Repurposing Drugs in Oncology (ReDO)—nitroglycerin as an anti-cancer agent
Vidula Sukhatme1, §*, Gauthier Bouche2, Lydie Meheus2, Vikas P Sukhatme1, 3 and Pan Pantziarka2, 4, §*
1GlobalCures, Inc, Newton MA 02459, USA
2Anticancer Fund, Brussels, 1853 Strombeek-Bever, Belgium
3Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA
4The George Pantziarka TP53 Trust, London KT1 2JP, UK
§Corresponding authors, *Lead authors
Correspondence to: Pan Pantziarka. Email: pan.pantziarka@anticancerfund.org
Abstract
Nitroglycerin (NTG), a drug that has been in clinical use for more than a century, has a range of actions which make it of particular interest in an oncological setting. It is generally accepted that the main mechanism of action of NTG is via the production of nitric oxide (NO), which improves cardiac oxygenation via multiple mechanisms including improved blood flow (vasodilation), decreased platelet aggregation, increased erythrocyte O2 release and decreased mitochondrial utilization of oxygen. Its vasoactive properties mean that it has the potential to exploit more fully the enhanced permeability and retention effect in delivering anti-cancer drugs to tumour tissues. Moreover NTG can reduce HIF-1α levels in hypoxic tumour tissues and this may have anti-angiogenic, pro-apoptotic and anti-efflux effects. Additionally NTG may enhance anti-tumour immunity. Pre-clinical and clinical data on these anti-cancer properties of NTG are summarised and discussed. While there is evidence of a positive action as a monotherapy in prostate cancer, there are mixed results in NSCLC where initially positive results have yet to be fully replicated. Based on the evidence presented, a case is made that further exploration of the clinical benefits that may accrue to cancer patients is warranted. Additionally, it is proposed that NTG may synergise with a number of other drugs, including other repurposed drugs, and these are discussed in the supplementary material appended to this paper.
Keywords: ReDO project, drug repurposing, nitroglycerin, hypoxia, EPR effect
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 27/08/2015; Received: 29/06/2015
Current usage
Introduction
Nitroglycerin (NTG), also called glyceryl trinitrate, is an organic nitrate with potent vasodilatory effects. It has a long history of use as a coronary vasodilator, most commonly used for prophylaxis and treatment of angina pectoris, but also used as a treatment for hypertension, congestive heart failure and for the induction of surgical hypotension. As with other medical organic nitrates, such as isosorbide dinitrate and isosorbide mononitrate, NTG acts as a nitric oxide (NO) donor, which is a key vasodilatory effector molecule. NTG is a prescription drug available globally as a generic.
Dosage
NTG is available in sublingual tablet and spray form for oral use, as a transdermal patch, as an ointment and as an intravenous infusion. For the prevention or treatment of angina attacks sublingual tablets are used at a dose of 0.3–1.0 mg, the dose repeated as required. The typical metered dose for sprays is 0.4 mg, with one or two doses sprayed under the tongue. Transdermal patches are used for longer term prophylaxis of angina, with typical dosages being 5 mg/day–15 mg/day. The ointment form of NTG is used for the treatment of anal fissure as well as prophylaxis of angina. For the treatment of anal fissure a 0.4% w/w ointment is used, whereas for angina the ointment is 2% w/w, giving a dose of approximately 0.8 mg per hour per inch of ointment.
Sublingual treatment, using either the spray or tablets, can provide rapid relief of angina symptoms, though the effect may only last 20–30 minutes. For longer term prophylaxis using transdermal patches or ointment, patients can develop tolerance, often after around two weeks of use. However, lowering the blood-nitrate concentration for extended periods can reverse tolerance. Therefore patients are advised to remove patches over-night or else to ensure they have 4 to 8 hours without patches or ointment in every 24 hours.
Toxicity
NTG has low systemic toxicity. Common side effects include headache, dizziness, postural hypotension and tachycardia. Less common effects include nausea, vomiting, heartburn, flushing, and rash. NTG is contraindicated for those with known hypersensitivity to nitrates, severe anaemia, increased intracranial pressure or some hypotensive conditions. Caution is also advised for patients being treated with PDE-5 inhibitors (e.g. sildenafil citrate, tadalafil, vardenafil hydrochloride). These drugs have been shown to potentiate the hypotensive effects of organic nitrates, including NTG.
Pharmacokinetics
NTG undergoes extensive hepatic and non-hepatic clearance with peak plasma concentrations achieved within 3–5 minutes for sublingual tablets or sprays, reaching 1.40 ng/ml (6.2 nM) to 1.97 ng/ml (8.7 nM) after 0.5 mg sublingual tablet and 3.96 ng/ml (17.4 nM) after 0.8 mg sublingual spray [1, 2]. For transdermal delivery, peak levels are achieved in around 60 minutes. For a transdermal system delivery at a dose of 10 mg/day, steady state levels achieved were 0.15 ± 0.12 ng/ml (0.7 ± 0.5 nM) [3, 4]. Note that all forms of NTG delivery are prone to high-levels of inter and intra-patient variability [3]. The two main metabolites of NTG are 1,2 and 1,3-glyceryl dinitrate (1,2-GDN and 1,3-GDN), which achieve higher plasma concentrations than the parent compound but which have lower vasodilatory activity; nevertheless these metabolites contribute to the pharmacological effects of NTG administration [5].
Pre-clinical evidence in cancer—in vitro and in vivo
NO is a ubiquitous signalling, regulatory and effector molecule with diverse effects in the human body, including the vascular, neuronal and immune systems. With respect to cancer, NO is known to have dichotomous effects, both pro- and anti-tumour, depending on concentration, microenvironment and cell type [6–8]. In particular, low concentrations of NO (<100 nM) are associated with increases in angiogenesis, proliferation and resistance to apoptosis, whereas high NO concentrations (>500 nM) are associated with increased cytotoxicity and apoptosis [6]. While this biphasic property of NO is complex, it has not inhibited exploration of its use as an anti-cancer agent.
Interest in the potential use of NTG in cancer treatment arose primarily from investigations into the increased vascular permeability exhibited by solid tumours. This phenomenon, possibly related to inflammatory responses to injury or infection [9], was seen by some researchers as a potential mechanism for the targeted delivery of chemotherapeutic agents to solid tumours [10, 11]. However, subsequent investigations of anticancer effects have not been limited to vascular permeability alone.
Matthews et al investigated the relationship between hypoxia, endogenous NO production and the chemosensitivity of human and murine cancer cell lines [12]. Human breast cancer (MDA-MB-231) and mouse melanoma (B16F10) cells were exposed to differing levels of O2 and then incubated with an inhibitor of NO production and treated with either doxorubicin or 5-fluorouracil. Results showed that hypoxia and inhibition of endogenous NO production rapidly induced resistance to both chemotherapeutic agents. The increase in cell survival on exposure to doxorubicin was mimicked under normoxic conditions by use of an inhibitor of endogenous NO synthase, suggesting that low NO levels contribute to hypoxia-related chemoresistance. This effect could be partially reversed using low doses of NTG (1 μM and 0.1 nM) or diethylenetriamine NO adduct (1 μM).
Hypoxia is a known driver of increased tumour invasiveness and the effect of NTG on this process was assessed by the same group, also using the MDA-MB-231 breast cancer cell line [13]. Hypoxic conditions increased Matrigel invasion five-fold compared to normoxia, an effect abolished by the use of NTG (again at the low dose of 1 μM and 0.1 nM) or sodium nitroprusside. Similarly, in vivo investigations on the effect of hypoxia on metastases in a melanoma model showed that NTG reversed the increase in metastatic nodules induced by hypoxic conditions [14].
Other in vitro investigations of hypoxia, chemosensitivity and NTG have included prostate cancer [15, 16] and breast cancer three dimensional tumour spheroids [17].
Escape from immunosurveillance may also be a consequence of tumour hypoxia, and this was investigated by Siemens et al in prostate cancer cell lines and a murine xenograft model (using the human PC-3 prostate cancer cell line) [18]. The study showed that impaired NO signalling, associated with hypoxia, increased tumour cell shedding of MHC class I chain-related (MIC) molecules and thus contributed to immune escape. This effect could be reversed with exogenous NO using NTG, and in the murine model transdermal patches, delivering NTG at a rate of 7.3 μg/h, were used to attenuate the growth of xenografted MIC-expressing human prostate tumours compared to placebo. Later studies further elucidated this effect using the human prostate cancer DU145 cell line implanted into athymic nu/nu mice treated with placebo or NTG patches and showed a similar reduction in the rate of tumour growth [19].
Maeda and colleagues first proposed the term ‘enhanced permeability and retention effect’ (EPR Effect) in a Japanese paper in 1987 to refer to the intratumoral accumulation of large molecules due to the increased vascular permeability of tumours and the lack of lymphatic drainage [20]. Modulation of these vascular features for therapeutic advantage have been investigated in a number of in vivo models and using a variety of agents by Maeda and co-workers starting in 1998 [21–23]. In vivo manipulation of the EPR effect using NTG in both rat (breast cancer) and mouse (S-180 sarcoma, fibrosarcoma and colon adenocarcinoma) models of cancer were investigated by Maeda and colleagues, who used topical application of NTG ointment to show an increased accumulation of anticancer drugs and other macromolecules [24]. Of note was that the increased accumulation of agents was shown to be consistent in different tumour types, anatomical sites and macromolecular agent.
The use of NTG in lung cancer has also been investigated in vivo, in particular in combination with the anti-folate chemotherapeutic drug pemetrexed [25]. Murine Lewis Lung Carcinoma cells were implanted into NCr C57/BL6 mice, and following tumour development were treated with either saline, NTG, pemetrexed, NTG pemetrexed or NTG pemetrexed or 1H-[1, 2, 4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ). Treatment with the combination of NTG and pemetrexed showed the greatest reduction in tumour growth, (tumour volume was approximately 20% of untreated control at day 15 after start of treatment, P < 0.05), an effect that was reversed by the addition of ODQ.
Human data
While the pre-clinical evidence is suggestive of an anti-cancer effect of NTG, in general in drug repurposing it is human data which is of greater translational significance [26]. In this case there are a number of clinical studies which are in line with the pre-clinical data.
Lung cancer
A randomised controlled Phase II trial of NTG in addition to vinorelbine and cisplatin in stage IIIB/IV inoperable non-small cell lung cancer (NSCLC) was initiated in Japan following an unpublished analysis of response rates comparing lung cancer patients with angina pectoris treated with NTG and patients with lung cancer only [27]. 120 treatment-naïve patients were randomised to four cycles of vinorelbine and cisplatin and placebo patch or vinorelbine and cisplatin with a transdermal NTG patch (25 mg/patient daily for five days per cycle, starting three days prior to chemotherapy). Primary end-points were response rate and time to disease progression. The response rate in the NTG arm was 72%, which was significantly (P < 0.001) higher than the 43% for patients in the placebo arm. The median time to progression was 327 days for the NTG arm, compared to 185 days for the placebo arm (HR = 2.1; 95% CI 1.3–3.2; P = 0.002). Median overall survival was 413 days (range 32–1380 days) in NTG, and 289 days (range 56–1117 days) in placebo arm, (HR = 2.5; 95% CI 1.6–3.9; P < 0.001).
Subsequent work by the same group examined the combination of NTG and docetaxel and carboplatin and the impact of chemosensitivity in two small cohorts of operable lung cancer patients [28]. In the first group, 17 patients with lung cancer and stable angina pectoris (of whom eight had been treated for more than 3 months with transdermal NTG patches delivering 25 mg/day), had their resected tumours immunohistochemically analysed to investigate the long-term effects of NTG treatment on HIF-1α, P-gp, VEGF, p53, and activated p53 (phosphorylated p53 at serine 15). Results showed that staining for HIF-1α, P-gp and VEGF was significantly lower in NTG treated patients compared to controls, although there was no difference between groups for p53. In the second cohort, 29 patients with advanced NSCLC were treated with transdermal NTG (at a dose of 25 mg/day) with each cycle of chemotherapy with docetaxel and carboplatin. The five-day treatment commenced three days prior to chemotherapy. Treatment with NTG resulted in lower plasma VEGF levels compared to baseline, which correlated with sensitivity to chemotherapy response. The response rate in these patients was 59%, which compares well to the typical response of 24–36% to this chemotherapy regimen in similar populations.
An on-going trial by the same group is investigating the use of NTG with paclitaxel and carboplatin in treatment-naïve Stage III/IV NSCLC patients, (NCT00616031 – current status of trial unknown according to ClinicalTrials.gov as at June 2015).
There are also Japanese case reports of the use of NTG with the anthracycline amrubicin in five heavily pre-treated patients with advanced NSCLC [29]. All five patients, suffering refractory and recurrent disease, had been treated with at least three prior lines of chemotherapy before being treated with amrubicin and transdermal NTG. Two of the five patients achieved partial response; one patient had received three prior regimens and the other four. Both patients experienced partial response with transdermal NTG at a dose of 10 mg/day.
The Japanese trial of NTG with vinorelbine plus cisplatin was replicated in a Phase II trial in Germany, using a similar protocol and in a similar patient population, although in this case oral vinorelbine was used [30]. Treatment-naïve patients with stage IIIB/IV NSCLC were randomised to oral vinorelbine and cisplatin with NTG or placebo patch. The NTG dose was 25 mg/day. The trial was terminated early due to lack of recruitment, with only 66 patients treated. While the results did not reach statistical significance, the overall response rate (35.3% vs. 18.8%) and the disease control rates (61.8% vs. 53.1%) were higher in the NTG arm than in the placebo arm, although there was no difference in progression free survival (PFS) or overall survival (OS). The discordance between the overall response rate and the lack of difference in PFS and OS measures is suggestive of predominantly transient responses induced by NTG.
Negative results were reported for the NVALT12 Phase II randomised open-label trial of paclitaxel, carboplatin and bevacizumab (PCB), with and without transdermal NTG in patients with stage IV non-squamous NSCLC. Patients randomised to the NTG arm (n = 111) were treated with PCB and NTG patches delivering 15 mg/day for 5 days (starting two days prior to each of four 3-week chemo cycles), the control arm (n = 112) received the same treatment without the NTG patch. Treatment with bevacizumab or bevacizumab NTG continued as maintenance therapy until disease progression [31]. The response rate was 30% for the NTG arm, compared to 45% for control, median PFS 5.0 months (4.2–5.8) for NTG and 6.8 months (5.6–7.3) for the control, with HR of 1.22 (95% CI 0.91–1.63). Median OS was 9.5 months (7.8–11.9) for NTG and 11.6 months (8.7–14) in control, with HR of 1.12 (95% CI 0.76–1.67).
Another negative trial has also reported results in NSCLC. The Australasian NITRO study was a large phase III trial of NTG in addition to first line chemotherapy in advanced NSCLC (stage III and IV). Results reported at ESMO 2014 showed that the trial was terminated early after the first interim analysis for no demonstrable advantage in terms of PFS with the addition of NTG. Patients in the NTG arm were treated with NTG patches (delivering 25 mg/day) in addition to a variety of first line regimens (mainly carboplatin with either paclitaxel or gemcitabine). Objective tumour responses were seen in 26% of NTG patients compared to 31% in the non-NTG patients, and stable disease was recorded in 49% in the NTG group versus 45% in the non-NTG group. Median overall survival was 10.8 months with NTG and 11.2 months without [32]. A meta-analysis, (excluding the data for cisplatin and vinorelbine), presented at the same meeting by the NITRO authors showed that the addition of NTG did not improve outcomes for NSCLC patients treated with carboplatin with either paclitaxel or gemcitabine.
In addition to looking at NTG with chemotherapy, there has also been investigation to ascertain whether NTG is effective with chemo-radiotherapy. A non-randomised Phase II trial in NSCLC of NTG, oral vinorelbine, cisplatin and concurrent radiotherapy has also reported results in Mexico [33]. Thirty-five patients were enrolled in this trial, of whom 63% achieved a clinical response after induction of chemotherapy, and 75% achieved a clinical response after chemo-radiotherapy. The computed 2-year PFS was 38.1% and median overall survival was 26.9 months, with a 2-year OS rate of 51.3% which was close to, but below, the target rate of 55% used in the design and powering of the trial, and compared to a 2-year actuarial survival rate of 30% in a similar patient population. A reduction of plasma VEGF levels was positively associated with improved OS in patients in the NTG arm, with the median OS for patients who achieved > 50% VEGF level reduction was 42.9 months (95%, CI 20.8–64.9) compared to 19.5 months (95% CI, 17.6–31.4) for patients with <50% reduction (P = 0.043).
The impact of NTG on chemo-radiotherapy is also being investigated in an on-going Phase II trial in the Netherlands (NCT01210378). Some initial results of this trial are discussed in the Our Take section of this paper.
There is some evidence to suggest that some of the clinical effects in NSCLC may be specific to NTG and not a feature of NO-donors in general. For example a trial compared two different chemotherapy regimens (irinotecan and cisplatin versus irinotecan and capecitabine), with and without the NO-donor isosorbide-5-mononitrate (ISM) [34]. While results showed that there was little difference between the two chemotherapy regimens in terms of overall survival, the addition of ISM did not show improvement in any treatment outcome (response rate, progression free survival or overall survival). The study authors speculated that the lack of efficacy may have been related to the lack of nitrate-induced mitochondrial ROS production compared to NTG, and that this may have contributed to these negative results.
Other cancers
In prostate cancer a prospective, open-label trial of 29 men with an increasing prostate-specific antigen (PSA) level after surgery or radiotherapy investigated the use of transdermal NTG [35]. Patients were enrolled on a 24-month trial to compare PSA doubling time (PSADT) before and after treatment initiation, as well as comparison with a matched control group that received no immediate treatment for their PSA recurrence. The trial used very low doses of NTG, using commercially produced patches cut-down to a custom size, delivering a dose of 0.033 mg/h continuously. PSA levels were monitored monthly for the first 6 months and then every 3 months for the rest of the trial. Of the 29 patients in the trial, 17 completed the full 24-months of treatment, and only 3 of 29 had documented disease progression (10%) by the end of the trial. The results showed that the treatment group had a calculated PSADT of 31.8 months, compared to the pre-treatment rate of 13.3 months or that of the matched control group at 12.8 months.
NTG has also been investigated as a potential agent to improve response in liver cancer patients treated with transcatheter arterial embolization (TAE) or transcatheter arterial chemoembolization (TACE) [36]. In a single-site randomised controlled study, 101 hepatocellular carcinoma (HCC) patients with early to middle stage disease (BCLC A or B), were randomised to receive TAE/TACE with or without NTG. Patients receiving TACE were treated with the addition of doxorubicin to the lipiodol carrier, otherwise the treatment was the same as for TAE. The NTG dose of 100 μg was injected via the catheter immediately prior to the injection of lipiodol or lipiodol/doxorubicin. Results showed that the addition of low dose NTG increased the volume of lipiodol retained in tumours, and led to a greater reduction in tumour diameter. Although these results were statistically significant, the improved overall response in the NTG arm (98.1% vs 92%) was not, (the 92% response rate in the control arm limited the ability to detect a significant increase).
Finally, a small open-label Phase I dose-escalation study investigated the safety and tolerability of transdermal NTG patches in the neoadjuvant treatment of resectable rectal cancer, alongside chemo-radiotherapy with 5-FU [37]. The trial concluded that the treatment was safe at the maximum tested dose of 0.6 mg/h, and recommended this dose be used in subsequent Phase II trials. While safety and tolerability were the key end-points, the trial also reported a pathologic complete response rate of 17%, a figure within the range of current standard of care.
Clinical trials
A number of clinical trials are currently (June 2015) investigating the addition of NTG to existing cancer treatments.
Trial NCT01210378 is a Phase II study investigating the use of transdermal NTG in addition to standard of care treatment (chemoradiotherapy) for NSCLC. Specifically the study aims to track tumour hypoxia and perfusion. The primary outcome is an increase in overall survival of 15% compared to historical controls after 2 years.
Trial NCT00616031 is a Phase II trial of paclitaxel and carboplatin with or without NTG in patients with previously untreated stage III or IV NSCLC. The trial is randomised and controlled with tumour response rate as the primary outcome.
Trial JPRN-UMIN000000813 is a Phase II trial of amrubicin and NTG in third-line chemotherapy in patients with NSCLC. This is a single arm, non-randomised study with PFS as the primary end point.
Trial JPRN-UMIN000001820 is also in NSCLC. This is a phase II trial of NTG with docetaxel in elderly untreated stage IIIB/IV NSCLC. This is a single arm trial with response rate as the primary end point and PFS as a secondary outcome.
Trial NCT01704274 is Phase III study of two different low-dose applications of transdermal NTG in men with biochemical evidence of recurrence (increasing PSA levels). This randomised placebo controlled trial will track markers of immune response in addition to PSA.
Mechanism of action
There are multiple mechanisms of action posited to explain the diverse anticancer effects of NTG. These include:
• Vasodilation leading to enhanced permeability and retention (EPR) effects
• Anti-hypoxic activity
• Immunomodulation
• Pro-apoptopic effects
NO release/vasodilation
Although NTG has more than 100 years of clinical use as a vasodilatory agent, through its action as a NO donor, understanding of the molecular basis for this effect has remained elusive. Multiple mechanisms and pathways for the conversion of NTG to NO or NO precursors have been elucidated, including xanthine oxidase, glutathione S-transferase, mitochondrial aldehyde dehydrogenase (ALDH2) and via the phosphatidylinositol 3-kinase (PI3K) pathway. Currently it is the latter two pathways which are garnering the most interest in that they can explain the generation of NO, and consequent vasodilation, at clinically relevant nanomolar doses of NTG.
In 2002 Stamler et al published a paper that reported a mechanism of activation via mitochondrial ALDH (ALDH2) [38]. They showed that ALDH2 generated the right mix of metabolites (1,2-GDN in preference to 1,3-GDN), released NO, caused vasodilation and could induce nitrate tolerance. However, the in vitro concentrations used were 0.1 μM–10 μM which are high, and nitrate tolerance was induced at extremely high concentrations (0.3 mM). Later work extended the analysis using ALDH2 knockout mice using very similar dose levels [39]. Other work looked at using ALDH2 inhibitors along with relevant doses of NTG in animals and noting the reduction in vasodilatory activity. Not every step in the process of going from NTG to NO was elucidated by this work, as the authors made clear in their papers. Mackenzie et al also found that in humans, ALDH2 is involved in the transformation of NTG and subsequent vasodilation but that it accounts for less than half of the total bioactivation [40].
Beretta et al identified a possible NTG bioactivation mechanism via cytosolic ALDH (ALDH1) in a bid to elucidate some of the missing steps in the ALDH2 mechanism [41]. Highly purified ALDH1 and ALDH2 and extremely high concentrations of NTG (100 μM and 10 μM for ALDH1 and 2 respectively) were used. At these high in vitro concentrations ALDH1 showed some bioactivation of NTG, but as the authors concluded: The low apparent GTN [NTG] affinity of ALDH1 is expected to limit the contribution of this isoform to GTN bioactivation in patients treated with therapeutically relevant low doses… Tsou et al also investigated ALDH1 (specifically ALDH1A1) in vitro [42]. Again very high concentrations of NTG were used – 0.1 mM and 1.0 mM – thereby casting doubt on the relevance of this in vivo.
Subsequently, work by Bonini et al in 2008 proposed an alternative explanation in which they used in vitro and in vivo experiments to show that signal transduction by NTG via endothelial NOS is the active mechanism of action at low nanomolar doses [43]. This mechanism of action views NTG as a signalling molecule that activates eNOS generation of NO at very small doses (1 nM) and at very rapid timescales (< 1 minute). In the same way that the Stamler group used ALDH2 inhibitors to show that ALDH2 inhibition or knockout mice reduced NTG activity, the Bonini group used NOS inhibitors and knockout mice. Subsequent work further elucidated the signalling mechanism, implicating PI3K/PKB/PTEN [44]. In this they also accept that at higher doses (> 50 nM) metabolic effects may predominate.
Regardless of the direct mechanisms of action, and it is likely that there are multiple redundant pathways operating given the physiological importance and ubiquity of NO and NO-signalling, clinically there are a number of observations that are of significance with respect to NTG and its vasodilatory activities. The first is that the NO-generating activity of NTG is substantially increased during hypoxia, effectively acting to target the effect to those tissues with low oxygenation such as solid tumours or cardiac infarct tissue [45, 46]. Secondly, given the physical characteristics of tumour-associated vasculature – increased permeability, chaotic organisation, abnormal structure [47] – there is evidence that increasing permeability using NTG can lead to the increased retention of macromolecules in tumour tissues (the EPR effect) [46, 48, 49].
Hypoxia
Tumour tissue is subject to both chronic and transient hypoxia related to the chaotic structure of the vasculature. Tumour hypoxia is associated with increased genomic instability and the development of more aggressive cancer phenotypes, resistance to treatment, increased metastatic potential and worse prognosis in a range of cancer types [50]. Hypoxia-inducible factor 1-alpha (HIF-1α) is the primary transcription factor involved in the cellular response to low-oxygen conditions, transcribing for pro-survival responses such as increased angiogenesis (including upregulation of VEGF), metabolic adaptation and increased proliferation [51]. Early in vitro evidence showed that NO was an inducer and stabiliser of HIF-1α in both hypoxic and normoxic inflammatory environments [52]. However, later work suggested that the effect was concentration dependent, and low NO concentrations inhibited HIF-1α stabilisation, but that at higher concentrations NO acted to stabilise it [53]. Other work has shown that that NO can suppress HIF-1α activity, and that exogenous NO-donors may act to inhibit HIF-1α expression and downstream targets [54–56].
There is clinical evidence of an effect of NTG on expression of HIF-1α, with immunostaining of tumour samples showing decreased levels of expression of HIF-1α and VEGF in NSCLC patients treated with NTG patches compared to non-treated controls [28]. Lower levels of HIF-1α were also correlated with increased response to chemotherapy with docetaxel and carboplatin. Note that this action on HIF-1α may also impart some anti-angiogenic activity following NTG treatment due to the down-stream reduction of VEGF expression.
Immunity
Recently there has been an increased investigation of the role of HIF-1α in the process of immune escape in cancer [57]. One possible mechanism links HIF-1α to an increased expression of metalloproteinase ADAM10 leading to a decrease in surface MHC class I chain-related molecule A (MICA) levels, which in turn leads to tumour cell resistance to lysis mediated by innate immune effectors [18]. In an in vivo mouse model transdermal NTG patches attenuated tumour growth via a mechanism dependent on adaptive immune effector cells, presumed to be due to inhibition of HIF-1α accumulation and hypoxia induced PD-L1 expression [58].
It should be noted that there are concerns regarding the effects of NO on immune function. NO is a key regulatory and effector molecule with diverse effects on immunity. NO generated by iNOS is known to be involved in the regulation of lymphocyte populations, particularly T cell sub-types, and is also a key component of the lytic action of macrophages [59–61]. The action of exogenous NO on immune function is complex; for example there is some in vitro evidence for a non-specific immunosuppressive action for NTG [62], and in a rat model of atherosclerosis repeated exposure to NTG acted on macrophages to up-regulate matrix metalloproteinase-9 (MMP-9) and down-regulate tissue inhibitor of metalloproteinase-1 (TIMP-1) [63]. Additionally, there is evidence that NO is a key mediator in T-cell suppression by myeloid derived suppressor cells (MDSC) [64, 65]. These issues are further discussed later in this paper.
Apoptosis
A final putative mechanism of action of NTG in cancer is through a direct pro-apoptotic effect. The earliest evidence for a direct cytotoxic effect of NTG was shown in a number of human leukaemia cell lines [66]. Apoptosis was associated with cytochrome c release and caspase activation in Jurkat leukemic cell lines. Subsequent work also showed an effect in human colon cancer cell lines (SW480, SW620, and HCT-116), again associated with caspase activation [67]. Analysis showed that NTG increased Fas-receptor expression and decreased levels of several endogenous inhibitors of apoptosis and thus sensitised cells to Fas-ligand-mediated apoptosis. This process has been shown to be connected to the S-nitrosylation of cysteine residues 199 and 304 in the cytoplasmic part of Fas, suggesting that treatment with NTG may restore sensitivity to Fas-L mediated tumour cell death [68].
Our take
The pre-clinical and clinical evidence outlined in the preceding sections, (and summarised in Table 1), along with multiple putative mechanisms of action, suggest that there is a clinical role for NTG in the treatment of cancer. In particular, there is evidence that the effect of NTG in the EPR effect warrants continued investigation as a potentiating agent for existing standard of care chemo and chemo-radiation protocols. While there are some clinical trials on-going in this respect, recent negative results in NSCLC suggest that additional attention is required in the selection of agents to pair with NTG, in the timing of NTG administration within this treatment combination, potential biomarkers of response and in the characteristics of the patient population.
The most negative of the NSCLC trials was the NVALT 12 study, which has been the first to combine NTG and bevacizumab, in addition to carboplatin and paclitaxel [31]. The combination of NTG and bevacizumab, which were both administered until disease progression, may be a problematic one in that there is evidence of contrary effects of each drug on vascular permeability. Whereas NTG is well-characterised as a vasodilator able to enhance the permeability of the tumour vasculature, there is strong evidence that bevacizumab reduces permeability [69–72]. It may well be that the impaired response in the trial was due to antagonism between NTG and bevacizumab, and that the cross-talk was bi-directional with NTG interfering with the therapeutic action of bevacizumab and vice versa. In particular one must note that bevacizumab decreases chemotherapy delivery to tumour [69] while NTG increases it [46]. Additionally, bevacizumab is known to induce hypoxia and increase expression of HIF-1α [73, 74], potentially mitigating some of the anti-hypoxic effects of NTG. One may also note that the NVALT 12 study used a lower dose of NTG (15 mg/day) than the other trials in NSCLC (25 mg/day).
Table 1. Summary of pre-clinical evidence by cancer type.
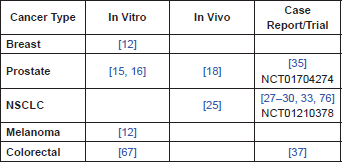
However, even if we exclude the NVALT 12 study due to the possible confounding with bevacizumab, there is a discrepancy between the early positive results in the Japanese trials, and the results from the non-Japanese trials that have also taken place. One possible explanation lies in the ethnic differences between patient populations. Some, (but not all [75]), studies, have suggested that polymorphisms in mitochondrial ALDH2, one of the enzymes presumed to be central in the bioactivation of NTG, have a different distribution between East Asian/Japanese populations and European populations and that these are clinically significant [40]. If this were the case it is conceivable that these differences are reflected in the divergent results in the NTG chemotherapy trials. However, further work is required to investigate whether the ALDH2 polymorphism/ethnic differences hypothesis is correct.
In the absence of a clear explanation for the discrepancy between the Japanese and non-Japanese results we must await the results from the on-going trials to ascertain the degree to which NTG co-treatment with chemotherapy or chemo-radiotherapy benefits patients with NSCLC in terms of PFS and OS. Tracking patients for metrics related to tumour hypoxia or VEGF status may also shed more light on the biological mechanisms involved in the response. One indication that the degree of hypoxia is relevant comes from data reported by Philippe Lambin and colleagues at the 3rd ESTRO Forum in April 2015 [76]. Based on preliminary data from trial NCT01210378 and additional in vitro analysis, the results showed that in patients with hypoxic tumours NTG reduced the hypoxic fraction and volume, and that this was associated with a reduced oxygen consumption rate. The authors suggest that patient selection criteria are required to identify those with hypoxic tumours who will benefit from NTG administration.
Another concern regarding the use of NTG is its action as an NO-donor and the effect this may have on cell mediated immunity. As discussed previously, there is evidence that NO may be implicated in the immunosuppressive activity of MDSC. While NO signalling is involved, there are some indications that the effect on MDSC is indirect and mediated by tumour iNOS-associated production of VEGF, which is pivotal in the MDSC induction from myeloid precursor cells [65]. In contrast there is evidence of a positive impact of NTG on immunosurveillance of tumours in an animal model [58]. Given the potentially dichotomous roles of NTG on immune status, it is suggested that in addition to further research, clinical application of NTG with agents that have anti-MDSC action should be investigated. A number of possibilities in this direction are discussed in the supplementary information.
We must also take note that while the EPR effect is well-established in animal models, clinical results have yet to meet expectations [77]. One key criticism of a simplistic reading of the EPR effect is that it ignores the effect of the increased interstitial pressure within tumours [78–80]. While the tumour vasculature may be more permeable than normal vasculature, large molecules may be unable to cross into, and therefore accumulate, into the interior of the tumour. A strategy to address this issue is therefore required if we are to maximise the potential of the EPR effect through the use of NTG. A set of proposals are made in the supplementary materials included with this paper for drug combinations which may address this issue.
Given the evidence as it stands, it is suggested that additional clinical trials of NTG are warranted in the following cancer types, and that NTG treatment may be best suited in tumours with large hypoxic fractions to capitalize on its effects on reducing HIF-1α:
• NSCLC
• Prostate cancer
• Colorectal cancer
• Breast cancer
• Pancreatic
Conclusion
It has long been acknowledged that treatment resistance and significant toxicity are two of the leading causes of treatment failure and subsequent mortality. The use of NTG offers an opportunity to address both of these issues. Hypoxia is associated with treatment resistance in both chemotherapy and radiotherapy; it is a driver of metastatic disease and the evolution of more aggressive tumour phenotypes. In targeting HIF-1α NTG acts as an anti-hypoxic agent, thus down-regulating the pro-survival pathways that contribute to treatment resistance. In addition by exploiting the EPR effect, NTG can help to target anti-cancer agents to disease, thus sparing host tissues and increasing the therapeutic effectiveness of a wide range of agents.
Finally, it is suggested that to maximise the potential of NTG in cancer therapy we look to expand the range of drugs with which it is combined. In doing so we can more fully address the specifics of tumour vasculature that the EPR seeks to address, and a range of proposals are made in the supplementary materials.
Author contributions
Primary authors: Vidula Sukhatme and Pan Pantziarka. Contributing authors: Gauthier Bouche, Lydie Meheus and Vikas P. Sukhatme. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. All the authors are associated with not for profit organisations that aim to repurpose drugs for oncology treatments.
References
1. Bashir A, Lewis MJ and Henderson AH (1982) Pharmacokinetic studies of various preparations of glyceryl trinitrate Br J Clin Pharmaco 14(6) 779–784 DOI: 10.1111/j.1365-2125.1982.tb02036.x
2. Jensen KM and Mikkelsen S (1997) Studies on the bioavailability of glyceryl trinitrate after sublingual administration of spray and tablet Arzneimittel-Forsch 47(6) 716–718
3. Curry SH and Aburawi SM (1984) Analysis, disposition and pharmacokinetics of nitroglycerin Biopharm Drug Dispos 6(3) 235–280 DOI: 10.1002/bdd.2510060302
4. Weber S, de Lauture D and Rey E et al (1987) The effects of moderate sustained exercise on the pharmacokinetics of nitroglycerine Br J Clin Pharmaco 23(1) 103–105 DOI: 10.1111/j.1365-2125.1987.tb03018.x
5. Salvemini D, Pistelli A and Anggard E (1993) Vascular and anti-platelet actions of 1,2- and 1,3-glyceryl dinitrate Br J Pharmaco 110(3) 937–942 DOI: 10.1111/j.1476-5381.1993.tb13903.x
6. Burke AJ, Sullivan FJ, Giles FJ and Glynn SA (2013) The yin and yang of nitric oxide in cancer progression Carcinogenesis 34(3) 503–512 DOI: 10.1093/carcin/bgt034 PMID: 23354310
7. Choudhari SK, Chaudhary M and Bagde S et al (2013) Nitric oxide and cancer: a review World J Surg Oncol 11(1) 118 DOI: 10.1186/1477-7819-11-118 PMID: 23718886 PMCID: 3669621
8. Fukumura D, Kashiwagi S and Jain RK (2006) The role of nitric oxide in tumour progression Nat Rev Cancer 6(7) 521–534 DOI: 10.1038/nrc1910 PMID: 16794635
9. Burke JF, Mark VH and Soloway AH et al (1966) The effect of antibody to L-phenylalanine mustard conjugate on malignant cells selectively marked through ‘early inflammatory-like’ vascular permeability Cancer Res 26(9) 1893–1904 PMID: 4162674
10. Matsumura Y and Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs Cancer Res 46(12 Pt 1) 6387–6392 PMID: 2946403
11. Seymour LW, Ulbrich K, Steyger PS and Brereton M et al (1994) Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma Br J Cancer 70(4) 636–641 DOI: 10.1038/bjc.1994.363 PMID: 7917909 PMCID: 2033419
12. Matthews NE, Adams MA and Maxwell LR et al (2001) Nitric oxide-mediated regulation of chemosensitivity in cancer cells J Natl Cancer Inst 93(24) 1879–1885 DOI: 10.1093/jnci/93.24.1879 PMID: 11752013
13. Postovit LM, Adams MA and Lash GE et al (2002) Oxygen-mediated regulation of tumor cell invasiveness Involvement of a nitric oxide signaling pathway J Biol Chem 277(38) 35730–35737 DOI: 10.1074/jbc.M204529200 PMID: 12107174
14. Postovit LM, Adams MA and Lash GE et al (2004) Nitric oxide-mediated regulation of hypoxia-induced B16F10 melanoma metastasis Int J Cancer 108(1) 47–53 DOI: 10.1002/ijc.11556
15. Frederiksen LJ, Siemens DR and Heaton JP et al (2003) Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate J Urol 170(3) 1003–1007 DOI: 10.1097/01.ju.0000081126.71235.e0 PMID: 12913759
16. Frederiksen LJ, Sullivan R and Maxwell LR et al (2007) Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin Cancer Res 13(7) 2199–2206 DOI: 10.1158/1078-0432.CCR-06-1807 PMID: 17404104
17. Muir CP, Adams MA and Graham CH (2006) Nitric oxide attenuates resistance to doxorubicin in three-dimensional aggregates of human breast carcinoma cells Breast Cancer Res Treat 96(2) 169–176 DOI: 10.1007/s10549-005-9076-9
18. Siemens DR, Hu N and Sheikhi AK et al (2008) Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: role of nitric oxide Cancer Res 68(12) 4746–1753 DOI: 10.1158/0008-5472.CAN-08-0054 PMID: 18559521
19. Barsoum IB, Hamilton TK and Li X et al (2011) Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: role of nitric oxide Cancer Res 71(24) 7433–7441 DOI: 10.1158/0008-5472.CAN-11-2104 PMID: 22006996
20. Matsumura Y, Oda T and Maeda H (1987) [General mechanism of intratumor accumulation of macromolecules: advantage of macromolecular therapeutics] Gan To Kagaku Ryoho 14(3 Pt 2) 821–829 PMID: 2952066
21. Wu J, Akaike T and Maeda H (1998) Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger Cancer Res 58(1) 159–165 PMID: 9426072
22. Tanaka S, Akaike T and Wu J et al (2003) Modulation of tumor-selective vascular blood flow and extravasation by the stable prostaglandin 12 analogue beraprost sodium J Drug Target 11(1) 45–52 DOI: 10.1080/1061186031000086072 PMID: 12852440
23. Iyer AK, Greish K and Seki T et al (2007) Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation J Drug Target 15(7–8) 496–506 DOI: 10.1080/10611860701498252 PMID: 17671896
24. Seki T, Fang J and Maeda H (2009) Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application Cancer Science 100(12) 2426–2430 DOI: 10.1111/j.1349-7006.2009.01323.x PMID: 19793083
25. Nagai H, Yasuda H and Hatachi Y et al (2012) Nitric oxide (NO) enhances pemetrexed cytotoxicity via NO-cGMP signaling in lung adenocarcinoma cells in vitro and in vivo Int J Oncol 41(1) 24–30 PMID: 22552400
26. Pantziarka P, Bouche G and Meheus L et al (2014) The repurposing drugs in oncology (ReDO) project Ecancermedicalscience 8 442 DOI: 10.3332/ecancer.2014.485 PMID: 25075216 PMCID: 4096030
27. Yasuda H, Yamaya M and Nakayama K et al (2006) Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer J Clin Oncol 24(4) 688–694 DOI: 10.1200/JCO.2005.04.0436 PMID: 16446342
28. Yasuda H, Nakayama K and Watanabe M et al (2006) Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma Clin Cancer Res 12(22) 6748–6757 DOI: 10.1158/1078-0432.CCR-06-1124 PMID: 17121895
29. Yasuda H, Nakayama K and Sasaki T et al (2007) Partial response by nitroglycerin plus amrubicin regimen in patients with refractory and recurrent advanced non-small cell lung cancer who had received at least third-line chemotherapy Cancer Ther 5 451–456
30. Reinmuth N, Meyer A and Hartwigsen D et al (2014) Randomized, double-blind phase II study to compare nitroglycerin plus oral vinorelbine plus cisplatin with oral vinorelbine plus cisplatin alone in patients with stage IIIB/IV non-small cell lung cancer (NSCLC) Lung Cancer 83(3) 363–368 DOI: 10.1016/j.lungcan.2014.01.001 PMID: 24462464
31. Dingemans A-MC (2014) A randomized phase II study of paclitaxel-carboplatin-bevacizumab (PCB) with or without nitroglycerin patches (NTG) in patients (pts) with stage IV nonsquamous non-small cell lung cancer (NSCLC): Nvalt 12 (NCT01171170) J Clin Oncol 32(5s) 8089
32. Davidson A, Veillard AS and Tognela A et al (2014) 1245PA phase 3 randomised trial of adding nitroglycerin to first line chemotherapy for advanced non-small cell lung cancer: the Australasian lung cancer trials group nitro trial RANDOMISED TRIAL OF ADDING NITROGLYCERIN TO FIRST LINE CHEMOTHERAPY FOR ADVANCED NON-SMALL CELL LUNG CANCER: THE AUSTRALASIAN LUNG CANCER TRIALS GROUP NITRO TRIAL Ann Oncol 25 (suppl 4) iv436–iv436
33. Arrieta O, Blake M and de la Mata-Moya MD et al (2014) Phase II study Concurrent chemotherapy and radiotherapy with nitroglycerin in locally advanced non-small cell lung cancer Radiother Oncol 1–5
34. Han JY, Nam BH and Kim HY et al (2012) A randomized phase II study of irinotecan plus cisplatin versus irinotecan plus capecitabine with or without isosorbide-5-mononitrate in advanced non-small-cell lung cancer Ann Oncol 23(11) 2925–2930 DOI: 10.1093/annonc/mds122 PMID: 22782331
35. Siemens DR, Heaton JPW and Adams MA et al (2009) Phase II study of nitric oxide donor for men with increasing prostate-specific antigen-level after surgery or radiotherapy for prostate cancer Urology 74(4) 878–883 DOI: 10.1016/j.urology.2009.03.004 PMID: 19476985
36. Liu Y, Chuang M and Tsai Y et al (2012) Nitroglycerine use in transcatheter arterial (chemo)embolization in patients with hepatocellular carcinoma and dual-energy CT assessment of Lipiodol retention Eur Radiol 22(10) 2193–2200 DOI: 10.1007/s00330-012-2484-4 PMID: 22618520
37. Illum H, Wang DH and Dowell JE et al (2015) Phase I dose escalation trial of nitroglycerin in addition to 5-fluorouracil and radiation therapy for neoadjuvant treatment of operable rectal cancer Surgery 1–6
38. Chen Z, Zhang J and Stamler JS (2002) Identification of the enzymatic mechanism of nitroglycerin bioactivation Proc Natl Acad Sci USA 99(12) 8306–8311 DOI: 10.1073/pnas.122225199 PMID: 12048254 PMCID: 123063
39. Chen Z, Foster MW and Zhang J et al (2005) An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation Proc Natl Acad Sci USA 102(34) 12159–12164 DOI: 10.1073/pnas.0503723102 PMID: 16103363 PMCID: 1189320
40. Mackenzie IS, Maki-Petaja KM and McEniery CM et al (2005) Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans Arterioscler Thromb Vasc Biol 25(9) 1891–1895 DOI: 10.1161/01.ATV.0000179599.71086.89 PMID: 16051882
41. Beretta M, Gruber K and Kollau A et al (2008) Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases J Biol Chem 283(26) 17873–17880 DOI: 10.1074/jbc.M801182200 PMID: 18450747 PMCID: 2440601
42. Tsou PS, Page NA and Lee SG et al (2011) Differential metabolism of organic nitrates by aldehyde dehydrogenase 1a1 and 2: substrate selectivity, enzyme inactivation, and active cysteine sites AAPS J 13(4) 548–555 DOI: 10.1208/s12248-011-9295-4 PMID: 21818694 PMCID: 3231853
43. Bonini MG, Stadler K and Silva SDO et al (2008) Constitutive nitric oxide synthase activation is a significant route for nitroglycerin-mediated vasodilation Proc Natl Acad Sci USA 105(25) 8569–8574 DOI: 10.1073/pnas.0708615105 PMID: 18562300 PMCID: 2438390
44. Mao M, Sudhahar V and Ansenberger-Fricano K et al (2012) Nitroglycerin drives endothelial nitric oxide synthase activation via the phosphatidylinositol 3-kinase/protein kinase B pathway Free Radic Biol Med 52(2) 427–435 DOI: 10.1016/j.freeradbiomed.2011.09.020 PMCID: 3432314
45. Agvald P, Adding LC and Artlich A et al (2002) Mechanisms of nitric oxide generation from nitroglycerin and endogenous sources during hypoxia in vivo Br J Pharmacol 135(2) 373–382 DOI: 10.1038/sj.bjp.0704489 PMID: 11815372 PMCID: 1573151
46. Maeda H (2010) Nitroglycerin enhances vascular blood flow and drug delivery in hypoxic tumor tissues: analogy between angina pectoris and solid tumors and enhancement of the EPR effect J Control Release 142(3) 296–298 DOI: 10.1016/j.jconrel.2010.01.002 PMID: 20074683
47. Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers J Clin Oncol 31(17) 2205–2218 DOI: 10.1200/JCO.2012.46.3653 PMID: 23669226 PMCID: 3731977
48. Maeda H (2012) Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc Jpn Acad Ser B Phys Biol Sci 88(3) 53–71 DOI: 10.2183/pjab.88.53 PMID: 22450535 PMCID: 3365245
49. Maeda H, Nakamura H and Fang J (2013) The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo Adv Drug Deliv Rev 65(1) 71–79 DOI: 10.1016/j.addr.2012.10.002
50. Wilson WR and Hay MP (2011) Targeting hypoxia in cancer therapy Nat Rev Cancer 11(6) 393–410 DOI: 10.1038/nrc3064 PMID: 21606941
51. Ke Q and Costa M (2006) Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol 70(5) 1469–1480 DOI: 10.1124/mol.106.027029 PMID: 16887934
52. Sandau KB, Fandrey J and Brüne B (2001) Accumulation of HIF-1alpha under the influence of nitric oxide Blood 97(4) 1009–1015 DOI: 10.1182/blood.V97.4.1009 PMID: 11159530
53. Mateo J, García-Lecea M and Cadenas S et al (2003) Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and mitochondria-independent pathways Biochem J 376(Pt 2) 537–544 DOI: 10.1042/BJ20031155 PMID: 14531732 PMCID: 1223794
54. Sogawa K, Numayama-Tsuruta K and Ema M et al (1998) Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia Proc Natl Acad Sci USA 95(13) 7368–7373 DOI: 10.1073/pnas.95.13.7368 PMID: 9636155 PMCID: 22620
55. Agani FH, Puchowicz M and Chavez JC et al (2002) Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia Am J Physiol Cell Physiol 283(1) C178–C186 DOI: 10.1152/ajpcell.00381.2001 PMID: 12055086
56. Cattaneo MG, Cappellini E and Benfante R et al (2011) Chronic deficiency of nitric oxide affects hypoxia inducible factor-1α (HIF-1α) stability and migration in human endothelial cells PLoS ONE 6(12) DOI: 10.1371/journal.pone.0029680
57. Imtiyaz HZ and Simon MC (2010) Hypoxia-inducible factors as essential regulators of inflammation Current topics in microbiology and immunology 345(215) pp 105–20 PMID: 20517715 PMCID: 3144567
58. Barsoum IB, Smallwood CA and Siemens DR et al (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells Cancer Res 74(3) 665–674 DOI: 10.1158/0008-5472.CAN-13-0992
59. Bogdan C (2011) Regulation of Lymphocytes by Nitric Oxide vol 677 Methods in Molecular Biology ed Cuturi MC and Anegon I (Clifton: New Jersey) pp 375–393 DOI: 10.1007/978-1-60761-869-0_24
60. Bogdan C (2001) Nitric oxide and the immune response Nat Immunol 2(10) 907–916 DOI: 10.1038/ni1001-907 PMID: 11577346
61. Holán V, Krulová M and Zajícová A et al (2002) Nitric oxide as a regulatory and effector molecule in the immune system Mol Immunol 38(12–13) 989–995 DOI: 10.1016/S0161-5890(02)00027-5 PMID: 12009578
62. Shoker AS, Yang H and Murabit MA et al (1997) Analysis of the in vitro effect of exogenous nitric oxide on human lymphocytes Mol Cell Biochem 171(1–2) 75–83 DOI: 10.1023/A:1006815430622 PMID: 9201699
63. Krishnatry AS, Fung SM and Brazeau DA (2011) Nitroglycerin alters matrix remodeling proteins in THP-1 human macrophages and plasma metalloproteinase activity in rats Nitric Oxide 24(2) 66–76 DOI: 10.1016/j.niox.2010.12.002 PMCID: 3039075
64. Wesolowski R, Markowitz J and Carson WE (2013) Myeloid derived suppressor cells – a new therapeutic target in the treatment of cancer J Immunother Cancer 1(1) 10 DOI: 10.1186/2051-1426-1-10
65. Jayaraman P, Parikh F and Lopez-Rivera E et al (2012) Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release J Immunol 188(11) 5365–5376 DOI: 10.4049/jimmunol.1103553 PMID: 22529296 PMCID: 3358566
66. Ushmorov A, Ratter F and Lehmann V et al (1999) Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome C release Blood 93(7) 2342–2352 PMID: 10090945
67. Millet A, Bettaieb A and Renaud F et al (2002) Influence of the nitric oxide donor glyceryl trinitrate on apoptotic pathways in human colon cancer cells Gastroenterol 123(1) 235–246 DOI: 10.1053/gast.2002.34310
68. Leon-Bollotte L, Subramaniam S and Cauvard O et al (2011) S-nitrosylation of the death receptor fas promotes fas ligandmediated apoptosis in cancer cells Gastroenterol 140(7) 2009–2018 2018.e1–4 DOI: 10.1053/j.gastro.2011.02.053
69. Van der Veldt AAM, Lubberink M and Bahce I et al (2012) Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs Cancer Cell 21(1) 82–91 DOI: 10.1016/j.ccr.2011.11.023 PMID: 22264790
70. Turley RS, Fontanella AN and Padussis JC et al (2012) Bevacizumab-induced alterations in vascular permeability and drug delivery: a novel approach to augment regional chemotherapy for in-transit melanoma Clinical Cancer Res 18(12) 3328–3339 DOI: 10.1158/1078-0432.CCR-11-3000
71. Dickson PV, Hamner JB and Sims TL et al (2007) Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy Clin Cancer Res 13(13) 3942–3950 DOI: 10.1158/1078-0432.CCR-07-0278 PMID: 17606728
72. Arjaans M, Oude Munnink TH and Oosting SF et al (2013) Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake Cancer Res 73(11) 3347–3355 DOI: 10.1158/0008-5472.CAN-12-3518 PMID: 23580572
73. Selvakumaran M, Yao KS and Feldman MD (2008) Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis Biochem Pharmacol 75(3) 627–638 DOI: 10.1016/j.bcp.2007.09.029 PMID: 18178171 PMCID: 2732335
74. Miyazaki S, Kikuchi H and Iino I et al (2014) Anti-VEGF antibody therapy induces tumor hypoxia and stanniocalcin 2 expression and potentiates growth of human colon cancer xenografts Int J Cancer 135(2) 295–307 DOI: 10.1002/ijc.28686 PMID: 24375080
75. Sakata S, Yoshihara T and Arima H et al (2011) Differential effects of organic nitrates on arterial diameter among healthy Japanese participants with different mitochondrial aldehyde dehydrogenase 2 genotypes: randomised crossover trial BMJ open 1(1) e000133 DOI: 10.1136/bmjopen-2011-000133 PMID: 22021773 PMCID: 3191425
76. Reyman B, Van Gisbergen M and Zegers K et al (2015) Nitroglycerin as a sensitizer in the treatment of non small cell lung cancer: from cells in vitro to phase 3 trial Radiother Oncol 115(Supp 1) S290–S291 DOI: 10.1016/S0167-8140(15)40591-2
77. Taurin S, Nehoff H and Greish K (2012) Anticancer nanomedicine and tumor vascular permeability; where is the missing link? J Control Release 164(3) 265–275 DOI: 10.1016/j.jconrel.2012.07.013
78. Nichols JW and Bae YH (2014) EPR: evidence and fallacy J Control Release 190C 451–464 DOI: 10.1016/j.jconrel.2014.03.057
79. Jain RK (1990) Vascular and interstitial barriers to delivery of therapeutic agents in tumors Cancer Metastasis Rev 9(3) 253–266 DOI: 10.1007/BF00046364 PMID: 2292138
80. Stohrer M, Boucher Y and Stangassinger M et al (2000) Oncotic pressure in solid tumors is elevated Cancer Res 60(15) 4251–4255 PMID: 10945638
Repurposing Drugs in Oncology (ReDO)—nitroglycerin as an anti-cancer agent—supplementary material
Introduction
The following drugs warrant further investigation in combination with nitroglycerin (NTG) and existing standard of care chemotherapy and radiotherapy protocols, both in pre-clinical studies and potentially in clinical trials. These combinations, listed in Table 1, have been selected on the basis of existing pre-clinical and clinical experience in each of the indications. In some cases these combinations replicate existing protocols currently being tested in clinical trials, but substitute known and repurposed drugs for the newer and/or more toxic agents currently being investigated. All these proposed combinations are expected to display relatively low toxicity and use low cost and generally available agents. The following drugs are not listed in order of priority.
Table 1. Proposed drug combinations with NTG and standard of care in different cancers.
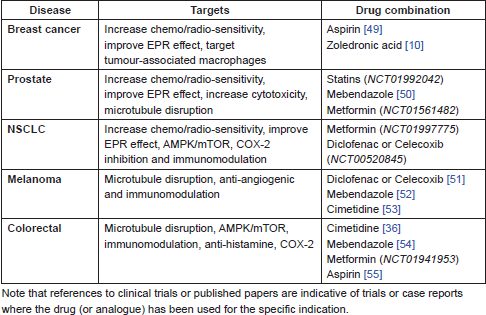
EPR
A number of existing non-cancer drugs with evidence of anticancer activity may benefit from the enhanced permeability and retention effects which NTG can induce. A selection is listed below.
• Mebendazole – The anti-helminthic benzimidazole mebendazole (MBZ) has strong pre-clinical evidence of anti-cancer effects with a multiple possible mechanisms of action and is currently being assessed in a number of clinical trials as an anti-cancer agent [1]. MBZ is known as a microtubule disrupting agent, though with lower toxicity than some of the more commonly used microtubule disrupters of the taxane or vinca alkaloid classes. One of the obstacles to tumour retention of anti-cancer drugs, even in the face of increased vessel permeability due to NTG administration, is the increased interstitial pressure in solid tumours [2]. There is some evidence that microtubule disrupting agents from the taxane class can reduce this pressure, thereby facilitating the increased absorption of anti-cancer agents [3]. The combination of NTG and MBZ may therefore be a synergistic combination to improve the EPR effect with existing standard of care treatments.
• Zoledronic Acid – Nitrogen-containing bisphosphonates, (zoledronate, ibandronate, pamidronate etc), have a long history of clinical use in cancer to prevent and treat skeletal-related events in multiple myeloma and bone metastases [4, 5]. This activity has been related directly to action on bone resorption, for which bisphosphonates are primarily used in the context of treating osteoporosis and other bone-related conditions. However, there has also been an increasing interest in bisphosphonates for their non-bone-resorptive effects in cancer, particularly with respect to effects on the immune system [6–8]. There is also clinical evidence of a positive effect when bisphosphonates are used as adjuvant therapy in breast cancer [9]. It has recently been shown that the extra-skeletal anticancer effects of zoledronic acid may be due to targeting of tumour-associated macrophages, and that zoledronic acid accumulates in tumour tissue due to leaky vasculature [10]. Using NTG to further enhance drug accumulation warrants investigation, particularly in breast cancer.
• Macromolecules – Maeda and colleagues propose the use of macro-molecular agents (>40 kDa) to fully exploit the EPR effect with NTG [11, 12]. Examples include anaerobic bacteria (for example Lactobacillus casei) [13], gold nanoparticles [14], paclitaxel albumin-bound nanoparticles (Abraxane) and other large molecular agents.
Anti-hypoxic
Hypoxia is implicated in resistance to both chemotherapy and radiotherapy in a wide range of cancer types. In this respect NTG has shown some evidence of positive effect and it is proposed that this be further investigated.
• Hormonal therapy for prostate cancer – A Phase II study has shown that very low dose transdermal NTG can slow PSA doubling time for men with relapsed disease [15]. Given these strong results, presumed to have been associated with a reduction in tumour hypoxia, further work is justified. Firstly the results should be replicated in a larger and fully randomised Phase III trial. Secondly, investigation of the effect of NTG on patients with hormone resistant disease is also warranted, particularly in light of the lack of toxicity and low costs associated with this treatment.
• Radiotherapy – There is some evidence that aberrant tumour vasculature and hypoxia are key drivers of resistance to the therapeutic effects of radiotherapy [16, 17]. Therefore addressing this issue of radioresistance is an important strategy in a number of cancers where radiotherapy is used primarily with curative intent, including head and neck cancers, cervical cancer, rectal cancer, oesophageal and gastric cancer. There is some evidence that NTG can improve radiosensitivity in NSCLC, possibly by its anti-hypoxic activity [18]. While this combination continues to be explored in NSCLC in trial NCT01210378, the mechanism of action appears not to be specific to lung cancer and therefore a rationale exists to explore the combination in other cancers.
• Celecoxib/NSAIDs – A number of different NSAIDs are being actively investigated clinically in cancer due to diverse positive effects in terms of anti-inflammatory, anti-angiogenic and possibly anti-hypoxic activity. For example there is pre-clinical evidence that the NSAID indomethacin and COX-2 inhibitor NS-398 exert an anti-hypoxic effect, in part by down-regulating expression of HIF1-α [19]. A more clinically relevant COX-2 inhibitor is celecoxib, which also shows evidence of anti-hypoxic activity [20, 21]. Celecoxib has shown positive results in a clinical trials in a number of different cancer types combined with standard of care treatments, including prostate [22], colorectal [23], and heavily pre-treated ovarian cancer [24]. Attacking tumour hypoxia via different mechanisms using NTG and a COX-2 inhibitor is an appealing prospect that is worthy of further investigation. Other options to target hypoxia in combination with NTG include mTOR inhibitors such as rapamycin [25] and temsirolimus [26], and/or metabolic agents such as metformin [27]. Additionally, there may also be an immunomodulatory aspect to celecoxib in that it may also be counter the possible MDSC promoting effects of NO by inhibiting production of MDSC [28–30].
NO donation
The NO generating activity of NTG may also be of some value in cancer therapy:
• Statins – There is both epidemiological and pre-clinical evidence of an anti-cancer effect of lipophilic statins, including simvastatin, lovastatin and fluvastatin [31]. A number of clinical trials are also currently investigating the addition of statins to existing standard of care treatments in a range of cancers, including prostate, NSCLC, glioblastoma, colorectal and gastric cancers. Multiple mechanisms of action have been proposed for the anti-cancer effects of statins, and these are summarised in [31]. However, there are some indications that in breast cancer cells the cytotoxic effect of statins is related to NO generation [32, 33]. It is possible therefore that the cytotoxic effects of statins may be potentiated by the NO-donating activity of NTG. Given the low toxicity of statins and the potential activity against cancer, the combination with NTG and standard of care treatments warrants exploration in animal models to establish that this posited synergism does exist and can be exploited using standard dosing of these drugs.
Immunomodulatory
There have been concerns regarding the possible effects of NO-donors on the immune system. Therefore it is of interest to investigate the effects of NTG with other agents to explore immunomodulatory activity. Pre-clinical evidence in this area is not sufficiently strong to warrant clinical trials and this may be a topic more suitable for initial exploration in animal models.
• Cimetidine – The classical H2 receptor antagonist (H2RA) cimetidine (Tagamet), primarily used to treat peptic ulcers and heartburn, has shown both in vitro, in vivo and clinical evidence of anti-cancer activity in a range of cancer types [34, 35]. Possessing a number of mechanisms of action, including cell adhesion, anti-angiogenesis and immunomodulation, there is especially strong evidence of a positive effect on survival when used peri-operatively for early stage colorectal cancer [36]. Given the concerns regarding the effect on cell mediated immunity of NTG, addressing this by targeting MDSC cells using cimetidine is an attractive prospect that warrants investigation in a clinical setting. In particular, the use of cimetidine and NTG in the peri-radiotherapy period would be an interesting avenue to explore with respect to maximising the potentially positive immune effects of radiotherapy [37].
Other approaches
An alternative method of exploiting the EPR effect is to paradoxically induce systemic hypertension, (using angiotensin II, epinephrine or other anti-hypotensive) during the administration of chemotherapy. The increase in hypertension induces in an increase in tumour blood flow volume that results in greater accumulation of chemotherapeutic drug, whereas in non-tumour tissues the increase in hypertension induces an increased blood flow velocity. This effect has been shown in animal models [38, 39] and in patients [40, 41], resulting both in increased tumour regression and in sparing non-tumour tissues from toxicity. Whether it is possible to combine this approach with the application of NTG in some way to further maximise on the EPR effect is an intriguing question that has yet to be considered.
Cancer stem cells
Finally, we speculate that NTG (independent of NO) may have an anti-cancer effect by inhibiting specific ALDH enzymes. It is known that NTG can cause deactivation of ALDH2; in fact long term clinical use of NTG gives rise to ‘nitrate tolerance’ where the drug no longer produces vasodilation. Withdrawal of the drug for a period of time restores its function. This phenomenon is attributed to the deactivation of the ALDH2 enzyme that at least partially catalyses the biotransformation of NTG. Wenzel et al showed that a single dose of NTG (0.8 mg) resulted in a 60% decrease in white blood cell ALDH2 activity [42]. Another study by Hink et al showed that in vivo treatment of cardiac bypass patients with NTG for more than 24 hours before surgery caused inhibition of ALDH2 [43]. These studies show that NTG administration results at least in intermittent inhibition of ALDH2.
The specificity of the Aldefluor assay, long assumed to be a specific inhibitor of ALDH1 has been opened to questioning and there is now some evidence that it may also inhibit other ALDH isoenzymes, including ALDH2 [44, 45]. This means that many studies which have assessed ALDH1 expression using only the Aldefluor assay may have not have been able to clearly differentiate between ALDH isoenzymes. This becomes significant in the context of the assessment of cancer cell stem populations using ALDH1 as a proxy marker. ALDH1 expression is associated with poor prognosis in breast [46], ovarian [47], esophageal [48] and other cancers.
In the case that ALDH2 is also a cancer stem cell marker then it is possible that inhibition of ALDH2 by clinically relevant doses of NTG may be a beneficial anticancer strategy.
References
1. Pantziarka P, Bouche G and Meheus L et al (2014) Repurposing drugs in oncology (ReDO)-mebendazole as an anti-cancer agent Ecancermedicalscience 8 443 DOI: 10.3332/ecancer.2014.485 PMID: 25075217 PMCID: 4096024
2. Nichols JW and Bae YH (2014) EPR: evidence and fallacy J Control Release 190C 451–464 DOI: 10.1016/j.jconrel.2014.03.057
3. Taghian AG, Abi-Raad R and Assaad SI et al (2005) Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications J Clin Oncol 23(9) 1951–1961 DOI: 10.1200/JCO.2005.08.119 PMID: 15774788
4. Coleman RE and McCloskey EV (2011) Bisphosphonates in oncology Bone 49(1) 71–76 DOI: 10.1016/j.bone.2011.02.003 PMID: 21320652
5. Lipton A (2007) Treatment of bone metastases and bone pain with bisphosphonates Support Cancer Ther 4(2) 92–100 DOI: 10.3816/SCT.2007.n.003
6. Laggner U, Lopez JS and Perera G et al (2009) Regression of melanoma metastases following treatment with the n-bisphosphonate zoledronate and localised radiotherapy Clinical Immunol 131(3) 367–373 DOI: 10.1016/j.clim.2009.01.008
7. Rogers TL and Holen I (2011) Tumour macrophages as potential targets of bisphosphonates J Transl Med 9(1) 177 DOI: 10.1186/1479-5876-9-177 PMID: 22005011 PMCID: 3215187
8. Santini D, Virzi V and Fratto ME et al (2010) Can we consider zoledronic acid a new antitumor agent? recent evidence in clinical setting Curr Cancer Drug Tar 10(1) 46–54 DOI: 10.2174/156800910790980223
9. He M, Fan W and Zhang X (2013) Adjuvant zoledronic acid therapy for patients with early stage breast cancer: an updated systematic review and meta-analysis J Hematol Oncol 6(1) 80 DOI: 10.1186/1756-8722-6-80 PMID: 24283946 PMCID: 3874690
10. Junankar S, Shay G and Jurczyluk J et al (2014) Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer Cancer Discov PMID: 25312016 PMCID: 4293349
11. Maeda H (2012) Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting Proc Jpn Acad Ser B Phys Biol Sci 88(3) 53–71 DOI: 10.2183/pjab.88.53 PMID: 22450535 PMCID: 3365245
12. Maeda H, Nakamura H and Fang J (2013) The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo Adv Drug Deliv Rev 65(1) 71–79 DOI: 10.1016/j.addr.2012.10.002
13. Fang J, Liao L and Yin H et al (2014) Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei J Pharm Sci 103(10) 3235–3243 DOI: 10.1002/jps.24083 PMID: 25041982
14. Lee J, Chatterjee DK and Lee MH et al (2014) Gold nanoparticles in breast cancer treatment: promise and potential pitfalls Cancer Lett 347(1) 46–53 DOI: 10.1016/j.canlet.2014.02.006 PMID: 24556077 PMCID: 4142062
15. Siemens DR, Heaton JPW and Adams MA et al (2009) Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer Urology 74(4) 878–883 DOI: 10.1016/j.urology.2009.03.004 PMID: 19476985
16. Multhoff G, Radons J and Vaupel P (2014) Critical role of aberrant angiogenesis in the development of tumor hypoxia and associated radioresistance Cancers 6(2) 813–828 DOI: 10.3390/cancers6020813 PMID: 24717239 PMCID: 4074805
17. Wilson WR and Hay MP (2011) Targeting hypoxia in cancer therapy Nat Rev Cancer 11(6) 393–410 DOI: 10.1038/nrc3064 PMID: 21606941
18. Arrieta O, Blake M and de la Mata-Moya MD et al (2014) Phase II study concurrent chemotherapy and radiotherapy with nitroglycerin in locally advanced non-small cell lung cancer Radiother Oncol 1–5
19. Jones MK, Szabó IL and Kawanaka H et al (2002) von Hippel Lindau tumor suppressor and HIF-1alpha: new targets of NSAIDs inhibition of hypoxia-induced angiogenesis FASEB J 16(2) 264–266 PMID: 11772947
20. Sui W, Zhang Y and Wang Z et al (2014) Antitumor effect of a selective COX-2 inhibitor, celecoxib, may be attributed to angiogenesis inhibition through modulating the PTEN/PI3K/Akt/HIF-1 pathway in an H22 murine hepatocarcinoma model Oncol Rep 31(5) 2252–2260 PMID: 24647425
21. Bocca C, Ievolella M and Autelli R et al (2014) Expression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness Expert Opinion Ther Targets 18(2) 121–135 DOI: 10.1517/14728222.2014.860447
22. Sooriakumaran P, Coley HM and Fox SB et al (2009) A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer Anticancer Res 29(5) 1483–1488 PMID: 19443354
23. Jin CH, Wang AH and Chen JM et al (2011) Observation of curative efficacy and prognosis following combination chemotherapy with celecoxib in the treatment of advanced colorectal cancer J Int Med Res 39(6) 2129–2140 DOI: 10.1177/147323001103900609
24. Legge F, Paglia A and DíAsta M et al (2011) Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients BMC Cancer 11 214 DOI: 10.1186/1471-2407-11-214 PMID: 21627839 PMCID: 3123659
25. Hirasawa T, Miyazawa M and Yasuda M et al (2013) Alterations of hypoxia-induced factor signaling pathway due to mammalian target of rapamycin (mTOR) suppression in ovarian clear cell adenocarcinoma: in vivo and in vitro explorations for clinical trial Int J Gynecol Cancer 23(7) 1210–1218 DOI: 10.1097/IGC.0b013e31829d2d51
26. Hudes GR, Berkenblit A and Feingold J et al (2009) Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma Semin Oncol 36(suppl 3) S26–S36 DOI: 10.1053/j.seminoncol.2009.10.013
27. Ranasinghe WKB, Sengupta S and Williams S et al (2014) The effects of nonspecific HIF1α inhibitors on development of castrate resistance and metastases in prostate cancer Cancer Med 3(2) 245–251 DOI: 10.1002/cam4.189 PMID: 24464861 PMCID: 3987074
28. Mao Y, Sarhan D and Steven A et al (2014) Inhibition of tumor-derived prostaglandin-E2 blocks the induction of myeloidderived suppressor cells and recovers natural killer cell activity Clin Cancer Res 20(15) 4096–4106 DOI: 10.1158/1078-0432.CCR-14-0635 PMID: 24907113
29. Sinha P, Clements VK and Fulton AM et al (2007) Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells Cancer Res 67(9) 4507–4513 DOI: 10.1158/0008-5472.CAN-06-4174 PMID: 17483367
30. Veltman JD, Lambers MEH and van Nimwegen M et al (2010) COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma Celecoxib influences MDSC function BMC Cancer 10 464 DOI: 10.1186/1471-2407-10-464
31. Osmak M (2012) Statins and cancer: current and future prospects Cancer Lett 324(1) 1–12 DOI: 10.1016/j.canlet.2012.04.011 PMID: 22542807
32. Kotamraju S and Williams CL et al (2007) Statin-induced breast cancer cell death: role of inducible nitric oxide and arginasedependent pathways Cancer Research 67(15) 7386–7394 DOI: 10.1158/0008-5472.CAN-07-0993 PMID: 17671209
33. Kanugula AK, Gollavilli PN and Vasamsetti SB et al (2014) Statin-induced inhibition of breast cancer proliferation and invasion involves attenuation of iron transport: intermediacy of nitric oxide and antioxidant defence mechanisms FEBS J 281(16) 3719–3738 DOI: 10.1111/febs.12893 PMID: 24964743
34. Pantziarka P, Bouche G and Meheus L et al (2014) Repurposing drugs in oncology (ReDO)-cimetidine as an anti-cancer agent Ecancermedicalscience 8 485 DOI: 10.3332/ecancer.2014.485 PMID: 25525463 PMCID: 4268104
35. Kubecova M, Kolostova K and Pinterova D et al (2011) Cimetidine: an anticancer drug? Eur J Pharm Sci 42(5) 439–444 DOI: 10.1016/j.ejps.2011.02.004 PMID: 21329756
36. Deva S and Jameson M (2012) Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer Cochrane Database Systematic Rev 8(8) CD007814
37. Ahmed MM, Hodge JW and Guha C et al (2013) Harnessing the potential of radiation-induced immune modulation for cancer therapy Cancer Immunol Res 1(5) 280–284 DOI: 10.1158/2326-6066.CIR-13-0141
38. Li CJ, Miyamoto Y and Kojima Y et al (1993) Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure Br J Cancer 67(5) 975–980 DOI: 10.1038/bjc.1993.179 PMID: 8494731 PMCID: 1968457
39. Favoulet P, Magnin G and Guilland JC et al (2001) Pre-clinical study of the epinephrine-cisplatin association for the treatment of intraperitoneal carcinomatosis Eur J Surg Oncol 27(1) 59–64 DOI: 10.1053/ejso.2000.1028 PMID: 11237494
40. Nagamitsu A, Greish K and Maeda H (2009) Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: cases of advanced solid tumors Jpn J Clin Oncol 39(11) 756–766 DOI: 10.1093/jjco/hyp074 PMID: 19596662
41. Goldberg JA, Murray T and Kerr DJ et al (1991) The use of angiotensin II as a potential method of targeting cytotoxic micro-spheres in patients with intrahepatic tumour Br J Cancer 63(2) 308–310 DOI: 10.1038/bjc.1991.71 PMID: 1997111 PMCID: 1971787
42. Wenzel P, Schulz E and Gori T et al (2009) Monitoring white blood cell mitochondrial aldehyde dehydrogenase activity: implications for nitrate therapy in humans J Pharm Exp Ther 330(1) 63–71 DOI: 10.1124/jpet.108.149716
43. Hink U, Daiber A and Kayhan N et al (2007) Oxidative inhibition of the mitochondrial aldehyde dehydrogenase promotes nitroglycerin tolerance in human bloodvVessels J Am Coll Cardiol 50(23) 2226–2232 DOI: 10.1016/j.jacc.2007.08.031 PMID: 18061070
44. Moreb JS, Ucar D and Han S et al (2012) The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance Chem Biol Interact 195(1) 52–60 DOI: 10.1016/j.cbi.2011.10.007 PMCID: 3350780
45. Marcato P, Dean CA and Giacomantonio CA et al (2011) Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform Cell Cycle (Georgetown, Tex.) 10(9) 1378–1384 DOI: 10.4161/cc.10.9.15486
46. Liu Y, Lv D, Duan J and Xu S et al (2014) ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis BMC Cancer 14(1) 444 DOI: 10.1186/1471-2407-14-444 PMID: 24938375 PMCID: 4070403
47. Kuroda T, Hirohash Y and Torigoe T et al (2013) ALDH1-high ovarian cancer stem-like cells can be isolated from serous and clear cell adenocarcinoma cells, and ALDH1 high fxpression Is associated with poor prognosis PLoS ONE 8(6) DOI: 10.1371/journal.pone.0065158
48. Yang L, Ren Y and Yu X et al (2013) ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma Mod Pathol 1–9
49. Fraser DM, Sullivan FM and Thompson AM et al (2014) Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study Br J Cancer 1–5
50. Briasoulis E, Aravantinos G and Kouvatseas G et al (2013) Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study BMC Cancer 13(1) 263 DOI: 10.1186/1471-2407-13-263 PMID: 23718900 PMCID: 3674943
51. Bhatt RS, Merchan J and Parker R et al (2010) A phase 2 pilot trial of low-dose, continuous infusion, or ëmetronomicí paclitaxel and oral celecoxib in patients with metastatic melanoma Cancer 116(7) 1751–1756 DOI: 10.1002/cncr.24902 PMID: 20120033 PMCID: 2847062
52. Doudican NA, Byron SA and Pollock PM et al (2013) XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts AntiCancer Drugs 24(2) 181–188 DOI: 10.1097/CAD.0b013e32835a43f1
53. Mandanas R, Schultz S and Scullin D et al (1991) Phase II trial of cimetidine in metastatic melanoma A Hoosier oncology group trial Am J Clin Oncol 14(5) 397–399 DOI: 10.1097/00000421-199110000-00007 PMID: 1951177
54. Nygren P and Larsson R (2013) Drug repositioning from bench to bedside: tumour remission by the antihelmintic drug mebendazole in refractory metastatic colon cancer Acta Oncol (September) 1–2 PMID: 24160353
55. Bastiaannet E, Sampieri K and Dekkers OM et al (2012) Use of aspirin postdiagnosis improves survival for colon cancer patients Br J Cancer 106(1532–1827 (Electronic)) 1564–1570






