative electron-beam radiation therapy boost in resected locally advanced rectal cancer: long-term results focused on topographic pattern of locoregional relapse Radiother Oncol 112(1) 52–8 https://doi.org/10.1016/j.radonc.2014.05.012 PMID: 24997989
61. Holman FA, Haddock MG, and Gunderson LL, et al (2016) Results of intraoperative electron beam radiotherapy containing multimodality treatment for locally unresectable T4 rectal cancer: a pooled analysis of the Mayo Clinic Rochester and Catharina Hospital Eindhoven J Gastrointest Oncol 7(6) 903–16 https://doi.org/10.21037/jgo.2016.07.01
62. Willett CG, Shellito PC, and Tepper JE, et al (1991) Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma J Clin Oncol 9(5) 843–9 https://doi.org/10.1200/JCO.1991.9.5.843 PMID: 2016628
63. Suzuki K, Gunderson LL, and Devine RM, et al (1995) Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer Cancer 75(4) 939–52 PMID: 7531113
64. Willett CG, Shellito PC, and Tepper JE, et al (1991) Intraoperative electron beam radiation therapy for recurrent locally advanced rectal or rectosigmoid carcinoma Cancer 67(6) 1504–8 PMID: 2001537
65. Haddock MG, Miller RC, and Nelson H, et al (2011) Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer Int J Radiat Oncol Biol Phys 79(1) 143–50 https://doi.org/10.1016/j.ijrobp.2009.10.046
66. Valentini V, Morganti AG, and De Franco A, et al (1999) Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma: prognostic factors and long term outcome Cancer 86(12) 2612–24 PMID: 10594856
67. Wiig JN, Tveit KM, and Poulsen JP, et al (2002) Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? A prospective study Radiother Oncol 62(2) 207–13 https://doi.org/10.1016/S0167-8140(01)00486-8 PMID: 11937248
68. Calvo FA, Sole CV, and Alvarez de Sierra P, et al (2013) Prognostic impact of external beam radiation therapy in patients treated with and without extended surgery and intraoperative electrons for locally recurrent rectal cancer: 16-year experience in a single institution Int J Radiat Oncol Biol Phys 86(5) 892–900 https://doi.org/10.1016/j.ijrobp.2013.04.008 PMID: 23845842
69. Dresen RC, Gosens MJ, and Martijn H, et al (2008) Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer Ann Surg Oncol 15(7) 1937–47 https://doi.org/10.1245/s10434-008-9896-z PMID: 18389321 PMCID: 2467498
70. Roeder F, Goetz JM, and Habl G, et al (2012) Intraoperative electron radiation therapy (IOERT) in the management of locally recurrent rectal cancer BMC Cancer 12 592 https://doi.org/10.1186/1471-2407-12-592 PMID: 23231663 PMCID: 3557137
71. Holman FA, Bosman SJ, and Haddock MG, et al (2017) Results of a pooled analysis of IOERT containing multimodality treatment for locally recurrent rectal cancer: results of 565 patients of two major treatment centres Eur J Surg Oncol 43(1) 107–17 https://doi.org/10.1016/j.ejso.2016.08.015
72. Huber FT, Stepan R, and Zimmermann F, et al (1996) Locally advanced rectal cancer: resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy Dis Colon Rectum 39(7) 774–9 https://doi.org/10.1007/BF02054443 PMID: 8674370</a>
73. Nuyttens JJ, Kolkman-Deurloo IK, and Vermaas M, et al (2004) High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer Int J Radiat Oncol Biol Phys 58(1) 106–12 https://doi.org/10.1016/S0360-3016(03)01494-9
74. Mirnezami R, Chang GJ, and Das P, et al (2013) Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications Surg Oncol 22(1) 22–35 https://doi.org/10.1016/j.suronc.2012.11.001
75. Alektiar KM, Zelefsky MJ, and Paty PB, et al (2000) High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer Int J Radiat Oncol Biol Phys 48(1) 219–26 https://doi.org/10.1016/S0360-3016(00)00634-9 PMID: 10924992
76. Lindel K, Willett CG, and Shellito PC, et al (2001) Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer Radiother Oncol 58(1) 83–7 https://doi.org/10.1016/S0167-8140(00)00309-1 PMID: 11165686
77. Edmonson JH, Petersen IA, and Shives TC, et al (2002) Chemotherapy, irradiation, and surgery for function-preserving therapy of primary extremity soft tissue sarcomas: initial treatment with ifosfamide, mitomycin, doxorubicin, and cisplatin plus granulocyte macrophage-colony-stimulating factor Cancer 94(3) 786–92 https://doi.org/10.1002/cncr.10259 PMID: 11857314
78. Azinovic I, Martinez Monge R, and Aristu JJ, et al (2003) Intraoperative radiotherapy electron boost followed by moderate doses of external beam radiotherapy in resected soft-tissue sarcoma of the extremities Radiother Oncol 67(3) 331–7 https://doi.org/10.1016/S0167-8140(03)00163-4 PMID: 12865183
79. Kretzler A, Molls M, and Gradinger R, et al (2004) Intraoperative radiotherapy of soft tissue sarcoma of the extremity Strahlenther Onkol 180(6) 365–70 https://doi.org/10.1007/s00066-004-1191-8 PMID: 15175871
80. Oertel S, Treiber M, and Zahlten-Hinguranage A, et al (2006) Intraoperative electron boost radiation followed by moderate doses of external beam radiotherapy in limb-sparing treatment of patients with extremity soft-tissue sarcoma Int J Radiat Oncol Biol Phys 64(5) 1416–23 https://doi.org/10.1016/j.ijrobp.2005.10.009 PMID: 16413697
81. Niewald M, Fleckenstein J, and Licht N, et al (2009) Intraoperative radiotherapy (IORT) combined with external beam radiotherapy (EBRT) for soft-tissue sarcomas—a retrospective evaluation of the Homburg experience in the years 1995–2007 Radiat Oncol 4 32 https://doi.org/10.1186/1748-717X-4-32
82. Call JA, Stafford SL, and Petersen IA, et al (2014) Use of intraoperative radiotherapy for upper-extremity soft-tissue sarcomas: analysis of disease outcomes and toxicity Am J Clin Oncol 37(1) 81–5 https://doi.org/10.1097/COC.0b013e31826b9b3d
83. Calvo FA, Sole CV, and Polo A, et al (2014) Limb-sparing management with surgical resection, external-beam and intraoperative electron-beam radiation therapy boost for patients with primary soft tissue sarcoma of the extremity: a multicentric pooled analysis of long-term outcomes Strahlenther Onkol 190(10) 891–8 https://doi.org/10.1007/s00066-014-0640-2 PMID: 24715241
84. Roeder F, Lehner B, and Schmitt T, et al (2014) Excellent local control with IOERT and postoperative EBRT in high grade extremity sarcoma: results from a subgroup analysis of a prospective trial BMC Cancer 14 350 https://doi.org/10.1186/1471-2407-14-350 PMID: 24885755 PMCID: 4032585
85. Roeder F, Lehner B, and Saleh-Ebrahimi L, et al (2016) Intraoperative electron radiation therapy combined with external beam radiation therapy and limb sparing surgery in extremity soft tissue sarcoma: a retrospective single center analysis of 183 cases Radiother Oncol 119(1) 22–9 https://doi.org/10.1016/j.radonc.2015.11.014
86. Sindelar WF, Kinsella TJ, and Chen PW, et al (1993) Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial Arch Surg 128(4) 402–10 https://doi.org/10.1001/archsurg.1993.01420160040005 PMID: 8457152
87. Alektiar KM, Hu K, and Anderson L, et al (2000( High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas Int J Radiat Oncol Biol Phys 47(1) 157–63 https://doi.org/10.1016/S0360-3016(99)00546-5 PMID: 10758318
88. Gieschen HL, Spiro IJ, and Suit HD, et al (2001) Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma Int J Radiat Oncol Biol Phys 50(1) 127–31 https://doi.org/10.1016/S0360-3016(00)01589-3 PMID: 11316555
89. Petersen IA, Haddock MG, and Donohue JH, et al (2002) Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas Int J Radiat Oncol Biol Phys 52(2) 469–75 https://doi.org/10.1016/S0360-3016(01)02595-0 PMID: 11872294
90. Bobin JY, Al-Lawati T, and Granero LE, et al (2003) Surgical management of retroperitoneal sarcomas associated with external and intraoperative electron beam radiotherapy Eur J Surg Oncol 29(8) 676–81 https://doi.org/10.1016/S0748-7983(03)00139-2 PMID: 14511617
91. Pierie JP, Betensky RA, and Choudry U, et al (2006) Outcomes in a series of 103 retroperitoneal sarcomas Eur J Surg Oncol 32(10) 1235–41 https://doi.org/10.1016/j.ejso.2006.07.002 PMID: 16919908
92. Krempien R, Roeder F, and Oertel S, et al (2006) Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma Int J Radiat Oncol Biol Phys 65(3) 773–9 https://doi.org/10.1016/j.ijrobp.2006.01.028 PMID: 16682152
93. Pawlik TM, Pisters PW, and Mikula L, et al (2006) Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma Ann Surg Oncol 13(4) 508–17 https://doi.org/10.1245/ASO.2006.05.035 PMID: 16491338
94. Ballo MT, Zagars GK, and Pollock RE, et al (2007) Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment Int J Radiat Oncol Biol Phys 67(1) 158–63 https://doi.org/10.1016/j.ijrobp.2006.08.025
95. Dziewirski W, Rutkowski P, and Nowecki ZI, et al (2006) Surgery combined with intraoperative brachytherapy in the treatment of retroperitoneal sarcomas Ann Surg Oncol 13(2) 245–52 https://doi.org/10.1245/ASO.2006.03.026 PMID: 16411144
96. Sweeting RS, Deal AM, and Llaguna OH, et al (2013) Intraoperative electron radiation therapy as an important treatment modality in retroperitoneal sarcoma J Surg Res 185(1) 245–9 https://doi.org/10.1016/j.jss.2013.05.015 PMID: 23769633 PMCID: 4166614
97. Roeder F, Ulrich A, and Habl G, et al (2014) Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: interim analysis BMC Cancer 14 617 https://doi.org/10.1186/1471-2407-14-617 PMID: 25163595 PMCID: 4156610
98. Stucky CC, Wasif N, and Ashman JB, et al (2014) Excellent local control with preoperative radiation therapy, surgical resection, and intra-operative electron radiation therapy for retroperitoneal sarcoma J Surg Oncol 109(8) 798–803 https://doi.org/10.1002/jso.23576 PMID: 24862926
99. Gronchi A, De Paoli A, and Dani C, et al (2014) Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: a phase I-II study from the Italian Sarcoma Group Eur J Cancer 50(4) 784–92 https://doi.org/10.1016/j.ejca.2013.11.021
100. Miller RC, Haddock MG, and Petersen IA, et al (2006) Intraoperative electron-beam radiotherapy and ureteral obstruction Int J Radiat Oncol Biol Phys 64(3) 792–8 https://doi.org/10.1016/j.ijrobp.2005.08.019
101. Calvo FA, Sole CV, and Cambeiro M, et al (2014) Prognostic value of external beam radiation therapy in patients treated with surgical resection and intraoperative electron beam radiation therapy for locally recurrent soft tissue sarcoma: a multicentric long-term outcome analysis Int J Radiat Oncol Biol Phys 88(1) 143–50 https://doi.org/10.1016/j.ijrobp.2013.10.021
102. Haase GM, Meagher DP, Jr., and McNeely LK, et al (1994) Electron beam intraoperative radiation therapy for pediatric neoplasms Cancer 74(2) 740–7 PMID: 8033056
103. Oertel S, Niethammer AG, and Krempien R, et al (2006) Combination of external-beam radiotherapy with intraoperative electron-beam therapy is effective in incompletely resected pediatric malignancies Int J Radiat Oncol Biol Phys 64(1) 235–41 https://doi.org/10.1016/j.ijrobp.2005.06.038
104. Goodman KA, Wolden SL, and LaQuaglia MP, et al (2003) Intraoperative high-dose-rate brachytherapy for pediatric solid tumors: a 10-year experience Brachytherapy 2(3) 139–46 https://doi.org/10.1016/S1538-4721(03)00135-1
105. Sole CV, Calvo FA, and Polo A, et al (2015) Intraoperative electron-beam radiation therapy for pediatric Ewing sarcomas and rhabdomyosarcomas: long-term outcomes Int J Radiat Oncol Biol, Phys 92(5) 1069–76 https://doi.org/10.1016/j.ijrobp.2015.04.048
106. Nag S, Tippin D, and Smith S, et al (2003) Intraoperative electron beam treatment for pediatric malignancies: The Ohio State University experience Med Pediatr Oncol 40(6) 360–6 https://doi.org/10.1002/mpo.10296 PMID: 12692803
107. Stauder MC, Laack NN, and Moir CR, et al (2011) Excellent local control and survival after intraoperative and external beam radiotherapy for pediatric solid tumors: long-term follow-up of the Mayo Clinic experience J Pediatr Hematol/Oncol 33(5) 350–5 https://doi.org/10.1097/MPH.0b013e3182148dad
108. Brunschwig A, Barber HR (1964) Extended pelvic exenteration for advanced cancer of the cervix. long survivals following added resection of involved small bowel Cancer 17 1267–70 PMID: 14236759
109. Karlen JR, Piver MS (1975) Reduction of mortality and morbidity associated with pelvic exenteration Gynecol Oncol 3(2) 164–7 https://doi.org/10.1016/0090-8258(75)90076-1 PMID: 1183867
110. Kiselow M, Butcher HR, Jr., Bricker EM (1967) Results of the radical surgical treatment of advanced pelvic cancer: a fifteen-year study Ann Surg 166(3) 428–36 PMID: 6039602 PMCID: 1477392
111. Symmonds RE, Pratt JH, Webb MJ (1975) Exenterative operations: experience with 198 patients Am J Obstet Gynecol 121(7) 907–18 https://doi.org/10.1016/0002-9378(75)90908-4 PMID: 1115180
112. Giorda G, Boz G, and Gadducci A, et al (2011) Multimodality approach in extra cervical locally advanced cervical cancer: chemoradiation, surgery and intra-operative radiation therapy A phase II trial Eur J Surg Oncol 37(5) 442–7 https://doi.org/10.1016/j.ejso.2011.02.011 PMID: 21492777
113. Garton GR, Gunderson LL, and Webb MJ, et al (1997) Intraoperative radiation therapy in gynecologic cancer: update of the experience at a single institution Int J Radiat Oncol Biol Phys 37(4) 839–43 https://doi.org/10.1016/S0360-3016(96)00546-9 PMID: 9128960
114. Gemignani ML, Alektiar KM, and Leitao M, et al (2001) Radical surgical resection and high-dose intraoperative radiation therapy (HDR-IORT) in patients with recurrent gynecologic cancers Int J Radiat Oncol Biol Phys 50(3) 687–94 https://doi.org/10.1016/S0360-3016(01)01507-3 PMID: 11395237
115. Haddock MG, Petersen IA, and Webb MJ,et al (1997) IORT for locally advanced gynecological malignancies Front Radiat Ther Oncol 31 256–9 https://doi.org/10.1159/000061131 PMID: 9263836
116. Martinez-Monge R, and Jurado M, et al (2001) Intraoperative electron beam radiotherapy during radical surgery for locally advanced and recurrent cervical cancer Gynecol Oncol 82(3) 538–43 https://doi.org/10.1006/gyno.2001.6329 PMID: 11520152
117. Sole CV, Calvo FA, and Lozano MA, et al (2014) External-beam radiation therapy after surgical resection and intraoperative electron-beam radiation therapy for oligorecurrent gynecological cancer Long-term outcome Strahlenther Onkol 190(2) 171–80 https://doi.org/10.1007/s00066-013-0472-5
118. Tran PT, Su Z, and Hara W, et al (2007) Long-term survivors using intraoperative radiotherapy for recurrent gynecologic malignancies Int J Radiat Oncol Biol Phys 69(2) 504–11 https://doi.org/10.1016/j.ijrobp.2007.03.021 PMID: 17560736
119. Barney BM, Petersen IA, and Dowdy SC, et al (2013) Intraoperative electron beam radiotherapy (IOERT) in the management of locally advanced or recurrent cervical cancer Radiat Oncol 8 80 https://doi.org/10.1186/1748-717X-8-80 PMID: 23566444 PMCID: 3641982
120. Calvo FA, Sole CV, and Lozano MA, et al (2013) Intraoperative electron beam radiotherapy and extended surgical resection for gynecological pelvic recurrent malignancies with and without external beam radiation therapy: long-term outcomes Gynecol Oncol 130(3) 537–44 https://doi.org/10.1016/j.ygyno.2013.05.016 PMID: 23707668
121. Foley OW, Rauh-Hain JA, and Clark RM, et al (2016) Intraoperative radiation therapy in the management of gynecologic malignancies Am J Clin Oncol 39(4) 329–34 https://doi.org/10.1097/COC.0000000000000063
122. Arians N, Foerster R, and Rom J, et al (2016) Outcome of patients with local recurrent gynecologic malignancies after resection combined with intraoperative electron radiation therapy (IOERT) Radiat Oncol 11 44 https://doi.org/10.1186/s13014-016-0622-x PMID: 26988089 PMCID: 4797348
123. Dowdy SC, Mariani A, and Cliby WA, et al (2006) Radical pelvic resection and intraoperative radiation therapy for recurrent endometrial cancer: technique and analysis of outcomes Gynecol Oncol 101(2) 280–6 https://doi.org/10.1016/j.ygyno.2005.10.018
124. Mahe MA, Gerard JP, and Dubois JB, et al (1996) Intraoperative radiation therapy in recurrent carcinoma of the uterine cervix: report of the French intraoperative group on 70 patients Int J Radiat Oncol Biol Phys 34(1) 21–6 https://doi.org/10.1016/0360-3016(95)02089-6 PMID: 12118553
125. Mahe MA, Romestaing P, and Gerard JP, et al (1997) Prognostic factors for local control in recurrent cervical carcinoma treated with IORT: report of the French IORT Group Front Radiat Ther Oncol 31 267–70 https://doi.org/10.1159/000061190 PMID: 9263839
126. del Carmen MG, McIntyre JF, and Fuller AF, et al (2000) Intraoperative radiation therapy in the treatment of pelvic gynecologic malignancies: a review of fifteen cases Gynecol Oncol 79(3) 457–62 https://doi.org/10.1006/gyno.2000.6002 PMID: 11104619
127. Backes FJ, Billingsley CC, and Martin DD, et al (2014) Does intra-operative radiation at the time of pelvic exenteration improve survival for patients with recurrent, previously irradiated cervical, vaginal, or vulvar cancer? Gynecol Oncol 135(1) 95–9 https://doi.org/10.1016/j.ygyno.2014.07.093 PMID: 25084510
128. Hallemeier CL, Karnes RJ, and Pisansky TM, et al (2013) Multimodality therapy including surgical resection and intraoperative electron radiotherapy for recurrent or advanced primary carcinoma of the urinary bladder or ureter Am J Clin Oncol 36(6) 596–600 https://doi.org/10.1097/COC.0b013e31825d52f7
129. Hallemeier CL, Choo R, and Davis BJ, et al (2012) Long-term outcomes after maximal surgical resection and intraoperative electron radiotherapy for locoregionally recurrent or locoregionally advanced primary renal cell carcinoma Int J Radiat Oncol Biol Phys 82 https://doi.org/10.1016/j.ijrobp.2011.02.026
130. Calvo FA, Sole CV, and Martinez-Monge R, et al (2013) Intraoperative EBRT and resection for renal cell carcinoma: twenty-year outcomes Strahlentherapie Onkol 189
131. Eble MJ, Staehler G, Wannenmacher M (1998) [The intraoperative radiotherapy (IORT) of locally spread and recurrent renal-cell carcinomas] Strahlentherapie Onkol 174(1) 30–6 https://doi.org/10.1007/BF03038225
132. Habl G, Uhl M, and Hensley F, et al (2013) Intraoperative electron radiation therapy (IOERT) in patients with locally recurrent renal cell carcinoma Radiat Oncol 8(1) 282 https://doi.org/10.1186/1748-717X-8-282 PMID: 24295293 PMCID: 3922867
133. Master VA, Gottschalk AR, and Kane C, et al (2005) Management of isolated renal fossa recurrence following radical nephrectomy J Urol 174 https://doi.org/10.1097/01.ju.0000165574.62188.d0
134. Paly JJ, Hallemeier CL, and Biggs PJ, et al (2012) Outcomes for a multi-institutional cohort of patients treated with intraoperative radiation therapy for advanced or recurrent renal cell carcinoma [abstract] Int J Oncol Biol Phys 84 https://doi.org/10.1016/j.ijrobp.2012.07.1123
135. Thompson IM, Valicenti RK, and Albertsen P, et al (2013) Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline J Urol 190(2) 441–9 https://doi.org/10.1016/j.juro.2013.05.032 PMID: 23707439
136. Rocco B, Jereczek-Fossa BA, and Matei DV, et al (2009) Intraoperative radiotherapy during radical prostatectomy for intermediate-risk to locally advanced prostate cancer: treatment technique and evaluation of perioperative and functional outcome vs standard radical prostatectomy, in a matched-pair analysis BJU Int 104(11) 1624–30 https://doi.org/10.1111/j.1464-410X.2009.08668.x PMID: 19624597
137. Higashi Y, Hyochi N, Tari K (1998) [Intraoperative radiotherapy combined with external beam radiation for prostate cancer without metastasis] Nihon Rinsho 56(8) 2177–80 PMID: 9750530
138. Kato S, Sakura M, and Kazumoto T, et al (1998) Intraoperative radiation therapy for locally advanced prostate cancer J JASTRO 10(3) 241–8
139. Krengli M, Terrone C, and Ballare A, et al (2010) Intraoperative radiotherapy during radical prostatectomy for locally advanced prostate cancer: technical and dosimetric aspects Int J Radiat Oncol Biol Phys 76(4) 1073–7 https://doi.org/10.1016/j.ijrobp.2009.03.037
140. Orecchia R, Jereczek-Fossa BA, and Ciocca M, et al (2007) Intraoperative radiotherapy for locally advanced prostate cancer: treatment technique and ultrasound-based analysis of dose distribution Anticancer Res 27(5b) 3471–6 PMID: 17972503
141. Saracino B, Gallucci M, and et al (2008) Phase I-II study of intraoperative radiation therapy (IORT) after radical prostatectomy for prostate cancer Int J Radiat Oncol Biol Phys 71(4) 1049–56 https://doi.org/10.1016/j.ijrobp.2007.11.076 PMID: 18325679
142. Wisbeck WM, Becher EM, Russell AH (1986) Adenocarcinoma of the stomach: autopsy observations with therapeutic implications for the radiation oncologist Radiother Oncol 7(1) 13–8 https://doi.org/10.1016/S0167-8140(86)80120-7 PMID: 3775075
143. Gunderson LL (2002) Gastric cancer—patterns of relapse after surgical resection Semin Radiat Oncol 12(2) 150–61 https://doi.org/10.1053/srao.2002.30817 PMID: 11979416
144. Macdonald JS, Smalley SR, and Benedetti J, et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction N Engl J Med 345(10) 725–30 https://doi.org/10.1056/NEJMoa010187 PMID: 11547741
145. Cunningham D, Allum WH, and Stenning SP, et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer New Engl J Med 355(1) 11–20 https://doi.org/10.1056/NEJMoa055531 PMID: 16822992
146. Wu CW, Hsiung CA, and Lo SS, et al (2006) Nodal dissection for patients with gastric cancer: a randomised controlled trial Lancet Oncol 7(4) 309–15 https://doi.org/10.1016/S1470-2045(06)70623-4 PMID: 16574546
147. Maruyama K, Sasako M, and Kinoshita T, et al (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer World J Surg 19(4) 532–6 https://doi.org/10.1007/BF00294714 PMID: 7676695
148. Fu S, Lu JJ, and Zhang Q, et al (2008) Intraoperative radiotherapy combined with adjuvant chemoradiotherapy for locally advanced gastric adenocarcinoma Int J Radiat Oncol, Biol, Phys 72(5) 1488–94 https://doi.org/10.1016/j.ijrobp.2008.03.012
149. Sindelar WF, Kinsella TJ, and Tepper JE, et al (1993) Randomized trial of intraoperative radiotherapy in carcinoma of the stomach Am J Surg 165(1) 178–86 https://doi.org/10.1016/S0002-9610(05)80423-4 PMID: 8418695
150. Zhang Q, Tey J, and Peng L, et al (2012) Adjuvant chemoradiotherapy with or without intraoperative radiotherapy for the treatment of resectable locally advanced gastric adenocarcinoma Radiother Oncol 102(1) 51–5 https://doi.org/10.1016/j.radonc.2011.10.008
151. Drognitz O, Henne K, and Weissenberger C, et al (2008) Long-term results after intraoperative radiation therapy for gastric cancer Int J Radiat Oncol Biol Phys 70(3) 715–21 https://doi.org/10.1016/j.ijrobp.2007.07.2331 PMID: 18164840
152. Ogata T, Araki K, and Matsuura K, et al (1995) A 10-year experience of intraoperative radiotherapy for gastric carcinoma and a new surgical method of creating a wider irradiation field for cases of total gastrectomy patients Int J Radiat Oncol Biol Phys 32(2) 341–7 https://doi.org/10.1016/0360-3016(94)00479-5 PMID: 7751175
153. Qin HL, Lin CH, Zhang XL (2006) Evaluation of intraoperative radiotherapy for gastric carcinoma with D2 and D3 surgical resection World J Gastroenterol 12(43) 7033–7 https://doi.org/10.3748/wjg.v12.i43.7033 PMID: 17109501 PMCID: 4087350
154. Skoropad VY, Berdov BA, and Mardynski YS, et al (2000) A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer Eur J Surg Oncol 26(8) 773–9 https://doi.org/10.1053/ejso.2000.1002 PMID: 11087644
155. Calvo FA, Sole CV, and Obregon R, et al (2013) Intraoperative radiotherapy for the treatment of resectable locally advanced gastric adenocarcinoma: topography of locoregional recurrences and long-term outcomes. Clin Transl Oncol 15(6) 443–9 https://doi.org/10.1007/s12094-012-0949-1
156. Coquard R, Ayzac L, and Gilly FN, et al (1997) Intraoperative radiation therapy combined with limited lymph node resection in gastric cancer: an alternative to extended dissection? Int J Radiat Oncol Biol Phys 39(5) 1093–8 https://doi.org/10.1016/S0360-3016(97)00386-6 PMID: 9392549
157. Bacalbasa N, Balescu I, and Calin M, et al (2014) Intraoperative radiation therapy in gastric cancer J Med life 7(2) 128–31 PMID: 25408715 PMCID: 4197496
158. Avizonis VN, Buzydlowski J, and Lanciano R, et al (1995) Treatment of adenocarcinoma of the stomach with resection, intraoperative radiotherapy, and adjuvant external beam radiation: a phase II study from Radiation Therapy Oncology Group 85-04 Ann Surg Oncol 2(4) 295–302 https://doi.org/10.1007/BF02307060 PMID: 7552617
159. Weese JL, Harbison SP, and Stiller GD, et al (2000) Neoadjuvant chemotherapy, radical resection with intraoperative radiation therapy (IORT): improved treatment for gastric adenocarcinoma Surgery 128(4) 564–71 https://doi.org/10.1067/msy.2000.108420 PMID: 11015089
160. Glehen O, Peyrat P, and Beaujard AC, et al (2003) Pattern of failures in gastric cancer patients with lymph node involvement treated by surgery, intraoperative and external beam radiotherapy Radiother Oncol 67(2) 171–5 https://doi.org/10.1016/S0167-8140(02)00344-4 PMID: 12812847
161. Miller RC, Haddock MG, and Gunderson LL, et al (2006) Intraoperative radiotherapy for treatment of locally advanced and recurrent esophageal and gastric adenocarcinomas Dis Esophagus 19(6) 487–95. https://doi.org/10.1111/j.1442-2050.2006.00626.x PMID: 17069594
162. Gourgou-Bourgade S, Bascoul-Mollevi C, and Desseigne F, et al (2013) Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial J Clin Oncol 31(1) 23–9 https://doi.org/10.1200/JCO.2012.44.4869
163. Hazard L, Tward JD, and Szabo A, et al (2007) Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the Surveillance, Epidemiology, and End Results (SEER) registry data Cancer 110(10) 2191–201 https://doi.org/10.1002/cncr.23047 PMID: 17918259
164. Von Hoff DD, Ervin T, and Arena FP, et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine N Engl J Med 369(18) 1691–703 https://doi.org/10.1056/NEJMoa1304369 PMID: 24131140 PMCID: 4631139
165. Alfieri S, Morganti AG, and Di Giorgio A, et al (2001) Improved survival and local control after intraoperative radiation therapy and postoperative radiotherapy: a multivariate analysis of 46 patients undergoing surgery for pancreatic head cancer Arch Surg 136(3) 343–7 https://doi.org/10.1001/archsurg.136.3.343 PMID: 11231859
166. Calvo FA, Sole CV, and Atahualpa F, et al (2013) Chemoradiation for resected pancreatic adenocarcinoma with or without intraoperative radiation therapy boost: long-term outcomes Pancreatology 13(6) 576–82 https://doi.org/10.1016/j.pan.2013.09.002 PMID: 24280572
167. Showalter TN, Rao AS, and Anne PR, et al (2009) Does intraoperative radiation therapy improve local tumor control in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma? A propensity score analysis Ann Surg Oncol 16(8) 2116–22 https://doi.org/10.1245/s10434-009-0498-1 PMID: 19437078
168. Valentini V, Calvo F, and Reni M, et al (2009) Intra-operative radiotherapy (IORT) in pancreatic cancer: joint analysis of the ISIORT-Europe experience Radiother Oncol 91(1) 54–9 https://doi.org/10.1016/j.radonc.2008.07.020
169. Messick C, Hardacre JM, and McGee MF, et al (2008) Early experience with intraoperative radiotherapy in patients with resected pancreatic adenocarcinoma Am J Surg 195(3) 308–11 https://doi.org/10.1016/j.amjsurg.2007.12.024 PMID: 18207129
170. Ogawa K, Karasawa K, and Ito Y, et al (2010) Intraoperative radiotherapy for resected pancreatic cancer: a multi-institutional retrospective analysis of 210 patients Int J Radiat Oncol Biol Phys 77(3) 734–42 https://doi.org/10.1016/j.ijrobp.2009.09.010 PMID: 20207498
171. Valentini V, Morganti AG, and Macchia G, et al (2008) Intraoperative radiation therapy in resected pancreatic carcinoma: long-term analysis Int J Radiat Oncol Biol Phys 70(4) 1094–9 https://doi.org/10.1016/j.ijrobp.2007.07.2346 PMID: 18313525
172. Kokubo M, Nishimura Y, Shibamoto Y, Sasai K, Kanamori S, Hosotani R, et al. Analysis of the clinical benefit of intraoperative radiotherapy in patients undergoing macroscopically curative resection for pancreatic cancer. International journal of radiation oncology, biology, physics. 2000;48(4):1081-7. https://doi.org/10.1016/S0360-3016(00)00673-8 PMID: 11072166
173. Reni M, Panucci MG, and Ferreri AJ, et al (2001) Effect on local control and survival of electron beam intraoperative irradiation for resectable pancreatic adenocarcinoma Int J Radiat Oncol Biol Phys 50(3) 651–8 https://doi.org/10.1016/S0360-3016(01)01470-5 PMID: 11395232
174. Cai S, Hong TS, and Goldberg SI, et al (2013) Updated long-term outcomes and prognostic factors for patients with unresectable locally advanced pancreatic cancer treated with intraoperative radiotherapy at the Massachusetts General Hospital, 1978 to 2010 Cancer 119(23) 4196–204 https://doi.org/10.1002/cncr.28329 PMID: 24006012 PMCID: 4403862
175. Chen Y, Che X, and Zhang J, et al (2016) Long-term results of intraoperative electron beam radiation therapy for nonmetastatic locally advanced pancreatic cancer: retrospective cohort study, 7-year experience with 247 patients at the National Cancer Center in China Medicine 95(38) e4861 https://doi.org/10.1097/MD.0000000000004861 PMID: 27661028 PMCID: 5044898
176. Jingu K, Tanabe T, and Nemoto K, et al (2012) Intraoperative radiotherapy for pancreatic cancer: 30-year experience in a single institution in Japan Int J Radiat Oncol Biol Phys 83(4) e507–11 https://doi.org/10.1016/j.ijrobp.2012.01.024 PMID: 22445002
177. Ma HB, Di ZL, and Wang XJ, et al (2004) Effect of intraoperative radiotherapy combined with external beam radiotherapy following internal drainage for advanced pancreatic carcinoma World J Gastroenterol 10(11) 1669–771 https://doi.org/10.3748/wjg.v10.i11.1669 PMID: 15162548 PMCID: 4572777
178. Mohiuddin M, Regine WF, and Stevens J, et al (1995) Combined intraoperative radiation and perioperative chemotherapy for unresectable cancers of the pancreas J Clin Oncol 13(11) 2764–8 https://doi.org/10.1200/JCO.1995.13.11.2764 PMID: 7595736
179. Nishimura Y, Hosotani R, and Shibamoto Y, et al (1997) External and intraoperative radiotherapy for resectable and unresectable pancreatic cancer: analysis of survival rates and complications Int J Radiat Oncol, Biol, Phys 39(1) 39–49 https://doi.org/10.1016/S0360-3016(97)00295-2
180. Willett CG, Del Castillo CF, and Shih HA, et al (2005)_ Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer Ann Surg 241(2) 295–9. https://doi.org/10.1097/01.sla.0000152016.40331.bb PMID: 15650640 PMCID: 1356915
181. Keane FK, Wo JY, and Ferrone CR, et al Intraoperative radiotherapy in the era of intensive neoadjuvant chemotherapy and chemoradiotherapy for pancreatic adenocarcinoma Am J Clin Oncol 9000
182. Conroy T, Bachet JB, and Ayav A, et al (2016) Current standards and new innovative approaches for treatment of pancreatic cancer Eur J Cancer 57 10–22 https://doi.org/10.1016/j.ejca.2015.12.026 PMID: 26851397
183. Hackert T, Sachsenmaier M, and Hinz U, et al (2016) Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg 264(3) 457–63 https://doi.org/10.1097/SLA.0000000000001850 PMID: 27355262






